Abstract
Antimicrobial peptides are cationic molecules, which participate in multiple aspects of the immune response including the control of inflammatory diseases, characteristic that make these molecules attractive as therapeutic tools. These peptides are produced in bacteria, insects, plants and vertebrates, and are classified together due to their capacity to directly inhibit the growth of microorganisms, and to regulate the immune response by inducing the secretion of chemokines and cytokines. Various families of antimicrobial peptides have been identified including the cathelicidins and defensins, the most investigated human antimicrobial peptides. This review will cover the main biological functions of antimicrobial and cell-penetrating peptides in inflammation, and describe the importance and utility of antimicrobial peptides as therapeutics for inflammatory diseases.
Keywords: Antimicrobial peptides, cathelicidins, defensins, inflammation
INTRODUCTION
The need for more effective inflammatory diseases therapies with safer side effect profiles has pushed researchers to devise new therapies including new immunomodulatory agents such as antimicrobial peptides. Mammalian antimicrobial peptides represent an efficient arm of the innate immune system. To date, over 1,500 antimicrobial peptides have been listed in different databases.[1,2] These peptides are classified based on secondary structural features, such as cathelicidins (with a linear α-helical structure), defensins (with a β-strand structure), and bactenecins (with a loop structure).[3,4,5] These peptides are also called cationic molecules because have a positive charge provided by arginine (Arg) and lysine (Lys) residues, and are small molecules (fewer than 100 amino acids in lenght). Currently, there are two main genetic categories for antimicrobial peptides in mammals: Cathelicidins and defensins.[6,7,8] Cathelicidins are characterized by an NH2-terminal signal peptide (a highly conserved cathelin domain) and a variable COOH-terminal antimicrobial domain that can be released from the precursor protein after cleavage by proteinases.[9,10,11] The first cathelicidin was isolated from mammalian myeloid cells, showing a conserved NH2 -terminal precursor protein (containing typically about 100 amino acid residues) and a variable COOH-terminal protein domain (10-40 amino acid residues).[12] Importantly, these authors demonstrated that the stability of the α-helix in the structure of this antimicrobial peptide is attributed to peptide concentration-dependent aggregation induced by ionic and hydrophobic interactions. To date, the only human cathelicidin identified is the human cationic antimicrobial protein with a molecular size of 18 kD (hCAP18).[13,14,15] The hCAP18 has 37 amino residues therefore this molecule is also termed LL-37.[16] LL-37 is mainly expressed by neutrophils[17] and epithelial cells.[18,19,20] A more recent study reported that the human cathelicidin LL-37 is a positively charged molecule (+6 at physiological pH of ~7.4) with a high content of Arg and Lys amino acids and adopts an α-helical structure in solutions with ionic composition similar to human plasma.[21] A study by Schaller-Bals et al.[22] showed that the cathelicidin LL-37 is present in the human organism at a very early stage of development, since has been detected at approximately 5 μg/ml in tracheal aspirates of infants during infection. The cathelicidin LL-37 is encoded by only one cathelicidin gene, which is located on chromosome 3 (3p21.3), and is expressed in the squamous epithelia of the airways, mouth, tongue, esophagus and intestine.[23,24] This peptide is constitutively synthesized in spleen, liver, stomach, intestine and bone marrow. Recently, it has been demonstrated that NADPH oxidase 2 interaction with Toll-like receptor (TLR) 2 is required for efficient secretion of cathelicidin.[25]
Defensins represent an important peptide family among antimicrobial peptides. Mammalian defensins are divided into three subfamilies, depending on the position of cysteines and disulfide bridges.[26,27] Currently, six α-defensins and four β-defensins (HBD1–4) have been well characterized.[28,29,30] However, 55 α-defensins,[31,32] and more than 90 β-defensin genes have been found in the human genome based on a computational search strategy.[33] Recently, γ-defensins have been described. Human neutrophil α-defensins (HNPs), isoforms 1-4 differ in a single N-terminal residue but have similar antimicrobial properties. The genes encoding HNPs are located in a contiguous segment of chromosome 8p23,[34] have three exons and two introns.[35] Furthermore, a HNP gene produces a 94-amino acid precursor structure that is made up of a signal peptide (19 amino acid residues from N-terminal) and the prodefensin (74 amino acid residues toward C-terminal). In regard to β-defensins, the first β-defensin was identified in bovine tongue,[36] whereas the first isolated human β-defensin-1(HBD-1) was discovered from hemofiltrates.[37] The second human β-defensin, HBD-2, was discovered in extracts of lesional scales from patients suffering from psoriasis.[38,39] Moreover, we have reported that M. bovis Bacillus Calmette-Guérin (BCG) infection of human cells induces HBD-2 mRNA expression in inflamed skin.[40] Regarding the characterization of human beta-defensin-3, Harder et al.[41] reported that this antimicrobial peptide is expressed mainly in skin and tonsils. β-defensin 4 is highly expressed in the testes and gastric antrum.[29] The main biological functions of cathelicidins and defensins in inflammatory responses, and the therapeutic implications of these peptides in inflammatory diseases are discussed in this revision.
The main role of antimicrobial peptides is the direct lysis of microorganisms through the electrostatic interaction with the cell target, followed by insertion into the plasma membrane accompanied by pore formation.[27] A second mechanism of action of these peptides involves the recognition of intracellular molecules such as DNA, DnaK chaperone, and mitochondria (histatin 5, which induces a decrease of mitochondrial ATP synthesis ending in cell death).
Antimicrobial peptides and inflammatory responses
The inflammatory response is a protective reaction by the host to eliminate injurious stimuli. At present, it is well known that cathelicidins have the capacity to induce secretion of cytokines to promote the recruitment of immune cells to the site of injury.[6,21] In addition, it has been demonstrated that the in vivo contribution of antimicrobial peptides to inflammatory responses depends on their capacity to induce the production of pro-inflammatory cytokines to enhance phagocytosis.[42] In this regard, it has been reported that the cathelicidin LL-37 induces the release of IL-1β, IL-8, TNF-α, IL-6 and granulocyte–macrophage colony stimulating factor (GM-CSF) by keratinocytes, and of TNF-α and IL-6 by immature dendritic cells.[43,44] In addition, it has been demonstrated that LL-37 promotes the expression of IL-1β from monocytes.[45] Furthermore, it has been reported that the antimicrobial peptide LL-37 increases immune responses by activation of the nuclear factor kappa B (NF-κB) pathway in human cells, and that this peptide at 50-100 μg/ml regulates a number of genes in the human epithelial cell line A549 and in the murine macrophage cell line RAW 264.7, some of which are pro-inflammatory mediators.[46] Importantly, it has been acknowledged that the cathelicidin LL-37 is found in various tissues at estimated concentrations of 2-5 μg/ml and at higher levels under inflammatory conditions.[47,48,49] Furthermore, Niyonsaba et al.[50] showed that the cathelicidin LL-37 induces direct chemotaxis for immune cells as another biological activity related to inflammation. In addition, it has been acknowledged that LL-37 has an important role in mast cell recruitment at inflammatory sites, since this antimicrobial peptide has the capacity to induce mast cell degranulation leading to release of inflammatory mediators.[51] More recently, it has been demonstrated the important role of neutrophil-secreted LL-37 in the induction of chemotactic migration of inflammatory cells in vivo.[52]
On the other hand, there is a growing body of evidence that α-defensins play an important role in regulating inflammation. In this context, it has been demonstrated that HNPs increase the production of TNF-α and IL-1β, and decrease the production of IL-10 in human monocytes and adhesion molecule expression in endothelial cells.[53] In support of this, Braff et al.[54] reported that HNP-1,-2, and-3 have an important role as immune modulators by increasing TNF-α and IL-1 secretion in human monocytes infected by bacteria. Recently, a study by Syeda et al.[55] showed that HNP can activate endothelial cells to produce vasoactive molecules such as endothelin-1, indicating that HNP can regulate inflammatory responses. Furthermore, a study by Soehnlein et al.[52] showed that HNP1-3 can up-regulate bacterial phagocytosis by human macrophages. The mechanism for how HNP1-3 influence phagocytosis lies in the capacity of these peptides to induce macrophage release of TNF-α and interferon (IFN)-γ.
At present, it has also been reported that β-defensins are effector molecules of inflammatory responses of the host by regulating the secretion of inflammatory cytokines and chemokines. In this regard, a study by Yang et al.[56] showed that HBD-2 shows immune stimulating properties by chemo-attracting immature dendritic cells and T cells to modify the adaptive immune reaction. In addition, it has been reported that the antimicrobial peptide HBD-2 is involved in wound repair by activating an intrinsic antibiotic mechanism in wounds and inflammation.[57] Furthermore, Niyonsaba and Ogawa[51] showed that human β-defensins increase the expression of TNF-α and IL-1 in human monocytes activated by bacteria. In addition, they also demonstrated that HBD2-4 can stimulate human keratinocytes to increase the secretion of pro-inflammatory cytokines and chemokines such as IL-6, IL-10, MCP-1 and macrophage inflammatory protein (MIP)-3α.[58] Importantly, a study by Nagaoka et al.[59] showed that HBD-3 is a potent modulator of inflammation due to their effects on neutrophil apoptosis. The mechanism for how HBD-3 induces their suppressive roles on neutrophil apoptosis lies in the capacity of this peptide to induce inhibition of caspase 3 activity. More recently, it has been acknowledged that HBD-2 can regulate inflammatory angiogenesis by stimulating migration, and tube formation of human umbilical vein endothelial cells.[60] Furthermore, they also demonstrated that HBD-2 can stimulate chemotaxis of human endothelial cells, promote wound healing and capillary-like tube formation of endothelial cells, indicating that HBD-2 could links inflammatory response and host defense through its pro-angiogenic activity.
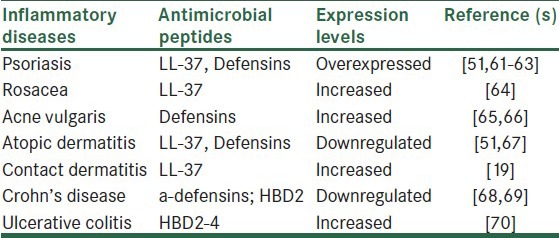
Importantly, a number of clinical studies have shown that several inflammatory diseases are associated with antimicrobial peptide production changes [Table 1]. In this regard, individuals with lower gene copy number of HBD-2 have a significant higher risk of developing Crohn's disease, which is an inflammatory disease of the small intestine and the colon.[68,69] In addition, single-nucleotide polymorphisms in transcription factor-4 are directly related to Crohn's disease incidence.[71] Data from this study indicate that defensins are directly associated with this disease. In regard to skin inflammatory diseases and antimicrobial peptides, it has been documented that patients with atopic dermatitis display an abnormally reduced antimicrobial peptide concentration in lesional skin.[51,67] It has been reported that patients with atopic eczema have a decreased expression of LL-37, HBD2 and HBD3.[67] Psoriasis is another human inflammatory skin disease associated with abnormal antimicrobial peptide expression.[51] In this regard, recent studies have demonstrated that the high levels of LL-37 in psoriasis forms complexes with human self-DNA to activate plasmacytoid dendritic cells.[61] These complexes triggers TLR9 in the cells to produce type I interferons. These interferons trigger maturation of myeloid dendritic cells to activate autoreactive Th1 or Th17 cells, resulting in sustained production of IL-17 and IL-22. The sustained production of these interleukins leads to the expression of LL-37 that forms a feedback loop to maintain the inflammatory responses in psoriasis.[62] In addition, it has been demonstrated that this human inflammatory disease is associated with increased β-defensin genomic copy number.[63] Importantly, patients with rosacea express higher levels of LL-37 on skin with an increased activity of a dermal serine-protease that induces inflammation.[64] Furthermore, it has been demonstrated that patients with acne vulgaris,[65,66] contact dermatitis,[19] and ulcerative colitis[42] have high expression of antimicrobial peptides in their inflammatory lesions. It is important to consider that up-regulation of defensin gene expression during inflammation depends on the activation of the NF-κB, which acts as a master switch for inflammation.[40] In this regard, our results have demonstrated that M. bovis Bacillus Calmette-Guérin (BCG) infection of human epithelial cells induces HBD-2 mRNA expression which is up-regulated by TNF-α produced from M. bovis BCG-infected cells, and is modulated by NF-kB.[70]
Table 1.
Inflammatory diseases associated with antimicrobial peptides production changes
Currently, the literature indicates that some antimicrobial peptides protect the skin from infections. In this regard, LL-37 is the only active form of cathelicidin in the skin, and the concept that endogenous expression of LL-37 protects from skin infections comes from the demonstration that nude mice for the CRAMP cathelicidin gene are much more susceptible to skin infection by group A Streptococcus than wild-type mice are.[72] Furthermore, it has been demonstrated that neonatal skin in humans expresses increased levels of antimicrobial peptides.[73] Importantly, these authors reported that the synergistic antimicrobial activity of LL-37 and HBD-2 efficiently kill the growth of group B Streptococcus, an important neonatal pathogen, indicating that these peptides provide innate immunity during development of cellular immune response mechanisms in the newborn period. In fact, a major criticism of antimicrobial peptide research is that many of the immunomodulatory effects observed in culture occurs at concentrations that are much higher than would be expected in vivo. However, the notion that in humans, the cathelicidin LL-37, in cooperation with other antimicrobial peptides such as HBD-2 prevents infection in digestive and pulmonary systems indicate that the immunomodulatory activity of the cathelicidin LL-37 can be augmented by other antimicrobial peptides. Murakami et al.[74] showed that cathelicidin expression in sweat represent an important innate defense system for the skin. In addition, data from Sayama et al.[75] demonstrated that the apoptosis signal-regulating kinase-1 (ASK1)-mediated keratinocyte differentiation regulates the production of both β-defensins and the cathelicidin LL37 as a new mechanisms of skin innate immunity.
On the other hand, data from Miles et al.[76] showed that α-defensins secreted by human neutrophils in chronic inflammation can inhibit the secretion of multiple pro-inflammatory cytokines, as an important anti-inflammatory mechanism involved in the prevention of damage to tissues. In addition, data from experiments with knockout mice highlight their anti-inflammatory effect of β-defensins. In support of this, it has recently been shown that mouse β-defensin 1 knockout mice when compared with wild-type animals died earlier after infection and also exhibited a grater inflammatory infiltrate early in disease, indicating that mouse β-defensin 1 plays a critical role in controlling inflammation in influenza infection.[77] Taken together, these studies suggest that antimicrobial peptides prime the inflammatory microenvironment by inducing both proinflammatory and anti-inflammatory properties.
Therapeutic implications of antimicrobial peptides in inflammatory diseases
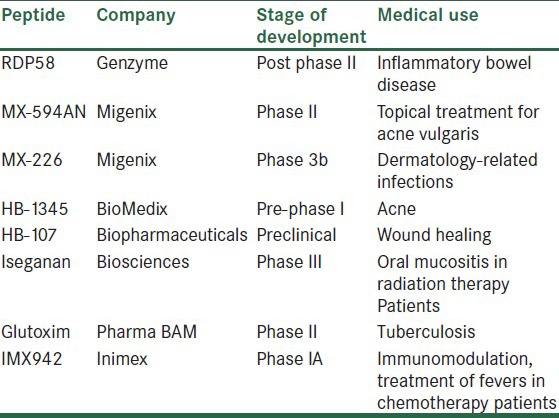
The concept of using antimicrobial peptides as therapeutic tools was first introduced in the late 1990s. Table 2 shows some antimicrobial peptides, which are being tested in inflammatory diseases. Antimicrobial peptides can be used to improve those diseases related with the non-functional endogenous peptides such as Crohn's. The use of antimicrobial peptides as therapeutic tools has as main advantages their rapid action against a broad array of infectious agents and their low tendency for resistance development. However, the tight regulation of the antimicrobial peptides should be considered, since it is well known that persistent amounts of these peptides may lead to a chronic inflammatory process, as it has been demonstrated for psoriasis. In addition, it is important to consider that the practical use of these peptides as therapeutic tools is limited by serious drawbacks concerning bioavailability, metabolic stability, immunogenicity, and production cost.[78] In this regard, the main disadvantage for the practical use of antimicrobial peptides is related to peptide large size. Therefore, the research in this field is currently devoted to develop smaller synthetic peptidomimetics.[79,80] In addition, a number of structural modifications leading to enhanced antimicrobial peptide biological lifetimes, reduction of cytotoxicity and therapeutic index have been proposed.
Table 2.
Antimicrobial peptides in commercial development which are being tested in inflammatory diseases
It is important to consider that patients with atopic dermatitis showed a significant increase in LL-37 expression after treatment with 4000 IU/d oral vitamin D for 21 days.[81] These data support the concept that vitamin D3 induces the expression of LL-37, and that this vitamin might be use in dermatology.[82] In fact, the observation that LL-37 expression is under the control of the vitamin D pathway indicates the use of this vitamin in novel therapies for the treatment of the chronic skin disease psoriasis.
At present, the systemic administration of LL-37 or its induction by vitamin D3 are being tested in the treatment of skin inflammatory diseases. The effectiveness of butyrate administration to induce antimicrobial production to counterattack inflammatory diseases is well documented in animal models.[83] Moreover, an alternative approach has been described using gene therapy methods. In this regard, the cutaneous adenoviral delivery of LL-37 has been an effective method in the treatment of burn wound infections.[84,85,86] Taken together, these data indicate that this is a promising research field with a great potential for the development of antimicrobial peptides as therapeutics that can aid in the control of inflammatory diseases.
CONCLUSIONS
Importantly, the capacity of antimicrobial peptides to regulate different responses connected with host defense, such as chemotaxis of inflammatory cells suggest a high level of integration of these effector molecules with specific innate immune responses. In this regard, these peptides can exert selective immunoregulatory activities on the host inflammatory response, indicating the clinical use of synthetic peptides and the development of analogues as therapeutic tools. An alternative approach is to induce the endogenous production of antimicrobial peptides to avoid adverse systemic reactions and the possible toxicity of synthetic peptides.
Although, the biological activities of antimicrobial peptides in different cell types and significance in the inflammatory responses in vivo clearly require further investigation, it is evident that the participation of antimicrobial peptides in inflammatory diseases illustrates the potential of these peptides as therapeutic agents. A deeper understanding of the processes that regulate antimicrobial expression will potentially assist the practical use of these peptides for therapeutic intervention in inflammatory diseases and could be possible to develop novel and more effective therapeutics for inflammatory diseases.
In summary, substantial progress has been achieves in the last decade with respect to the functional significance of the involvement of antimicrobial peptides in inflammation, and this progress may result in the potential use of antimicrobial peptides as therapeutics that enhance the innate immune defense system.
ACKNOWLEDGEMENTS
PMS is an EDI, COFAA, and SNI fellow.
Footnotes
Source of Support: Escuela Nacional de Ciencias Biologicas, Instituto Politecnico Nacional, Mexico, Kumari
Conflict of Interest: None declared
REFERENCES
- 1.Fjell CD, Hancock RE, Cherkasov A. AMPer: A database and an automated discovery tool for antimicrobial peptides. Bioinformatics. 2007;23:1148–55. doi: 10.1093/bioinformatics/btm068. [DOI] [PubMed] [Google Scholar]
- 2.Wang G, Li X, Wang Z. APD2: The updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 2009;37:D933–7. doi: 10.1093/nar/gkn823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boman HG. Antimicrobial peptides: Basic facts and emerging concepts. J Intern Med. 2003;254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 4.van’t Hof W, Veerman E, Helmerhorst E, Amerongen AV. Antimicrobial peptides: Properties and applicability. Biol Chem. 2001;382:597–619. doi: 10.1515/BC.2001.072. [DOI] [PubMed] [Google Scholar]
- 5.McDermott AM. Defensins and other antimicrobial peptides at the ocular surface. Ocul Surf. 2004;2:229–47. doi: 10.1016/s1542-0124(12)70111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- 7.Ganz T. Defensins: Antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–20. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 8.Lehrer RI. Primate defensins. Nat Rev Microbiol. 2004;2:727–38. doi: 10.1038/nrmicro976. [DOI] [PubMed] [Google Scholar]
- 9.Bals R. Epithelial peptides in host defense against infection. Respir Res. 2000;1:141–50. doi: 10.1186/rr25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckmann L. Defence molecules in intestinal innate immunity against bacterial infections. Curr Opin Gastroenterol. 2005;21:147–51. doi: 10.1097/01.mog.0000153311.97832.8c. [DOI] [PubMed] [Google Scholar]
- 11.Zanetti M, Gennaro R, Romeo D. Cathelicidins: A novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
- 12.Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: Relevance to the molecular basis for its non-cell-selective activity. Biochem J. 1999;341:501–13. [PMC free article] [PubMed] [Google Scholar]
- 13.Agerberth B, Gunne H, Odeberg J, Kogner P, Boman H, Gudmundsson G. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci U S A. 1995;92:195–9. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Smet K, Contreras R. Human antimicrobial peptides: Defensins, cathelicidins and histatins. Biotechnol Lett. 2005;27:1337–47. doi: 10.1007/s10529-005-0936-5. [DOI] [PubMed] [Google Scholar]
- 15.Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC. Human CAP18: A novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–7. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gudmundsson GH, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R. The human gene FALL-39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem. 1996;238:325–32. doi: 10.1111/j.1432-1033.1996.0325z.x. [DOI] [PubMed] [Google Scholar]
- 17.Cowland JB, Johnsen AH, Borregaad N. hCAP-18, a cathelin/probactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995;368:173–6. doi: 10.1016/0014-5793(95)00634-l. [DOI] [PubMed] [Google Scholar]
- 18.Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci U S A. 1998;95:9541–6. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frohm M, Agerberth B, Ahangari G, Stâhle-Bäckdahl M, Lidén S, Wigzell H, et al. The expression of the gene coding for the antimicrobial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–63. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson MF, Sandstedt B, Sorensen O, Weber G, Borregaard N, Ståhle-Bäckdahl M. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun. 1999;67:2561–6. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1408–25. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 22.Schaller-Bals S, Schulze A, Bals R. Increased levels of antimicrobial peptides in tracheal aspirates of newborn infants during infection. Am J Respir Crit Care Med. 2002;165:992–5. doi: 10.1164/ajrccm.165.7.200110-020. [DOI] [PubMed] [Google Scholar]
- 23.Malm J, Sorensen O, Persson T, Frohm-Nilsson M, Johansson B, Bjartell A, et al. The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect Immun. 2000;68:4297–302. doi: 10.1128/iai.68.7.4297-4302.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Méndez-Samperio P. The human cathelicidin hCAP18/LL-37: A multifunctional peptide involved in mycobacterial infections. Peptides. 2010;31:1791–8. doi: 10.1016/j.peptides.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Yang CS, Shin DM, Kim KM, Lee ZW, Lee CH, Park SG, et al. NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. J Immunol. 2009;182:3696–705. doi: 10.4049/jimmunol.0802217. [DOI] [PubMed] [Google Scholar]
- 26.Hoover DM, Rajashankar KR, Blumenthal R, Puri A, Oppenheim JJ, Chertov O, et al. The structure of human beta-defensin-2 shows evidence of higher order oligomerization. J Biol Chem. 2000;275:32911–8. doi: 10.1074/jbc.M006098200. [DOI] [PubMed] [Google Scholar]
- 27.Lehrer R, Lichtenstein A, Ganz T. Defensins: Antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–28. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 28.Ganz T, Lehrer RI. Defensins. Pharmacol Ther. 1995;66:191–205. doi: 10.1016/0163-7258(94)00076-f. [DOI] [PubMed] [Google Scholar]
- 29.García JR, Krause A, Schulz S, Rodríguez-Jiménez FJ, Klüver E, Adermann K, et al. Human b-defensin 4: A novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001;10:1819–21. [PubMed] [Google Scholar]
- 30.Goldman MJ, Anderson GM, Stolzenberg ED, Kari UP, Zassloff M. Human b-defensin-1 is a salt-sensitive antibiotic in the lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–60. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Jimenez FJ, Krause A, Schulz S, Forssmann WG, Conejo-Garcia JR, Schreeb R, et al. Distribution of new human beta-defensin genes clustered on chromosome 20 in functionally different segments of epididymis. Genomics. 2003;81:175–83. doi: 10.1016/s0888-7543(02)00034-4. [DOI] [PubMed] [Google Scholar]
- 32.Schutte BC, Mitros JP, Bartlett JA, Walters JD, Jia HP, Welsh MJ, et al. Discovery of five conserved beta-defensin gene clusters using a computational search strategy. Proc Natl Acad Sci U S A. 2002;99:2129–33. doi: 10.1073/pnas.042692699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schutte BC, McCray PB. Beta-defensins in lung host defense. Annu Rev Physiol. 2002;64:709–48. doi: 10.1146/annurev.physiol.64.081501.134340. [DOI] [PubMed] [Google Scholar]
- 34.Sparkes RS, Kronenberg M, Heinzmann C, Daher KA, Klisak I, Ganz T, et al. Assignment of defensin gene(s) to human chromosome 8p23. Genomics. 1989;5:240–4. doi: 10.1016/0888-7543(89)90052-9. [DOI] [PubMed] [Google Scholar]
- 35.Linzmeier R, Michaelson D, Liu L, Ganz T. The structure of neutrophil defensin genes. FEBS Lett. 1993;321:267–73. doi: 10.1016/0014-5793(93)80122-b. [DOI] [PubMed] [Google Scholar]
- 36.Diamond G, Zasloff M, Eck H, Brasseur M, Maloy WL, Bevins CL. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa:peptide isolation and cloning of a cDNA. Proc Natl Acad Sci U S A. 1991;88:3952–6. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bensch K, Raida M, Magert HJ, Schulz-Knappe P, Forssmann WG. HBD-1: A novel b-defensin from human plasma. FEBS Lett. 1995;368:331–5. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 38.Harder J, Bartels J, Christophers E, Schröder JM. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 39.Schröder JM, Harder J. Human beta-defensin-2. Int J Biochem Cell Biol. 1999;31:645–51. doi: 10.1016/s1357-2725(99)00013-8. [DOI] [PubMed] [Google Scholar]
- 40.Méndez-Samperio P, Miranda E, Trejo A. Mycobacterium bovis Bacillus Calmette-Guérin (BCG) stimulates human beta-defensin-2 gene transcription in human epithelial cells. Cell Immunol. 2006;239:61–6. doi: 10.1016/j.cellimm.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Harder J, Bartels J, Christophers E, Schröder JM. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–13. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 42.Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- 43.Davidson DJ, Currie AJ, Reid GS, Bowdish DM, MacDonald KL, Ma RC, et al. The cationic antimicrobial peptide LL-37 Modulates dendritic cell differentiation and dendritic cell induced T cell polarization. J Immunol. 2004;172:1146–56. doi: 10.4049/jimmunol.172.2.1146. [DOI] [PubMed] [Google Scholar]
- 44.Braff MH, Hawkins MA, Di Nardo A, Lopez-Garcia B, Howell MD, Wong C, et al. Structure–function relationships among human cathelicidin peptides: Dissociation of antimicrobial properties from host immunostimulatory activities. J Immunol. 2005;174:4271–8. doi: 10.4049/jimmunol.174.7.4271. [DOI] [PubMed] [Google Scholar]
- 45.Agerberth B, Gudmundsson GH. Host antimicrobial defence peptides in human disease. Curr Top Microbiol Immunol. 2006;306:67–90. doi: 10.1007/3-540-29916-5_3. [DOI] [PubMed] [Google Scholar]
- 46.Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169:3883–91. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- 47.Bowdish DM, Davidson DJ, Hancock RE. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr Protein Pept Sci. 2005;6:35–51. doi: 10.2174/1389203053027494. [DOI] [PubMed] [Google Scholar]
- 48.Hase K, Murakami M, Iimura M, Cole SP, Horibe Y, Ohtake T, et al. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology. 2003;125:1613–25. doi: 10.1053/j.gastro.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 49.Woo JS, Jeong JY, Hwang YJ, Chae SW, Hwang SJ, Lee HM. Expression of cathelicidin in human salivary glands. Arch Otolaryngol Head Neck Surg. 2003;129:211–4. doi: 10.1001/archotol.129.2.211. [DOI] [PubMed] [Google Scholar]
- 50.Niyonsaba F, Iwabuchi K, Someya A, Hirata M, Matsuda H, Ogawa H, et al. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology. 2002;106:20–6. doi: 10.1046/j.1365-2567.2002.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niyonsaba F, Ogawa H. Protective roles of the skin against infection: Implication of naturally occurring human antimicrobial agents beta-defensins, cathelicidin LL-37 and lysozyme. J Dermatol Sci. 2005;40:157–68. doi: 10.1016/j.jdermsci.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 52.Soehnlein O, Zernecke A, Eriksson E, Rothfuchs AG, Pham CT, Herwald H, et al. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112:1461–71. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaly YV, Paleolog EM, Kolesnikova TS, Tikhonov I, Petratchenko EV, Voitenok N. Neutrophil alpha-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur Cytokine Netw. 2000;11:257–66. [PubMed] [Google Scholar]
- 54.Braff MH, Bardan A, Nizet V, Gallo RL. Cutaneous defense mechanisms by antimicrobial peptides. J Invest Dermatol. 2005;125:9–13. doi: 10.1111/j.0022-202X.2004.23587.x. [DOI] [PubMed] [Google Scholar]
- 55.Syeda F, Tullis E, Slutsky AS, Zhang H. Human neutrophil peptides upregulate expression of COX-2 and endothelin-1 by inducing oxidative stress. Am J Physiol Heart Circ Physiol. 2008;294:H2769–74. doi: 10.1152/ajpheart.00211.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmid P, Grenet O, Medina J, Chibout SD, Osborne C, Cox DA. An intrinsic antibiotic mechanismin wounds and tissue-engineered skin. J Invest Dermatol. 2001;116:471–2. doi: 10.1046/j.1523-1747.2001.01279.x. [DOI] [PubMed] [Google Scholar]
- 57.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, et al. Beta-defensins: Linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 58.Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, et al. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol. 2007;127:594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- 59.Nagaoka I, Niyonsaba F, Tsutsumi-Ishii Y, Tamura H, Hirata M. Evaluation of the effect of human beta-defensins on neutrophil apoptosis. Int Immunol. 2006;20:543–53. doi: 10.1093/intimm/dxn012. [DOI] [PubMed] [Google Scholar]
- 60.Baroni A, Donnarumma G, Paoletti I, Longanesi-Cattani I, Bifulco K, Tufano MA, et al. Antimicrobial human beta defensin-2 stimulates migration, proliferation and tube formation of human umbilical vein endothelial cells. Peptides. 2009;30:267–72. doi: 10.1016/j.peptides.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–9. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 62.Lai Y, Gallo RL. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–41. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hollox EJ, Huffmeier U, Zeeuwen PL, Palla R, Lascorz J, Rodijk-Olthuis D, et al. Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet. 2008;40:23–5. doi: 10.1038/ng.2007.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosácea. Nat Med. 2007;13:975–80. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 65.Di Nardo A, Braff MH, Taylor KR, Na C, Granstein RD, McInturff JE, et al. Cathelicidin antimicrobial peptides block dendritic cell TLR4 activation and allergic contact sensitization. J Immunol. 2007;178:1829–34. doi: 10.4049/jimmunol.178.3.1829. [DOI] [PubMed] [Google Scholar]
- 66.Marta Guarna M, Coulson R, Rubinchik E. Anti-inflammatory activity of cationic peptides: Application to the treatment of acne vulguris. FEMS Microbiol Lett. 2006;257:1–6. doi: 10.1111/j.1574-6968.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 67.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 68.Fellermann K, Stange DE, Schaeffeler E, Schmalzl H, Wehkamp J, Bevins CL, et al. A chromosome 8 gene-cluster polymorphism with low human beta-defensin 2 gene copy number predisposes to Crohn disease of the colon. Am J Hum Genet. 2006;79:439–48. doi: 10.1086/505915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wehkamp J, Harder J, Weichenthal M, Mueller O, Herrlinger KR, Fellermann K, et al. Inducible and constitutive beta-defensins are differentially expressed in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2003;9:215–23. doi: 10.1097/00054725-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 70.Fahlgren A, Hammarstrom S, Danielsson A, Hammarstrom ML. Beta-Defensin-3 and -4 in intestinal epithelial cells display increased mRNA expression in ulcerative colitis. Clin Exp Immunol. 2004;137:379–85. doi: 10.1111/j.1365-2249.2004.02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wehkamp J, Wang G, Kubler I, Nuding S, Gregorieff A, Schnabel A, et al. The Paneth cell alpha-defensin deficiency of ileal Crohn's disease is linked to Wnt/Tcf-4. J Immunol. 2007;179:3109–18. doi: 10.4049/jimmunol.179.5.3109. [DOI] [PubMed] [Google Scholar]
- 72.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–7. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 73.Dorschner RA, Lin KH, Murakami M, Gallo RL. Neonatal skin in mice and humans expresses increased levels of antimicrobial peptides: Innate immunity during development of the adaptive response. Pediatr Res. 2003;53:566–72. doi: 10.1203/01.PDR.0000057205.64451.B7. [DOI] [PubMed] [Google Scholar]
- 74.Murakami M, Ohtake T, Dorschner RA, Schittek B, Garbe C, Gallo RL. Cathelicidin nti-microbial peptide expression in sweat, an innate defense system for the skin. J Invest Dermatol. 2002;119:1090–5. doi: 10.1046/j.1523-1747.2002.19507.x. [DOI] [PubMed] [Google Scholar]
- 75.Sayama K, Komatsuzawa H, Yamasaki K, Shirakata Y, Hanakawa Y, Ouhara K, et al. New mechanisms of skin innate immunity: ASK1-mediated keratinocyte differentiation regulates the expression of beta-defensins, LL37, and TLR2. Eur J Immunol. 2005;35:1886–95. doi: 10.1002/eji.200425827. [DOI] [PubMed] [Google Scholar]
- 76.Miles K, Clarke DJ, Lu W, Sibinska Z, Beaumont PE, Davidson DJ, et al. Dying and necrotic neutrophils are anti-inflammatory secondary to the release of alpha-defensins. J Immunol. 2009;183:2122–32. doi: 10.4049/jimmunol.0804187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ryan L, Diamond G. Mouse beta-defensin-1 plays a role in influenza innate immunity. J Immunol. 2010;184:37–49. [Google Scholar]
- 78.Sharma KR, Reddy PR, Tegge W, Jain R. Discovery of Trp-His and His-Arg analogues as new structural classes of short antimicrobial peptides. J Med Chem. 2009;52:7421–31. doi: 10.1021/jm900622d. [DOI] [PubMed] [Google Scholar]
- 79.Rodziewicz-Motowidlo S, Mickiewicz B, Greber K, Sikorska E, Szultka L, Kamysz E, et al. Antimicrobial and conformational studies of the active and inactive analogues of the protegrin-1 peptide. FEBS J. 2010;277:1010–22. doi: 10.1111/j.1742-4658.2009.07544.x. [DOI] [PubMed] [Google Scholar]
- 80.Saviello RM, Malfi S, Campiglia P, Cavalli A, Grieco P, Novellino E, et al. A New insight into the mechanism of action of the temporin antimicrobial peptides. Biochemistry. 2010;49:1477–85. doi: 10.1021/bi902166d. [DOI] [PubMed] [Google Scholar]
- 81.Hata TR, Kotol P, Jackson M, Nguyen M, Paik A, Udall D, et al. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol. 2008;122:829–31. doi: 10.1016/j.jaci.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Segaert S. Vitamin D regulation of cathelicidin in the skin: Toward a renaissance of vitamin D in dermatology? J Invest Dermotol. 2008;128:773–5. doi: 10.1038/jid.2008.35. [DOI] [PubMed] [Google Scholar]
- 83.Schwab M, Reynders V, Shastri Y, Loitsch S, Stein J, Schröder O. Role of nuclear hormone receptors in butyrate-mediated up-regulation of the antimicrobial peptide cathelicidin in epithelial colorectal cells. Mol Immunol. 2007;44:2107–14. doi: 10.1016/j.molimm.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 84.Jacobsen F, Mittler D, Hirsch T, Gerhards A, Lehnhardt M, Voss B, et al. Transient cutaneous adenoviral gene therapy with human host defense peptide hCAP-18/LL-37 is effective for the treatment of burn wound Infections. Gene Ther. 2005;12:1494–502. doi: 10.1038/sj.gt.3302568. [DOI] [PubMed] [Google Scholar]
- 85.Steinstraesser L, Kraneburg U, Jacobsen F, Al-Benna S. Host defense peptides and their antimicrobial-immunomodulatory duality. Immunobiology. 2011;216:322–33. doi: 10.1016/j.imbio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 86.Yeung AT, Gellatly SL, Hancock RE. Multifunctional cationic host defence peptides and their clinical applications. Cell Mol Life Sci. 2011;68:2161–76. doi: 10.1007/s00018-011-0710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


