Abstract
Background:
Acute myeloid leukemia (AML) is the most common type of leukemia. In this study, outcome of intensive chemotherapy in patients treated in a large urban public university hospital in a developing country was investigated.
Materials and Methods:
The records of all patients treated for AML with 3 + 7 protocol from 2002 to 2010 were analyzed.
Results:
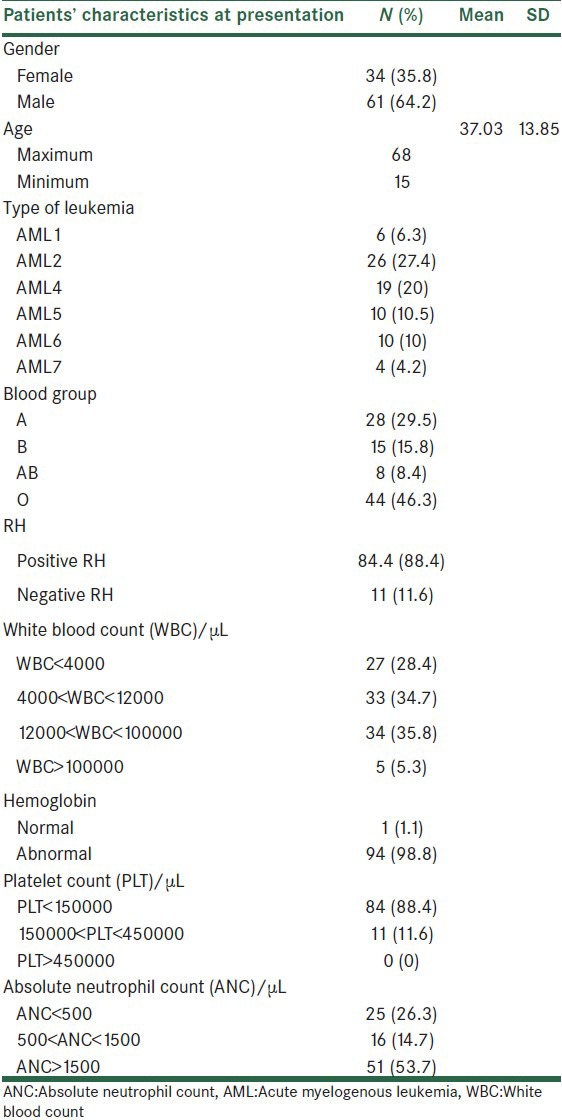
Among 95 patients, 34 (35.8%) were female and 61 (64.2%) were male patients. Patients’ median age was 37 years, ranging 15-68 years. Complete remission (CR) was observed in 56 (58.9%) of patients treated with this protocol. Median overall survival was 13 months (95% CI: 8.8-17.1 months). The 1-year AML survival rate was 51%, and 2-year survival rate was 26%.
Conclusion:
Our study shows that in our center in Iran, CR rates and median overall survival rates after induction chemotherapy are less than developed countries.
Keywords: Acute myeloid leukemia, remission, survival, treatment
INTRODUCTION
Acute myelogenous leukemia (AML) is a malignant disease of the bone marrow in which hematopoietic precursors are arrested in an early stage of development. Most AML subtypes are distinguished from other related blood disorders by the presence of more than 20% blasts in the bone marrow.[1]
AML is the most common type of leukemia.[2] The age-adjusted incidence rate of AML is 3.5 per 100,000 men and women per year.[2]
Current standard induction treatment for AML involves drug regimens with two or more agents that include an anthracycline antibiotic or an anthraquinone and cytarabine.[3] Remission rates in the studies cited range from approximately 55-80% in adult subjects.[3,4,5,6]
These figures are derived from studies conducted in North America and Western Europe and may not reflect the pattern of disease in the developing world. It is known that there are some important differences among patients due to ethnic, socioeconomic, and environmental factors.[7]
In this investigation, remission rates and long-term outcomes of AML in adults in our center are reported.
MATERIALS AND METHODS
The Seyed al Shohada Hospital is a referral hematology center in Isfahan. Patients are referred from center and west part of Iran. In this report, we present the clinicopathological features, remission rates, and long-term outcomes of AML in adults in our center from 2002 to 2010.
We reviewed the charts of all adult patients with primary AML, admitted between March 2002 and March 2010 in this center.
Patients with a history of myelodysplasia, other antecedent hematologic cancers, and those previously exposed to cytotoxic chemotherapy or radiation therapy were excluded.
AML was diagnosed by studying peripheral blood smears, bone marrow aspiration, and biopsy slides stained with Wright, Periodic acid-Schiff stain (PAS) and myeloperoxidase. All slides were independently reviewed by two experienced hematologist and pathologist, who are not involved in the study. Immunophenotypic and cytogenetic studies were performed at diagnosis on bone marrow samples. AML was defined as the presence of more than 20% of myeloblasts in the bone marrow.[8] Subtype of AML was recorded according to French-American-British (FAB) classification.[9] Complete remission (CR) was defined as the absence of abnormal clinical symptoms and having less than 5% of myeloblasts in the bone marrow, absence of blasts with Auer rods; absence of extramedullary disease; absolute neutrophil count >1.0 × 109/L (1000/μL); platelet count >100 × 109/L (100,000/μL); independence of red cell transfusions.[10]
Induction regimen consisted of ara-C 100 mg/m2 for 7 days plus an anthracycline for 3 days, either daunorubicin 45-60 mg/m2 or idarubicin 12 mg/m2 (3 + 7).[11]
Platelet support was provided in the case of bleeding, or whenever the platelet count was less than 20 × 109/L. Platelets were collected from random donors. Red cell transfusions were administered to maintain the hemoglobin above 9 g/dL or on clinical grounds.
Empiric antibiotics were given to all febrile patients (two or more temperature readings above 38°C in a 24-h period, or a single reading of 38.5°C or higher) with neutropenia (fewer than 0.5 × 109/L neutrophils) following an initial clinical and laboratory evaluation. Carbapenem or combination of ceftazidime and amikacin was used.
The inclusion of another antibiotic (vancomycin or anti-anaerobe) was dictated by clinical findings. If fever persisted at day 7, intravenous amphotericin B was added to antibiotics. Antibiotic and antifungal therapy was continued until the number of granulocytes was greater than 0.5 × 109/L.
RESULTS
Among 95 patients, 34 patients (35.8%) were females and 61 (64.2%) were males. Patients’ median age was 37 years, ranging 15-68 years.
Table 1 shows patients’ characteristics at the time of hospital admission.
Table 1.
Patients’ characteristics at the time of hospital admission
Cytogenetic study was performed in 14 patients. In nine of these patients, cytogenetic was normal.
All 95 patients received 3 + 7 regime. Sixty-eight patients received daunorubicin 45 mg/m2, 13 patients treated with daunorubicin 60 mg/m2, and another 14 patients received idarubicin 12 mg/m2.
Adverse effects of induction chemotherapy were pulmonary failure, renal failure, increase in liver enzymes, and oral ulcers [Table 2].
Table 2.
Nonhematological toxicity of induction chemotherapy

Febrile neutropenia occurred in 88.4% of patients. All febrile patients received antibiotics, and 40% of febrile patients treated with empiric amphotericin B.
Only 20% of patients had a microbiological focus of infection. The most common infection sites were central venous catheter (6.4%), urinary tract infection (4.3%), and gastrointestinal infection (3.2%).
Overall, 73 patients (76.8%) were transfused with platelets, and 87 (88.4%) received packed cell transfusion.
Two patients expired during induction chemotherapy and mortality rate was 2.1%. One patient expired 26th day of chemotherapy due to uncontrolled infection without any response to chemotherapy in bone marrow evaluation of day 14, and another one died in 10th day of treatment and the cause of death was indeterminate.
CR was attained in 56 (58.9%) patients. Thirty-four (35.8%) patients had no response to chemotherapy in day 28 evaluation. One patient died before day 28 and in another four patients, bone marrow evaluation was not performed due to lack of patient consent.
Final outcome of all enrolled patients was evaluated 6 months after study endpoint.
At this time, 27 (28.4%) of patients were alive. Mortality rate was 70.5% and outcome of one patient was not evaluable.
Median overall survival was 13 months (95% CI: 8.8-17.1 months). The 1-year AML survival rate was 51% and 2-year survival rate was 26%.
Figure 1 shows the Kaplan–Meier curve of overall survival in AML patients.
Figure 1.
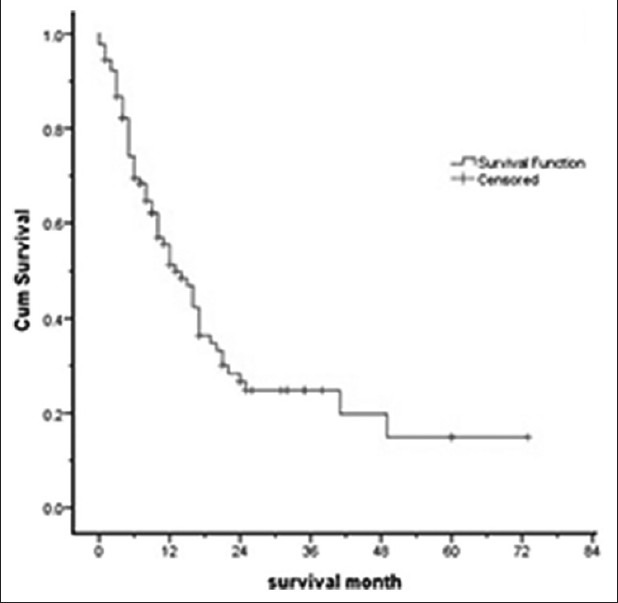
Kaplan – Meier curve of overall survival in acute myelogenous leukemia (AML) patients
Bone marrow transplantation was performed in 11 (11.6%) of patients. At the time of outcome evaluation, six of transplanted patients were alive.
DISCUSSION
There is very little information available from developing Asian countries including Iran about the results of AML treatment. We performed this retrospective study to find out the outcome of AML treatment in a large referral Hematology/Oncology Hospital in central part of Iran.
Characteristics of AML patients including age, gender ratio, and subtypes of AML in this study were not different from similar studies.[12]
Three days of an anthracycline (e.g. daunorubicin, at least 60 mg/m2, idarubicin, 10-12 mg/m2, or the anthracenedione mitoxantrone, 10-12 mg/m2) and 7 days of cytarabine (100-200 mg/m2 continuous IV) (3 + 7) currently remain the standard for induction therapy. With such regimes, CR is achieved in 60-80% of younger adults.[8] No other intervention has been convincingly shown to be better.[13]
In our study, CR rate after induction chemotherapy was 58.9%, less than reported rates in developed countries clinical trials.[8]
In a similar study from northwest of Iran, after chemotherapy with 3 + 7 regimen, CR rate was 52.5%.[12]
In another study in Brazil, another developing country, CR rate reported to be 56% for subset of patients who had been treated with 3 + 7 regime.[14]
It seems that reported remission rate in developed country is different from that of developing countries.
One of the facts that could be effective in decreasing CR rate in our study is the protocol of treatment in our hospital. AML patients regardless of bone marrow findings in day 14 received only one course of 3 + 7 regime for treatment.
Recent data from the Eastern Cooperative Oncology Group (ECOG) suggest that patients who receive a second cycle of induction therapy on day 14, based on the presence of unequivocal residual leukemia, and subsequently achieve a CR, have a prognosis that is similar to those achieving CR with one cycle of induction.[15] Based on these findings, the standard protocol in many centers is administration of second course of 3 + 7 in patients who had unequivocal residual leukemia blasts in bone marrow evaluation of day 14 or 1 week after treatment.[16]
In our center, there are no standard clean rooms for neutropenic patients and concern of severe infections after second course of induction chemotherapy was a major factor for approval of this protocol.
Another factor that could explain lower rate of CR in our center is the dosage of anthracycline in our center.
The traditional dose of daunorubicin (45 mg/m2 for 3 days) is no longer appropriate as induction therapy for AML.[17] A recent randomized trial for younger patients under age 60 years reported a significantly higher CR rate for patients receiving 90 mg/m2 of daunorubicin compared with 45 mg/m2.[4] The overall survival was also improved with the higher dose of daunorubicin.[4]
The dose of daunorubicin in our patients was 45-60 mg/m2.
Based on these data, increasing the dose of daunorubicin to 90 mg/m2 would be an appropriate recommendation for treating AML patients.
Cytogenetic study is a mandatory test in diagnosis and treatment of AML patients.[8]
However, in our study, this test was done only in 11% of patients. The major causes of this finding were unavailability of this test in our center and lack of insurance cover for this expensive test.
Another finding is low rate of transplantation (only 11%) in our patients.
This low rate could explain the lower rate of overall survival of patients in comparisons with other studies.
Transplantation in Iran is completely covered by insurance, but the major limiting factor for doing transplantation is long waiting list of major center of transplantation in Tehran, capital of Iran, and long distance between center of Iran and transplantation center that is a major prohibitive factor for doing transplantation.
In conclusion, our study shows that in our center in Iran, unavailability of cytogenetic and molecular studies make many problems in diagnosis and risk stratification of patients. CR rates and median overall survival rates after induction chemotherapy are less than developed countries.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood. 2009;114:937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 2.Howlader NN, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al., editors. Bethesda, MD: National Cancer Institute; 2011. SEER Cancer Statistics Review, 1975-2008. Available from: http://www.seercancergov/csr/1975_2008/(based on November 2010 SEER data submission, posted to the SEER web site) [Google Scholar]
- 3.Wiernik PH, Banks PL, Case DC, Jr, Arlin ZA, Periman PO, Todd MB, et al. Cytarabine plus idarubicin or daunorubicin as induction and consolidation therapy for previously untreated adult patients with acute myeloid leukemia. Blood. 1992;79:313–9. [PubMed] [Google Scholar]
- 4.Fernandez HF, Sun Z, Yao X, Litzow MR, Luger SM, Paietta EM, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:1249–59. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flasshove M, Meusers P, Schütte J, Noppeney R, Beelen DW, Sohrab S, et al. Long-term survival after induction therapy with idarubicin and cytosine arabinoside for de novo acute myeloid leukemia. Ann Hematol. 2000;79:533–42. doi: 10.1007/s002770000193. [DOI] [PubMed] [Google Scholar]
- 6.Holowiecki J, Grosicki S, Robak T, Kyrcz-Krzemien S, Giebel S, Hellmann A, et al. Addition of cladribine to daunorubicin and cytarabine increases complete remission rate after a single course of induction treatment in acute myeloid leukemia. Multicenter, phase III study. Leukemia. 2004;18:989–97. doi: 10.1038/sj.leu.2403336. [DOI] [PubMed] [Google Scholar]
- 7.Deschler B, Lübbert M. Acute myeloid leukemia: Epidemiology and etiology. Cancer. 2006;107:2099–107. doi: 10.1002/cncr.22233. [DOI] [PubMed] [Google Scholar]
- 8.Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European Leukemia Net. Blood. 2010;115:453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 9.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976;33:451–8. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 10.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Bishop JF. The treatment of adult acute myeloid leukemia. Semin Oncol. 1997;24:57–69. [PubMed] [Google Scholar]
- 12.Eivazi-Ziaei J. Control of acute myeloid leukemia morbidity in northwest Iran. Asian Pac J Cancer Prev. 2005;6:472–3. [PubMed] [Google Scholar]
- 13.Löwenberg B, Griffin JD, Tallman MS. Acute myeloid leukemia and acute promyelocytic leukemia. Hematology Am Soc Hematol Educ Program. 2003:82–101. [PubMed] [Google Scholar]
- 14.Pulcheri W, Spector N, Nucci M, de Morais JC, Pimenta G, de Oliveira HP. The treatment of acute myeloid leukemia in Brazil: Progress and obstacles. Haematologica. 1995;80:130–5. [PubMed] [Google Scholar]
- 15.Rowe JM, Kim HT, Cassileth PA, Lazarus HM, Litzow MR, Wiernik PH, et al. Adult patients with acute myeloid leukemia who achieve complete remission after 1 or 2 cycles of induction have a similar prognosis: A report on 1980 patients registered to 6 studies conducted by the Eastern Cooperative Oncology Group. Cancer. 2010;116:5012–21. doi: 10.1002/cncr.25263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acute myeloid leukemia. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) 2012. [Last cited on 6 Feb 2012]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/aml.pdf .
- 17.Rowe JM, Tallman MS. How I treat acute myeloid leukemia. Blood. 2010;116:3147–56. doi: 10.1182/blood-2010-05-260117. [DOI] [PubMed] [Google Scholar]


