Abstract
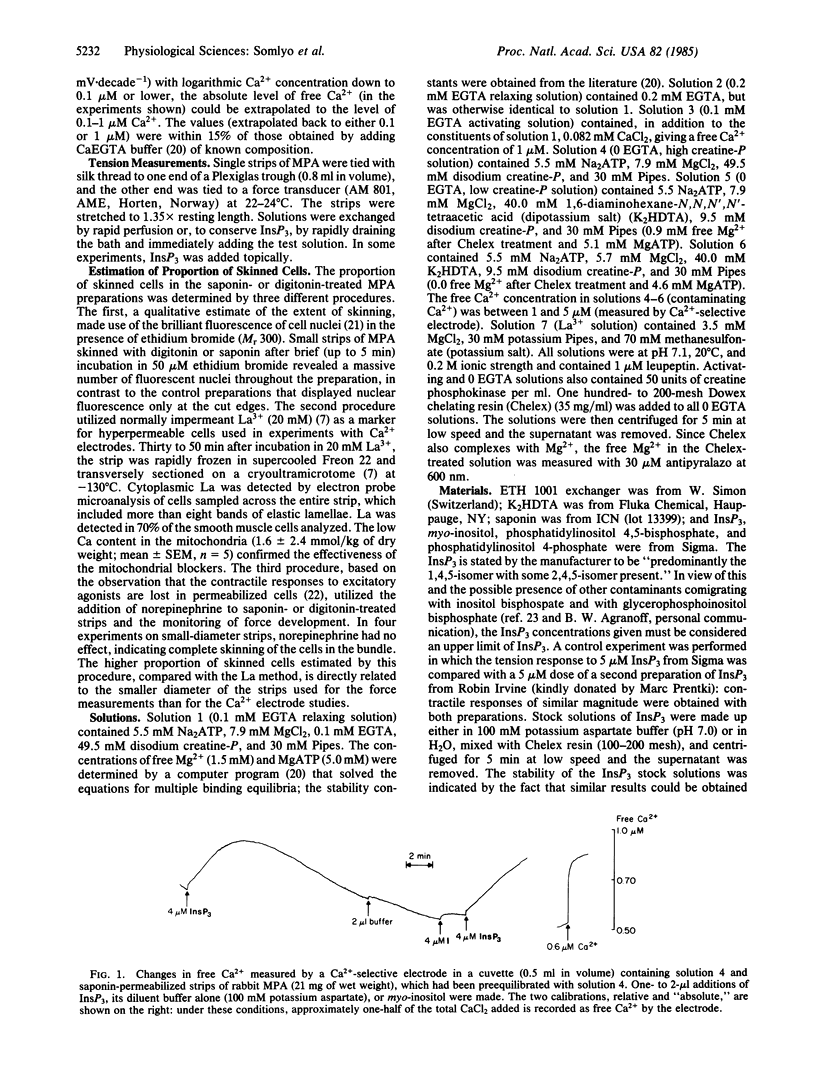
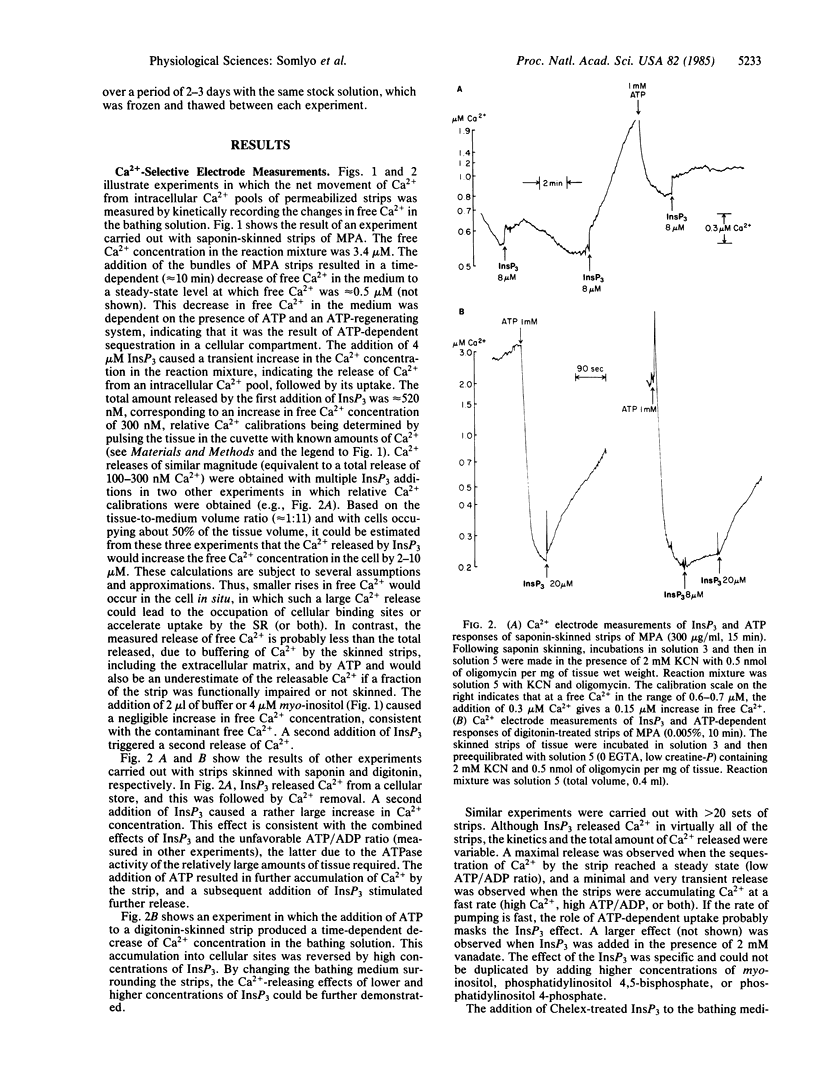
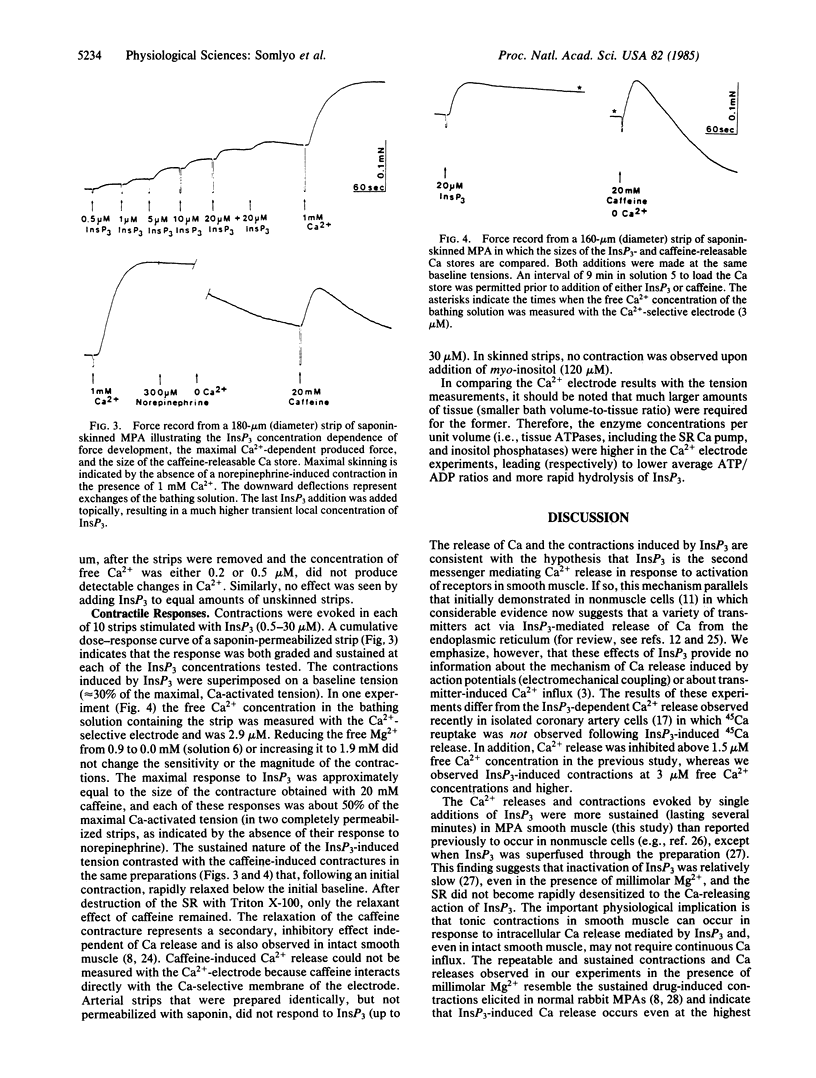
Inositol 1,4,5-trisphosphate (InsP3) caused Ca release and tension development in rabbit main pulmonary artery smooth muscle permeabilized with saponin or digitonin. Both of these responses to single additions of InsP3 (0.5-30 microM) were repeatable and occurred in the presence of 0.0-1.9 mM free Mg2+. Sustained contractions were induced by InsP3. The amount of Ca released by InsP3, measured with a Ca2+-selective electrode, was also estimated to be sufficient to stimulate contraction in intact smooth muscle. Ca release was not influenced by inhibitors of mitochondrial oxidative phosphorylation. The uptake of Ca2+ from the medium into the InsP3-sensitive pool was ATP-dependent. The present results support the hypothesis that, in smooth muscle, InsP3 is the messenger, or one of the messengers, involved in transmitter-induced (pharmacomechanical) Ca release from the sarcoplasmic reticulum, which is the intracellular Ca store identified previously as the source of Ca released by norepinephrine in main pulmonary artery.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar R. A., Abdel-Latif A. A. Carbachol causes rapid phosphodiesteratic cleavage of phosphatidylinositol 4,5-bisphosphate and accumulation of inositol phosphates in rabbit iris smooth muscle; prazosin inhibits noradrenaline- and ionophore A23187-stimulated accumulation of inositol phosphates. Biochem J. 1984 Nov 15;224(1):291–300. doi: 10.1042/bj2240291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron C. B., Cunningham M., Strauss J. F., 3rd, Coburn R. F. Pharmacomechanical coupling in smooth muscle may involve phosphatidylinositol metabolism. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6899–6903. doi: 10.1073/pnas.81.21.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bond M., Kitazawa T., Somlyo A. P., Somlyo A. V. Release and recycling of calcium by the sarcoplasmic reticulum in guinea-pig portal vein smooth muscle. J Physiol. 1984 Oct;355:677–695. doi: 10.1113/jphysiol.1984.sp015445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. Electro- and pharmacomechanical coupling in vascular smooth muscle. Chest. 1980 Jul;78(1 Suppl):150–156. doi: 10.1378/chest.78.1_supplement.150. [DOI] [PubMed] [Google Scholar]
- Coburn R. F., Yamaguchi T. Membrane potential-dependent and-independent tension in the canine tracheal muscle. J Pharmacol Exp Ther. 1977 May;201(2):276–284. [PubMed] [Google Scholar]
- Devine C. E., Somlyo A. V., Somlyo A. P. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972 Mar;52(3):690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., Yagi S., Iino M. Tension-pCa relation and sarcoplasmic reticulum responses in chemically skinned smooth muscle fibers. Fed Proc. 1982 May;41(7):2245–2250. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fiskum G., Craig S. W., Decker G. L., Lehninger A. L. The cytoskeleton of digitonin-treated rat hepatocytes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3430–3434. doi: 10.1073/pnas.77.6.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts B. D. Involvement of guanine nucleotide-binding protein in the gating of Ca2+ by receptors. Nature. 1983 Nov 3;306(5938):64–66. doi: 10.1038/306064a0. [DOI] [PubMed] [Google Scholar]
- HOKIN M. R., HOKIN L. E. Enzyme secretion and the incorporation of P32 into phospholipides of pancreas slices. J Biol Chem. 1953 Aug;203(2):967–977. [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Downes C. P. Inositol trisphosphates in carbachol-stimulated rat parotid glands. Biochem J. 1984 Oct 1;223(1):237–243. doi: 10.1042/bj2230237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Differences and similarities in the noradrenaline- and caffeine-induced mechanical responses in the rabbit mesenteric artery. J Physiol. 1983 Apr;337:609–629. doi: 10.1113/jphysiol.1983.sp014645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. M., Michell R. H. The relationship of calcium to receptor-controlled stimulation of phosphatidylinositol turnover. Effects of acetylcholine, adrenaline, calcium ions, cinchocaine and a bivalent cation ionophore on rat parotid-gland fragments. Biochem J. 1975 Jun;148(3):479–485. doi: 10.1042/bj1480479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina E. G., Briley P. A., De Robertis E. Effect of adrenergic agonists on phosphatidylinositol labelling in heart and aorta. Biochim Biophys Acta. 1976 Jun 22;431(3):624–630. doi: 10.1016/0005-2760(76)90226-5. [DOI] [PubMed] [Google Scholar]
- Leijten P. A., van Breemen C. The effects of caffeine on the noradrenaline-sensitive calcium store in rabbit aorta. J Physiol. 1984 Dec;357:327–339. doi: 10.1113/jphysiol.1984.sp015502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martonosi A. N. Mechanisms of Ca2+ release from sarcoplasmic reticulum of skeletal muscle. Physiol Rev. 1984 Oct;64(4):1240–1320. doi: 10.1152/physrev.1984.64.4.1240. [DOI] [PubMed] [Google Scholar]
- Prentki M., Wollheim C. B., Lew P. D. Ca2+ homeostasis in permeabilized human neutrophils. Characterization of Ca2+-sequestering pools and the action of inositol 1,4,5-triphosphate. J Biol Chem. 1984 Nov 25;259(22):13777–13782. [PubMed] [Google Scholar]
- Somlyo A. P. Cell physiology: cellular site of calcium regulation. Nature. 1984 Jun 7;309(5968):516–517. doi: 10.1038/309516b0. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Devine C. E., Somlyo A. V., North S. R. Sarcoplasmic reticulum and the temperature-dependent contraction of smooth muscle in calcium-free solutions. J Cell Biol. 1971 Dec;51(3):722–741. doi: 10.1083/jcb.51.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Shuman H., Endo M. Calcium and monovalent ions in smooth muscle. Fed Proc. 1982 Oct;41(12):2883–2890. [PubMed] [Google Scholar]
- Somlyo A. V., Somlyo A. P. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J Pharmacol Exp Ther. 1968 Jan;159(1):129–145. [PubMed] [Google Scholar]
- Somlyo A. V., Vinall P., Somlyo A. P. Excitation-contraction coupling and electrical events in two types of vascular smooth muscle. Microvasc Res. 1969 Oct;1(4):354–373. doi: 10.1016/0026-2862(69)90014-4. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Hashimoto T., Kuriyama H. Inositol 1,4,5-trisphosphate releases Ca2+ from intracellular store sites in skinned single cells of porcine coronary artery. Biochem Biophys Res Commun. 1984 Apr 30;120(2):481–485. doi: 10.1016/0006-291x(84)91279-8. [DOI] [PubMed] [Google Scholar]
- Takenawa T. Inositol phospholipids in stimulated smooth muscles. Cell Calcium. 1982 Oct;3(4-5):359–368. doi: 10.1016/0143-4160(82)90023-9. [DOI] [PubMed] [Google Scholar]
- Wuytack F., Casteels R. Demonstration of a (Ca2+ + Mg2+)-ATPase activity probably related to Ca2+ transport in the microsomal fraction of porcine coronary artery smooth muscle. Biochim Biophys Acta. 1980 Jan 25;595(2):257–263. doi: 10.1016/0005-2736(80)90088-7. [DOI] [PubMed] [Google Scholar]


