Abstract
Detailed pathologic analysis has delineated a close association between intra-tumoral CD8+ cytotoxic T cells and favorable clinical outcomes in diverse cancers. Conversely, the presence at tumor sites of negative immune regulatory elements, such as FoxP3+ T cells (Tregs) and PD-1/PD-L1 co-stimulatory molecules, is closely associated with inferior patient survival. Together, these results indicate the importance of the balance between cytotoxic and regulatory pathways in the tumor microenvironment as a critical determinant of prognosis. This immune index also provides a framework for devising therapeutic strategies to enlarge the population of anti-tumor cytotoxic T cells and attenuate immune regulation. Among these approaches, vaccination with irradiated, autologous tumor cells engineered to secrete granulocyte-macrophage colony stimulating factor (GM-CSF) followed by antibody blockade of cytotoxic T lymphocyte associated antigen-4 (CTLA-4) provides clinical benefits for some advanced-course melanoma patients. The extent of tumor necrosis in post-treatment biopsies is linearly related to the natural logarithm of the ratio of CD8+ T cells to FoxP3+ Tregs. These findings reveal a concordance between the immune signature of tumor protection in endogenous and therapy-induced responses, strongly supporting Martin Mihm’s original insights.
Introduction
Dr. Martin Mihm’s pioneering characterization of tumor-infiltrating lymphocytes in malignant melanoma has catalyzed research on the host response in the tumor microenvironment. Cancer cells may evoke recognition by both the innate and adaptive immune systems (1). The innate response, which involves granulocytes, macrophages, NK, NKT, and dendritic cells, is the first to be triggered and exploits germ-line encoded pattern recognition receptors to detect stress-induced molecules on tumor cells. The adaptive response, which involves CD4+ and CD8+ T lymphocytes and antibody-producing B cells, is slower to evolve but manifests long-term memory, reflecting the selection and expansion of rare lymphocytes that are specific for tumor-associated antigens.
Substantial evidence indicates that the immune system participates in cancer pathogenesis and may contribute to either disease progression or inhibition of tumor growth (2). These dual roles reflect the complex interplay of innate and adaptive immune elements with cancer cells and non-transformed stromal elements in the tumor microenvironment. These immune interactions resemble the dynamics of wounds that fail to heal, underscoring the oncogenic risks of recurrent tissue injury and abortive attempts at tissue repair (3). While the role of smoldering inflammation in tumor promotion is discussed elsewhere (4, 5), here we highlight clinical evidence, inspired by Dr. Mihm’s pioneering investigations, linking some anti-tumor immune responses with favorable patient outcomes.
Prognostic importance of intra-tumoral lymphocytes
There is compelling evidence that malignant melanoma can evoke immune responses in some patients. Drs. Martin Mihm and Wallace Clark first showed that the radial growth phase of primary melanoma typically elicits a significant dermal lymphocyte reaction that can effectuate partial tumor destruction (6). Clonal T cell expansions have been documented in primary regressing melanoma, and these lymphocytes manifest cytotoxicity towards autologous, cultured melanoma cells (7, 8). CD4+ and CD8+ T cells that react with melanoma cells can be detected in the blood, lymph nodes, and metastases of many patients (9). Moreover, in rare cases, widely disseminated melanoma may undergo spontaneous regression, accompanied by a diffuse infiltrate of lymphocytes, plasma cells, and macrophages (10).
Years ago, these findings prompted Drs. Clark and Mihm to explore the relationship between host responses to melanoma and survival. Through careful morphologic analysis, they established that dense intra-tumoral (but not peri-tumoral) T-cell infiltrates in the vertical growth phase of primary melanoma are tightly correlated with prolonged survival and a reduced incidence of metastatic disease (11, 12). Dr. Mihm and colleagues further showed that brisk T-cell reactions in melanomas that metastasize to regional lymph nodes are similarly predictors for improved survival when compared to lesions that fail to elicit infiltrates (13). These provocative results are currently being extended in several international cohort studies of thousands of patients with long-term clinical follow-up.
Dr. Mihm’s pioneering investigations have motivated several other groups to explore a potential link between T-cell infiltrates and patient outcome. Remarkably similar correlations between tumor-associated T cell infiltrates and prolonged survival have been delineated in ovarian (14), colon (15–17), renal cell (18), and non-small cell lung carcinomas (19) as well as follicular lymphomas (20).
However, an important issue is why these host responses fail to prevent disease development. Multiple immunosuppressive mechanisms in the tumor microenvironment may restrain the breadth and magnitude of host cytotoxic reactions (2). Prominent among these are a small population of professional regulatory T cells characterized by expression of the fork-headed winged-helix transcription factor FoxP3 (21–25). These lymphocytes develop naturally in the thymus or may be generated in the periphery in the presence of non-inflammatory antigen exposure and TGF-β (26, 27).
FoxP3+ regulatory T cells (Tregs) inhibit tumor-specific CD8+ T-cell killing and restrict the effector functions of NK cells and CD1d-restricted NKT cells (28, 29); in murine models, depleting these cells enhances immune-mediated tumor destruction (30, 31). The clinical relevance of Treg-mediated immune suppression is illustrated by the shorter survival of patients harboring the largest numbers of intra-tumoral FoxP3+ cells. Indeed, lymphocyte infiltrates rich in CD8+ cytotoxic T cells but deficient in FoxP3+ regulatory T cells are tightly correlated with improved patient outcomes following standard oncologic therapy, highlighting the balance of these subsets as a critical determinant of protective immunity (32–35).
Complementing these findings are recent investigations establishing a critical role for the PD-1/PD-L1 co-stimulatory pathway in restraining the action of anti-tumor cytotoxic T cells (36). The increased expression of PD-1 or PD-L1 on tumor and/or infiltrating T cells in ovarian cancer (37), pancreatic cancer (38), renal carcinoma (39), and hepatocellular carcinoma (40) is linked with inferior survival. It will be of particular interest to determine whether PD-1/PD-L1 expression could further refine the prognostic value of the ratio of CD8+ to FoxP3+ T cells.
Therapy-induced anti-tumor immune responses
The analysis of endogenous host responses raises the possibility that therapeutic strategies that augment anti-tumor cytotoxic T cells and inhibit FoxP3+ Tregs might potentiate protective immunity. Towards this end, we found that vaccination with irradiated GM-CSF-secreting tumor cells stimulated potent, specific, and long-term protective immunity in several murine tumor models (41). Immunization elicited a dense local infiltrate of macrophages, granulocytes, and CD11b+ dendritic cells that expressed high levels of B7-1, B7-2, MHC class II, and CD1d (42). This reaction resulted in the efficient phagocytosis and processing of dying tumor by the elicited dendritic cells, which in turn migrated to the regional lymph nodes to stimulate tumor-specific lymphocytes. CD4+ and CD8+ T cells, CD1d-restricted invariant NKT cells, and antibodies mediated protective immunity (41–46).
These pre-clinical experiments provided the foundation for Phase I clinical trials of vaccination with irradiated, autologous melanoma cells engineered to secrete GM-CSF in stage IV metastatic melanoma patients (47, 48). Surgically excised tumors were processed to single cells, introduced into short-term culture, and transduced with a replication-defective retrovirus or adenovirus encoding GM-CSF (typically achieving three log increases in cytokine production). The tumors were then irradiated and cryopreserved. No serious toxicity was detected. Most patients developed strong local response to the vaccine characterized by dense infiltrates of dendritic cells, macrophages, eosinophils, and lymphocytes. Though metastatic lesions resected prior to vaccination contained minimal immune infiltrates, metastatic lesions resected following vaccination revealed dense infiltrates of CD4+ and CD8+ T lymphocytes and plasma cells with extensive tumor destruction (at least 80%), fibrosis, and edema in approximately two-thirds of the lesions examined. We also found targeted destruction of the tumor vasculature by activated lymphocytes, eosinophils, and neutrophils. T lymphocytes harvested from the necrotic lesions produced a broad range of cytokines and manifested cytotoxicity to autologous tumor cells. High titers of IgG antibodies reactive with melanoma determinants were identified by flow cytometry and western analysis. Although objective responses were uncommon, approximately 20% of immunized stage IV patients achieved long-term survival in excess of five years. Investigation of blood and tumor samples from vaccinated patients yielded multiple targets of immune-mediated tumor destruction (49).
Despite these reactions, most immunized patients eventually succumbed to progressive disease, indicating that additional defects remain to be addressed. Substantial evidence implicates a critical role for CTLA-4 in attenuating anti-tumor immunity (50). CD28 engagement by B7-1 or B7-2 provides an important co-stimulatory signal for effector T cells, but the subsequent triggering of CTLA-4 by these ligands decreases cytokine production and restrains cellular proliferation (51–53). Moreover, regulatory T cells constitutively express CTLA-4, which is required for their function (54, 55). Although young mice deficient in CTLA-4 develop lethal autoimmunity (56–58), Allison and colleagues showed that transient anti-CTLA-4 antibody blockade increased tumor immunity, with only a partial loss of tolerance to normal tissues (59). CTLA-4 inhibition, either as monotherapy or in conjunction with chemotherapy, provoked tumor regression in a variety of immunogenic models, while combinations with GM-CSF-secreting tumor cell vaccines resulted in synergistic effects in poorly immunogenic models (60–66). Therapeutic immunity was associated with an expansion of CD8+ cytotoxic T cells and inhibition of Tregs at tumor challenge sites (67).
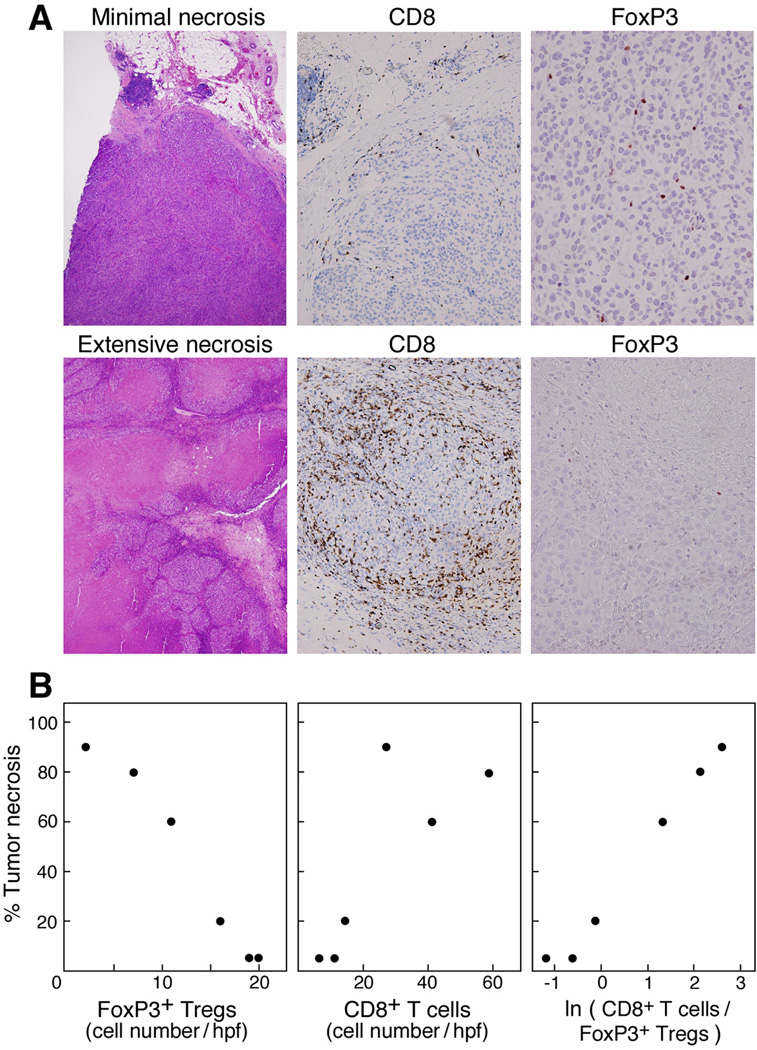
Based on these pre-clinical studies, we administered a fully human anti-CTLA-4 mAb (Ipilumimab; 3 mg/kg at 2–3 month intervals) to 14 metastatic melanoma patients who were previously immunized with irradiated, autologous, GM-CSF-secreting tumor cells (beginning one month after vaccination) (68, 69). Inflammatory toxicities were restricted to a single case of grade-II colitis, a single case of asymptomatic hilar adenopathy (resembling sarcoidosis) that resolved without therapy, and 13 cases of grade I-II skin rashes. Notwithstanding the absence of grade III-IV toxicities, 10 patients achieved clinically meaningful anti-tumor effects, which included four partial responses (53+, 43+, 41+, 20 months) and six stable diseases (46+, 45+, 18, 11, 9, 6 months). Pathologic examination of metastases resected following therapy revealed CD4+ and CD8+ T cells, CD20+ immunoglobulin-producing B cells, and granulocytes juxtaposed to dying melanoma cells. Quantitative analysis of intra-tumoral infiltrates showed that the extent of tumor necrosis was linearly related to the natural logarithm of the ratio of CD8+ T cells to FoxP3+ Tregs, suggesting that the balance between these subsets may be a critical determinant of therapeutic effect (Figure 1).
Figure 1.
The ratio of tumor-infiltrating CD8+ T cells to FoxP3+ Tregs following administration of GM-CSF-secreting melanoma vaccines and anti-CTLA-4 antibody infusion is tightly correlated with the extent of tumor necrosis in advanced melanoma patients. (A). Top, minimal necrosis of melanoma metastasis; bottom, extensive necrosis of melanoma metastasis. (Magnification: H&E ×4; CD8, ×20; FoxP3, ×40.) (B). Numbers of intra-tumoral FoxP3+ Tregs and CD8+ T cells versus tumor necrosis. Reprinted from (69)
Conclusions
Dr. Martin Mihm’s careful morphologic examination of early-stage melanomas first highlighted the prognostic importance of tumor-infiltrating lymphocytes. Further detailed studies of the tumor microenvironment have begun to unravel the major pathways that dictate the outcome of the anti-tumor immune response. The balance of cytotoxic and regulatory pathways has emerged as a major index of tumor destruction in both endogenous and therapy-induced reactions. Additional histologic, immune, and genetic characterization of tumor-infiltrating lymphocytes is likely to prove instrumental to the development of clinically efficacious immunotherapies.
Footnotes
Conflicts of interest
To be confirmed.
References
- 1.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4(1):11. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 2.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 3.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 4.Dougan M, Dranoff G. Inciting inflammation: the RAGE about tumor promotion. J Exp Med. 2008;205(2):267. doi: 10.1084/jem.20080136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5(10):749. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 6.Clark W, From L, Bernardino E, Mihm M. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29:705. [PubMed] [Google Scholar]
- 7.Ferradini L, Mackensen A, Genevee C, et al. Analysis of T cell receptor variability in tumor-infiltrating lymphocytes from a human regressive melanoma. J Clin Invest. 1993;91:1183. doi: 10.1172/JCI116278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackensen A, Carcelain G, Viel S, et al. Direct evidence to support the immunosurveillance concept in a human regressive melanoma. J Clin Invest. 1994;93:1397. doi: 10.1172/JCI117116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 10.Bulkley G, Cohen M, Banks P, Char D, Ketcham A. Long-term spontaneous regression of malignant melanoma with visceral metastases: Report of a case with immunologic profile. Cancer. 1975;36:485. doi: 10.1002/1097-0142(197508)36:2<485::aid-cncr2820360227>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.Clark W, Elder D, Guerry D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 12.Clemente C, Mihm M, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Mihm M, Clemente C, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases-a histopathologic prognostic indicator and an expression of local immune response. Lab Invest. 1996;74:43. [PubMed] [Google Scholar]
- 14.Zhang L, Conejo-Garcia J, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Eng J Med. 2003;348:203. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 15.Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491. [PubMed] [Google Scholar]
- 16.Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 17.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 18.Nakano O, Sato M, Naito Y, et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61(13):5132. [PubMed] [Google Scholar]
- 19.Dieu-Nosjean MC, Antoine M, Danel C, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26(27):4410. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 20.Dave SS, Wright G, Tan B, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351(21):2159. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- 21.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27(1):68. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 22.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 23.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22(3):329. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Ochs HD, Ziegler SF, Torgerson TR. FOXP3 acts as a rheostat of the immune response. Immunol Rev. 2005;203:156. doi: 10.1111/j.0105-2896.2005.00231.x. [DOI] [PubMed] [Google Scholar]
- 25.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6(4):345. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 26.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199(10):1401. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shevach E. CD4+CD25+ Suppressor T cells: More Questions than Answers. Nat Rev Immunol. 2002;2:389. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 28.Chen ML, Pittet MJ, Gorelik L, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci U S A. 2005;102(2):419. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishikawa H, Kato T, Tanida K, et al. CD4+ CD25+ T cells responding to serologically defined autoantigens suppress antitumor immune responses. Proc Natl Acad Sci U S A. 2003;100(19):10902. doi: 10.1073/pnas.1834479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163(10):5211. [PubMed] [Google Scholar]
- 31.Yu P, Lee Y, Liu W, et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201(5):779. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 33.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102(51):18538. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25(18):2586. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 35.Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 36.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 37.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104(9):3360. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13(7):2151. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 39.Thompson RH, Dong H, Lohse CM, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13(6):1757. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 40.Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15(3):971. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 41.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or flt3-ligand. Cancer Res. 2000;60:3239. [PubMed] [Google Scholar]
- 43.Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 44.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll H, Levitsky H. The central role of CD4+ T cells in the antitumor immune response. J Exp Med. 1998;188:2357. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reilly R, Machiels J-P, Emens L, et al. The collaboration of both humoral and cellular HER-2/neu-targeted immune responses is required for the complete eradication of HER-2/neu-expressing tumors. Cancer Res. 2001;61:880. [PubMed] [Google Scholar]
- 46.Gillessen S, Naumov YN, Nieuwenhuis EE, et al. CD1d-restricted T cells regulate dendritic cell function and antitumor immunity in a granulocyte-macrophage colony-stimulating factor-dependent fashion. Proc Natl Acad Sci USA. 2003;100(15):8874. doi: 10.1073/pnas.1033098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soiffer R, Lynch T, Mihm M, et al. Vaccination with irradiated, autologous melanoma cells engineered to secrete human granulocyte-macrophage colony stimulating factor generates potent anti-tumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci USA. 1998;95:13141. doi: 10.1073/pnas.95.22.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soiffer R, Hodi FS, Haluska F, et al. Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanoma. J Clin Oncol. 2003;21(17):3343. doi: 10.1200/JCO.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Hodi FS, Dranoff G. Combinatorial cancer immunotherapy. Adv Immunol. 2006;90:337. doi: 10.1016/S0065-2776(06)90009-1. [DOI] [PubMed] [Google Scholar]
- 50.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 51.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7(4):445. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 52.Doyle AM, Mullen AC, Villarino AV, et al. Induction of cytotoxic T lymphocyte antigen 4 (CTLA-4) restricts clonal expansion of helper T cells. J Exp Med. 2001;194(7):893. doi: 10.1084/jem.194.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 54.Hori S, Takahashi T, Sakaguchi S. Control of autoimmunity by naturally arising regulatory CD4+ T cells. Adv Immunol. 2003;81:331. doi: 10.1016/s0065-2776(03)81008-8. [DOI] [PubMed] [Google Scholar]
- 55.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 56.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 57.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 58.Chambers CA, Sullivan TJ, Allison JP. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity. 1997;7(6):885. doi: 10.1016/s1074-7613(00)80406-9. [DOI] [PubMed] [Google Scholar]
- 59.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 60.Yang YF, Zou JP, Mu J, et al. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: the effect is manifested only at the restricted tumor-bearing stages. Cancer Res. 1997;57(18):4036. [PubMed] [Google Scholar]
- 61.Kwon ED, Hurwitz AA, Foster BA, et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci USA. 1997;94(15):8099. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mokyr MB, Kalinichenko T, Gorelik L, Bluestone JA. Realization of the therapeutic potential of CTLA-4 blockade in low-dose chemotherapy-treated tumor-bearing mice. Cancer Res. 1998;58(23):5301. [PubMed] [Google Scholar]
- 63.van Elsas A, Hurwitz A, Allison J. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Elsas A, Sutmuller RP, Hurwitz AA, et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med. 2001;194(4):481. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hurwitz A, Yu T, Leach D, Allison J. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci USA. 1998;95:10067. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hurwitz AA, Foster BA, Kwon ED, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60(9):2444. [PubMed] [Google Scholar]
- 67.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116(7):1935. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA. 2003;100(8):4712. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105(8):3005. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]