Abstract
Background:
Drug-resistant strain of Herpes simplex virus type 1 (HSV-I) has increased the interest in the use of natural substances.
Aims:
This study was aimed to determine minimum inhibitory concentration of hydroalchoholic extract of a traditionally used herbal plant, Quercus persica L., on HSV-1 replication on baby hamster kidney (BHK) cells.
Setting:
The study was conducted in Shahrekord University of Medical Sciences, Iran.
Design:
This was an experimental study.
Materials and Methods:
BHK cells were grown in monolayer culture with Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal calf serum and plated onto 48-well culture plates. Fifty percent cytotoxic concentration (CC50%) of Q. persica L. on BHK cells was determined. Subsequently, 50% inhibitory concentration (IC50%) of the extract on replication of HSV-1 both in interacellular and exteracellular cases was assessed.
Statistical Analysis:
Statistic Probit model was used for statistical analysis. The dose-dependent effect of antiviral activity of the extracts was determined by linear regression.
Results:
Q. persica L. had no cytotoxic effect on this cell line. There was significant relationship between the concentration of the extract and cell death (P<0.01). IC50s of Q. persica L. on HSV-1, before and after attachment to BHK cells were 1.02 and 0.257 μg/mL, respectively. There was significant relationship between the concentration of this extract and inhibition of cytopathic effect (CPE) (P<0.05). Antioxidant capacity of the extract was 67.5%.
Conclusions:
The hydroalchoholic extract of Q. persica L. is potentially an appropriate and promising anti herpetic herbal medicine.
Keywords: Acorn, antiviral, cytopathic effect, herbal medicine, inhibitory concentration, mode of action
INTRODUCTION
Herpes simplex virus type 1 (HSV-1) infections are very common and the virus is an important pathogen for humans. HSV-1 initially replicates in mucoepithelial cells, causes disease at the site of infection, and then establishes latent infection of the innervating neurons. The most common infections of the virus include gingivostomatitis and pharyngitis.[1] Following latency establishment, HSV-1 causes recurrent infections, most commonly herpetic labialis. Herpetic keratitis leads to permanent scaring, corneal damage, and blindness. HSV-1 also causes encephalitis leading to destruction of the temporal lobe, seizure, and focal neurologic abnormalities.[2]
Since 1970s, acyclovir has been an effective available medication and it is still the most commonly used drug for HSV-1.[3] This agent is used to shorten the course and decrease the severity of these clinical symptoms and may suppress the virus itself.[2] The other anti-herpetic agents licensed currently for the treatment of herpes virus infections include acyclovir and its derivatives, foscarnet and cidofovir, all of which inhibit herpes virus DNA polymerases.[4] Some of these antiviral agents might produce toxic side-effects. In addition, the emergence of virus strains resistant to commonly used anti-herpes virus drugs is of importance, particularly in immunocompromised patients.[5] Thus, new antiviral agents exhibiting different mechanisms of action are urgently needed.[6] Medicinal plants have been used for many years for the treatment of human diseases and a number of herbal medicines have been developed into therapeutic agents. In Iran, there are a large number of different medicinal plants, such as Quercus persica with antimicrobial activities.[7]
Q. persica is a tree in the genus Quercus “oak”, of which about 600 species exist. The genus is native to the northern hemisphere and includes deciduous and evergreen species extending from cold latitudes to tropical Asia and the Americas.
A large region of forest in the north-west of Iran is covered by various oak species, mainly dominated by Q. persica.[8] In this region, oak leaves are an important source of forage for goats during periods of the year when quality and quantity of pasture herbages is limited. Quercus species have been reported to contain high levels of tannins in both hydrolysable[9] and condensed[10] forms.
The oak is an important source of wood and fiber. The oak fruit is a nut called an acorn, borne in a cup-like structure known as a cupule. Each acorn usually contains one seed and rarely two or three ones. Oak fruit include starch, protein, oil, and tannins. This fruit is useful in treatment of anemia, diarrhea, etc, and the other one application is livestock feeding.
Acorn has been utilized in Europe, Asia, North Africa, Middle East, and North America over the last 6,000 years.[11] Acorns are an important food source of starch, oil, and protein for North American Indians, especially in the east and west where sloping topography limited cereal crop production.[12] Nowadays, acorns are still consumed especially in Korea, China, and Japan.[13] Acorns are naturally high in tannins,[14] amylose, amylopectin, and high molecular weight and viscosity substances.[15] Later studies of several Quercus sp. found acorns containing 48–85% carbohydrates (dry weight with most varieties over 72%),[16,17] and starch content of 59% (dry weight)[18] with starch described as beige to yellow-brown in color.[17] Amylase activity on acorn starch has also been reported.[19]
Q. persica has been shown to have antibacterial activities and is said to have antiviral effect, too. However, there is not scientific data supporting the efficiency of this plant on viral infections. Therefore, this study was aimed to evaluate in vitro antiviral activity of Quercqus persica L., against HSV-1 using BHK cells.
MATERIALS AND METHODS
Folin-denis reagent
This reagent was prepared freshly by adding 10 g sodium tungstate and 2 g phosphomolybdic acid in 75 mL distilled water in a suitable flask and further adding 5 mL phosphoric acid. The mixture was refluxed for 2 h and made up to one liter with water. The reagent was protected from exposure to light.
Sodium carbonate solution
350 g sodium carbonate was dissolved in 1 L of water at 70-80°C and filter through glasswool after allowing it to stand overnight.
Standard tannic acid solution
100 mg tannic acid was dissolved in 100 mL of distilled water.
Working standard solution
5 mL of the stock solution was diluted to 100 mL with distilled water. 1 mL contains 50 μg tannic acid.
Extract preparation
Q. persica L. fruits of the Chaharmahal and Bakhtiari province were purchased from a grocery in Shahrekord city. The fruits were characterized by a botanist (Mortaza Rafieian) and a specimen was kept in Herbarium unit in Medical Plants Research Centre of Shahrekord University of Medical Sciences, Iran Herbarium number: 325).
Then, the fruits were washed, dried, and powdered. The powdered fruits of Q. persica L. were added 500 mL of 70% ethanol and incubated for 48 h at room temperature. Subsequently, the mixture was filtered and the solvent (ethanol) was separated from the solution by distillation at 40°C. Five milliliters of the solution was incubated for 48 h at 40°C until bone dried. The extractable percent was 38%.
The dried extract was dissolved at a concentration of 20 mg/mL in water containing 10% dimethyl sulfoxide. This stock preparation was filtered using a 0.45 filter and stored at –20°C until using.
The extract was standardized by measuring the antioxidant activity, total flavonoids, tannins, and total phenolic compounds of Q. persica L. extract.
Antioxidant activity of Q. persica L.
Antioxidant activity of the extract was determined using the ferric thiocyanate method as described previously.[20] Briefly, 500 μg of the extract was dissolved in ethanol and added to a reaction mixture containing 2.88 mL of 2.5% linoleic acid and 9 mL of 40 mM phosphate buffer. The mixture was incubated at 40°C for 96 h and every 12 h, 0.1 mL of it was diluted with 9.7 mL of 75% ethanol, 0.1 mL of ammonium thiocyanate and 0.1 mL of FeCl2. The absorbance of the samples was measured at 500 nm and the percent of inhibition (the capacity to inhibit the peroxide formation in linoleic acid) was determined using the following equation (A high inhibition percent indicates a high antioxidant activity). Ethanol with sample and without reagents was used as negative control.
Percent of inhibition = [1– (absorbance of sample)/(absorbance of control] ×100.
Determination of total flavonoid compounds of Q. persica L. extract
The total flavonoid compounds in the extract were determined using the colorimetric method as described by Chang and colleagues.[21] In brief, 0.5 mL of the extract or Rutin (standard flavonoid compound) was mixed with 1.5 mL of methanol, 0.1 mL of 10% aluminum chloride, 0.1 mL of 1 M potassium acetate and 2.8 mL of distilled water and left at room temperature for 30 min. The absorbance of the reaction mixture was measured at 415 nm prepared using Rutin solutions at concentrations of 25 to 500 ppm in methanol. The experiment was repeated for three times. The total amount of flavonoid was expressed in terms of Rutin equivalent (mg/g), which is a common reference compound.
Determination of tannin compounds of Q. persica L. extract
The tannin compounds in the extract were determined colorimetrically with the Folin–Denis reagent, using the method as described by.[22] Brief, the procedure used is as follows:
5 g Powdered material and 75 mL water were transferred to a 250 mL conical flask and boiled for 30 min. Then it was centrifuged at 2,000 rpm for 20 min. The supernatant was collected in a 100 mL volumetric flask and made up the volume.
0.1 mL of the sample extract was transferred to a 10 mL volumetric flask containing 7.5 mL water.
5 mL of Folin-Denis reagent and 1 mL of sodium carbonate solution was added and diluted to 10 mL with water. The absorbance was read at 700 nm after 30 min.
If absorbance was greater than 0.7, a 1 + 4 dilution of the sample was made.
The blank was prepared with water instead of the sample.
A standard graph was prepared by using 0-100 mg tannic acid.
The tannin content of the samples was calculated as tannic acid equivalents from the standard graph.
Determination of total phenolic compounds of Q. persica L. extract
The total phenolic compounds in the extract were determined colorimetrically with the Folin–Ciocalteu reagent, using the method described by Kim and coworkers.[23] Briefly, 5 mL of the extract or Gallic acid (standard phenolic compound) was mixed with Folin Ciocalteu reagent (1:10 diluted with distilled water) and aqueous Na2 CO3 (4 mL, 1 M). The mixtures were allowed to stand for 15 min and the total phenols were determined by colorimetry at 765 nm. A standard curve was prepared using 0, 50, 100, 150, 200, 250 mg/L solutions of Gallic acid in methanol: Water (50:50, v/v). Total phenol values were expressed in terms of Gallic acid equivalent (mg/g), which is a common reference compound. The experiment was repeated for three times.
Cell culture and viruses
Baby hamster kidney (BHK) cells were used in this study. Cells were grown in DMEM supplemented with glutamine, antibiotics, and 10% fetal bovine serum (FBS). Maintenance medium contained 1% FBS (MEM). The American Type Culture Collection (ATCC) laboratory strain, HSV-1 strain F was used in this assay to determine binding and post binding inhibition of HSV-1 to the cells. Virus stock was kept at −70°C until use.
Cytotoxicity assay
The cytotoxicity was determined by the inhibition of BHK growth as previously described.[6] Briefly, BHK cells were seeded at a density of 5×104 cells/well in 48-well plates and incubated at 37°C for 2 days. The culture medium was replaced with fresh medium containing test compounds at various concentrations, and the cells were further incubated for 2 days. The cells were trypsinized and the number of viable cells was determined by Trypan blue exclusion. The concentration of compound that reduced cell viability by 50% was determined from a standard curve relating percent cell viability to the compound concentration using SPSS15 Software and Probit statistical method.
Inhibition of binding of HSV-1 to BHK cells by herbal extracts
In this experiment, a modified procedure was used to assess the inhibition of HSV-1 binding to BHK cells by the herbal extracts as previously described.[24] Briefly, the binding assay was performed at 4°C to allow the virus to bind to cellular receptors but not enter the cell. Thus, all plates were cooled on ice and incubated at 4°C for 30 min. Serial two-fold dilutions of the herbal extract mixed with cooled HSV-1 strain F and were added to the BHK cells in microtiter plates. Infected and uninfected wells were used as positive and negative control, respectively. The plates were incubated at 4°C for 2 h to allow the virus to bind to the cells. All wells were washed four times with cold phosphate buffer saline (PBS) to remove the extracts and unbound virus. Modified Eagle's Medium (MEM) supplemented with 3% fecal calf serum was added to each well and the plates were incubated at 37°C for 48 h to cytopathic effect (CPE) was appeared. Subsequently, the degree of CPE in the wells was calculated using a standard carve comparing with that of control. The 50% inhibitory concentration (IC50%) was determined from a curve relating CPE inhibition to the concentration of each extract.
Postbinding inhibition of HSV-1 to BHK cells by herbal extracts
To evaluate the post binding inhibition of the extracts on HSV-1, BHK cells were grown in microtiter plates. The plates were cooled on ice for 30 min and inoculated with a chilled suspension of HSV-1. The plates were incubated at 4°C for 2 h to allow the virus to bind to the cellular receptors and washed four times with cold PBS to remove any unbound viruses. The temperature of the plates was shifted to 37°C and treated with eight serial two-fold dilutions of the herbal extracts. Subsequently, MEM supplemented with 3% fecal calf serum was added to each well and the plates were incubated at 37°C for 48 h until CPE was appeared. The degree of CPE in the wells was calculated using a standard carve comparing with that of control. The IC50% was determined from a curve relating CPE inhibition to the concentration of each extract.
Statistics
Statistic Probit model and SPSS was used in this study for statistical analysis. The dose-dependent effect of antiviral activity of the extracts was determined by linear regression.
RESULTS
Antioxidant capacity, flavonoids, phenolic, and tannin compounds of Q. persica L.
Antioxidant capacity (the percentage of inhibition or the capacity to inhibit the peroxide formation in linoleic acid) was found to be 67.5%. The amounts of tannins, flavonoids, and phenolic compounds in extract were found to be 123, 56.4, and 216 mg/g Q. persica powder, respectively.
Cytotoxicity of the extracts on BHK cells
Based on Probit analysis, surprisingly, Q. persica L. showed no cytotoxic effect on this cell line up to 200 mg/mL concentration. The analysis showed significant relationship between the concentration of the extract and cell death with more increase the extract concentration, the more cell death was shown (P<0.01).
Anti-HSV-1 activity of Q. persica L. on HSV-1 before and after cellular attachment
Based on Probit analysis, IC50 of Q. persica L. on HSV-1 before and after attachment to BHK cells was 1.02 and 0.257 ug/mL, respectively [Figure 1]. Based on the model, with the increasing of the extract concentration, the percentage of inhibition of the cytopathic effect (CPE) was increased (P<0.05). Based on these results, Q. persica L. showed strong activity against HSV-1.
Figure 1.
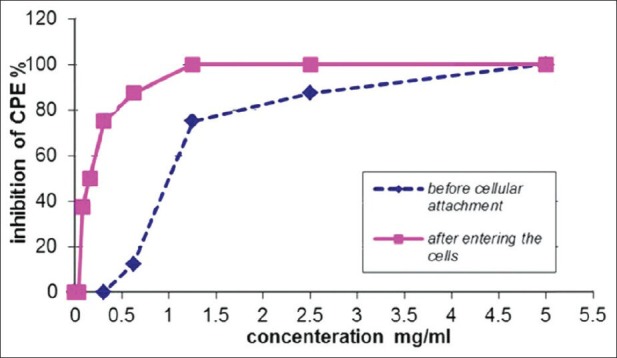
The inhibitory concentration of extract on HSV-1 before and after attachment to BHK cells.
DISCUSSION
There is currently increasing interest to use natural products in treating and preventing medical problems. At present, due to induction of resistance of pathogens to chemical drugs and the prevalence of the fatal different infections, the search for biological active extracts based traditionally used plants is extensively carried out.[25]
Human herpes viruses are found worldwide and are among the most frequent causes of viral infections particularly in immunosuppressed patients.[25]
During the past two decade, a better understanding of the replication and disease causing state of herpes simplex virus type 1 and 2 (HSV-1 and HSV-2), has been achieved due the development of potent antiviral compounds that target these viruses.
Most of the antiviral drugs have toxicities. In addition, the increased and prolonged use of these compounds in clinical setting, especially for the treatment of immunosuppressed patients, has led to the emergence of viral resistance against most of these drugs.[26]
The mechanism of virus inhibition by the most herbal extracts includes inhibition of viral adsorption to the cell surface and interfering with post absorption of viral particles including penetration, uncoating and replication of the virus inside the host cell.[27,28] This study was aimed to evaluate in vitro antiviral activity of hydroalcoholic extract of Q. persica L., against HSV-1. This herbal extract has a long history of use as a traditional herbal medicine and it has been used for treating of inflammatory diseases.[29]
In this study, Q. persica L. surprisingly exhibited the in vitro anti-HSV-1 activity without cytotoxicity, as exhibited no cytotoxic effect on this cell line up to concentration of 200 mg/mL. This extract showed high degree of protection by inhibition of HSV-1 replication as indicated by relative absence or reduction of CPE in virus inhibition assay before and after cellular attachment in concentrations of 1.02 and 0.257, respectively [Figure 1]. It has been suggested that tannin-based components of oat fruits have remarkable effect on virus replication with different mechanisms and with the minimum cytotoxicity.[30] As the extract used in this study is a tannin-rich component, its effects could be attributed to this component.
The results obtained by the percentage of CPE inhibition when the extract was applied with pre viral treatment the extract had acceptable virucidal activity (1.02 μg/mL) either by its direct effect on the virus or forming a complex with the virus preventing from being adsorbed to its binding sites on the cells, most probably preventing the virus to attach to the cellular biding sites [Figure 1], in consistent with some published results.[31] Q. persica L. also showed high degree of inhibitory effect on HSV-1 replication after cellular attachment as revealed by the percentage of CPE inhibition [Figure 1]. These results agreed with the study on an essential oil which has antiviral activity against an acyclovir resistant strain of HSV-1 (HSV-1-Acv). This essential oil is capable to exert a direct virucidal effect on HSV by interfering with targets located at the end of the viral replication cycle.[31]
For using in this study, the extract was prepared with 80% ethanol and, after drying, tested without further purification. It has been suggested that more purification of the extract might increase its antiviral activity.[32] As the extract was not completely purified, it probably showed its partial antiviral activity and it should be possible to increase the anti-HSV-1 activity through purification of the extract.
Generally, many antiviral compounds can be found in botanical sources which have the ability to inhibit human DNA and RNA viruses which cause serious diseases to humans without damaging or affecting the host cells. From this investigation, we hope to open a way for several studies in this field on the promising effectiveness of herbal extracts to be used with or without commercial therapeutic agents against human viral infections.
The results of this study showed a high level of phenolic compounds, particularly tannins in Q. persica L. extract, which have antioxidant activity. In fact there are some published reports indicating antiviral,[33] antibacterial[34] and anti-inflammatory[35] potentials of Q. persica L. These effects of the Q. persica L. have been attributed to its phenolic contents particularly to its flavonoid and tannin compounds.[21,36] It should be accepted that the benefits of phytochemicals extend far beyond both their phenolic contents and antioxidant capabilities.[24,25] Scientific investigations on Q. persica L. are proceeding rapidly in many directions. Further research at the molecular level is needed to precisely clarify the mechanism of Q. persica L.
CONCLUSIONS
Our results, herein, introduce an herbal extract with high potential activity against HSV-1 with low cytotoxicity which is most probably due to its phenolic compounds, particularly to its tannin compounds. Therefore, it would be promising candidate for developing a new anti-HSV-1 agent without or with minimum cytotoxicity.
Footnotes
Source of Support: Research deputy of Shahrekord University of Medical Sciences.
Conflict of Interest: None declared.
REFERENCES
- 1.Khan MT, Ather A, Thompson KD, Gambari R. Extracts and molecules from medicinal plants against Herpes simplex viruses. Antiviral Res. 2005;67:107–19. doi: 10.1016/j.antiviral.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Whitley RJ, Roizman B. Herpes simplex virus infections. Lancet. 2001;357:1513–8. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 3.Brady RC, Bernstein DI. Treatment of herpes simplex virus infections. Antiviral Res. 2004;61:73–81. doi: 10.1016/j.antiviral.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Whitley RJ, Levin M, Barton N, Hershey BJ, Davis G, Keeney RE, et al. Infections caused by Herpes simplex in the immunocompromised host: Natural history and topical acyclovir therapy. J Infect Dis. 1984;150:323–9. doi: 10.1093/infdis/150.3.323. [DOI] [PubMed] [Google Scholar]
- 5.De Clercq E. Antiviral drugs in current clinical use. J Clin Virol. 2004;30:115–33. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Kurokawa M, Ochiai H, Nagasaka K, Neki M, Xu H, Kadota S, et al. Antiviral traditional medicines against herpes simplex virus (HSV-1), poliovirus, and measles virus in vitro and their therapeutic efficacies for HSV-1 infection in mice. Antiviral Res. 1993;22:175–88. doi: 10.1016/0166-3542(93)90094-y. [DOI] [PubMed] [Google Scholar]
- 7.Teimouri M, Korori S, Moraghebi F, Matinizadeh M. Comparison antibacterial activity of quercus persica and quercus ilex. Iranian J Pharm Res. 2004;3:76–7. [Google Scholar]
- 8.Fattahi M. Study of Zagros oak forests and the most important factor of its destruction. Research Institute of Forest and Rangeland (RIFR) 1994:135. [Google Scholar]
- 9.Makkar HP. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rum Res. 2003;49:241–56. [Google Scholar]
- 10.Makkar HP, Dawra RK, Singh B. Tannin levels in leaves of some oak species at different stages of maturity. J Sci Food Agric. 1991;54:513–9. [Google Scholar]
- 11.Bainbridge DA. Quercus, a multi-purpose tree for temperate climates. Intl Tree Crops J. 1986;3:291–8. [Google Scholar]
- 12.Goodrum PD. Food of the Indians, acorn bread. Pac Hist. 1973;17:77–80. [Google Scholar]
- 13.Bainbridge DA. Use of acorns for food in California: Past, present and future, Symposium on Multiple-use Management of California's Hardwoods. 1986:12–4. [Google Scholar]
- 14.Nakabayashi T. Tannins of fruits and vegetables. Adsorption of polyphenolics with insoluble polyvinylpyrrolidone (polyclar) Jpn J Food Sci Technol. 1972;19:84–90. [Google Scholar]
- 15.Hirst EL, Jones JK, Roudier AJ. Structure of acorn starch. J Chem Soc. 1948;104:1779–83. doi: 10.1039/jr9480001779. [DOI] [PubMed] [Google Scholar]
- 16.Ofcarcik RP, Burns EE. Chemical and physical properties of selected acorns. J Food Sci. 1971;36:576–8. [Google Scholar]
- 17.Kim JO, Lee MJ. Studies on some physicochemical properties of the acorn starch. Kor J Food Sci Technol. 1976;8:230–5. [Google Scholar]
- 18.Saffarzadeh A, Vincze L, Csapó J. Determination of the chemical composition of acorn (Quercus branti), Pistacia atlantica and Pistacia khinjuk seeds as non conventional feedstuffs. Acta Agraria Kaposváriensis. 1999;3:59–69. [Google Scholar]
- 19.Kekos D, Macris BJ. Production and characterization of amylase from Calvatia gigantea. Appl Environ Microbiol. 1983;45:935–41. doi: 10.1128/aem.45.3.935-941.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masude T, Isobe J, Jitoe A, Nakamati N. Antioxidative curcuminoids from rhizomes of Curcuma xanthorrhiza. Phytochemistry. 1992;31:3645–7. [Google Scholar]
- 21.Chang C, Yang M, Wen H, Chern J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–82. [Google Scholar]
- 22.Schanderl SH. New York: Academic Press; 1970. Methods in Food Analysis; p. 709. [Google Scholar]
- 23.Kim DO, Jeong SW, Lee CY. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003;51:321–6. [Google Scholar]
- 24.Ferrea G, Canessa A, Sampietro F, Crudani M, Romussi G, Bassetti D. In vitro activity of a Combretum micranthum extract against Herpes simplex virus types 1 and 2. Antiviral Res. 1993;21:317–25. doi: 10.1016/0166-3542(93)90010-g. [DOI] [PubMed] [Google Scholar]
- 25.Rabindran R, Muthulakshmi P, Ganapathy T, Doraiswamy S. Induction of resistance in rice to rice Tungro Virus using horsegram (Vigna unguiculata Walp. Sub sp. unguiculata) seed sprout extract Madras Agric J. 2003;90:286–8. [Google Scholar]
- 26.Villarreal EC. Current and potential therapies for the treatment of herpes virus infections. Prog Drug Res. 2003;60:263–307. doi: 10.1007/978-3-0348-8012-1_8. [DOI] [PubMed] [Google Scholar]
- 27.Amoros M, Lurton E, Boustie J, Girre L. Comparison of the antiherpes simplex virus activities of propolis and 3-methyl-But-2 Enyl cafferate. J Nat Prod. 1994;57:644–7. doi: 10.1021/np50107a013. [DOI] [PubMed] [Google Scholar]
- 28.Barakat AB, Shoman SA, Dina N, Omar R, Alfarouk R. Antiviral activity and mode of action of Dianthus caryophyllus L. and Lupinus termes L. Seed extracts against in vitro herpes simplex and hepatitis A viruses infection. J Microbiol Antimicrob. 2010;2:23–9. [Google Scholar]
- 29.Muliawan SY, Shamala Devi LS, Hashim O, Yusof R. Inhibitory potential of Quercus lusitanica extract on Dengue virus type 2 replication. Southeast Asian J Trop Med Public Health. 2006;37:132–5. [PubMed] [Google Scholar]
- 30.Buzzini P, Arapitsas P, Goretti M, Branda E, Turchetti B, Pinelli P. Antimicrobial and Antiviral Activity of Hydrolysable Tannins. Mini Rev Med Chem. 2008;8:1179–87. doi: 10.2174/138955708786140990. [DOI] [PubMed] [Google Scholar]
- 31.Keyaerts E, Vijgen L, Pannecouque C, Van DE, Peumans W, Egberink H, et al. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res. 2007;75:179–87. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deig EF, Ehresmann DW, Hatch MT, Riedlinger DJ. Inhibition of herpesvirus eplication by marine algae extracts. Antimicrob Agents Chemother. 1974;6:524–5. doi: 10.1128/aac.6.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baba M, Pauwels R, Balzarini J, Arnout J, Desmyter J, De Clercq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob Agents Chemother. 1988;32:1742–5. doi: 10.1128/aac.32.11.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muliawan Y, Shamala SY, Devi LS, Hashim O, Yusof R. Inhibitory potential of Quercus lusitanica extract on Dengue Virus type 2 replication. Southeast Asian J Trop Med Public Health. 2006;37:132–5. [PubMed] [Google Scholar]
- 35.Kiarostami KH. Evaluation of the antibacterial effects of Quercus persica and Quercus castaneifolia in tissue culture and perfect plant. J Sci. 1998;11:1–8. [Google Scholar]
- 36.Chang C, Yang M, Wen H, Chern J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–82. [Google Scholar]


