Abstract
The aim of this study was to learn the toxicity and efficacy of adding 4 doses of rituximab to a standard platinum-based salvage regimen for relapsed CD20+ B-cell non-Hodgkin lymphoma. Patients were treated with rituximab 375 mg/m2 days 1,8,15, 22 (cycle 1 only); cisplatin 100 mg/m2 over 24 h on day 3, cytosine arabinoside 2 g/m2 IV every 12 h × two doses on day 4, dexamethasone 40 mg PO/IV days 3–6, and G-CSF days 5–14. The ORR was 82% (47/57) with 33% (19/57) complete remissions and 49% (28/57) partial remissions. The duration of response (DR) for the 47 responders was 10.5 months (95% CI: 5.3–16.8). The median time to progression (TTP) was 10.3 months (95% CI: 5.3–14.0), the median event-free survival (EFS) was 5.3 months (95% CI: 3.9–11.0), and the median overall survival was 30.5 months (95% CI: 17.8–60.6). We conclude that rituximab can be safely added to standard DHAP.
Keywords: Non-Hodgkin lymphoma, salvage chemotherapy, transplant, rituximab
Introduction
Patients with newly diagnosed large cell B-cell non-Hodgkin lymphoma (NHL) typically are treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP). With this program, approximately 60% of patients are cured [1 – 4] leaving 40% of patients that will eventually require additional therapy. For patients under the age of 75 years who are otherwise in good health, the standard of care is to provide high-dose therapy with autologous stem cell transplant (SCT) [5] for patients with chemotherapy sensitive relapsed disease or patients with chemotherapy sensitive disease who achieve less than a complete response to initial therapy. The efficacy of autologous SCT is greater when performed after a first relapse rather than after multiple relapses [6].
The most common salvage regimens in current use are cisplatin, cytosine arabinoside and dexamethasone (DHAP) or ifosfamide, carboplatin and etoposide (ICE) [7 – 12]. Although these regimens are effective in inducing an anti-tumour response, there are still approximately 35% of patients that are refractory and few patients are cured with DHAP or ICE unless they are able to proceed to SCT. Patients with relapsed large cell NHL have a 37% ORR when treated with single-agent rituximab [13]. This activity of rituximab, the lack of myelosuppression and the need to improve salvage therapy for patients with relapsed NHL provided the rationale to study the toxicity and efficacy of adding a course of rituximab to the DHAP regimen. Improving the ORR with rituximab-based salvage regimens could potentially increase the number of patients eligible for SCT and reduce the number of regimens required to demonstrate sensitive disease.
Patients and methods
Patient eligibility
Eligible patients were those with relapsed or refractory CD20+ B-cell NHL suitable for treatment with a platinum-based regimen. A tumour biopsy to document relapse was required ≤ 6 weeks of enrolment. Patients had measurable disease (at least one lesion ≥ 1.5 × 1.5 cm), ECOG performance status 0–2, absolute neutrophil count (ANC) ≥ 1500, platelet count ≥ 75,000, total bilirubin ≤ 2mg/dL and a creatinine ≤ 1.5 the upper normal limit (UNL) of the laboratory. Patients with known HIV infection, other active malignancies or involvement of the central nervous system with NHL were ineligible. All patients underwent computed tomography (CT) scans of the chest, abdomen and pelvis; and a bone marrow exam pre-treatment; PET scans were not required. All patients provided written informed consent and the trial was approved by the Institutional Review Board at each individual study site.
Study design
The treatment regimen was rituximab 375mg/m2 IV days 1, 8, 15 and 22; dexamethasone 40 mg PO/IV days 3–6; cisplatin 100 mg/m2 IV by continuous infusion for 24 h on day 3; cytosine arabinoside 2000 mg/m2 every 12 h × two doses on day 4; and granulocyte (G-CSF) or granulocyte macrophage colony stimulating factor (GM-CSF) subcutaneously days 5–14. A cycle was 21 days and patients received 2 cycles of treatment, with rituximab administered fully in cycle one and only on day 1 of cycle 2 for a total of 4 doses. Cycle 2 was administered at full dose if the ANC was ≥1500, platelet count ≥75,000 and the creatinine was <2 × UNL. If these criteria were not met, the treatment was delayed up to 2 weeks and then the cisplatin and cytosine arabinoside were reduced by 50%.
Response was assessed using the International Working Group criteria [14]. After cycle 1, patients were restaged with CT and those without progression received cycle two and were again restaged. After completing 2 cycles of treatment and going off study, responders (CR and PR) could proceed to SCT or further treatment at physician and patient discretion. Patients were followed for time to progression (TTP), event-free survival (EFS) and overall survival (OS).
Statistical methods
This study was a one-stage phase II trial with an interim analysis based on a Simon optimal design [15]. A success was defined as an objective status of CR/CRu or a PR within the first two cycles of R-DHAP. The hypothesis was that adding a course of rituximab to the standard DHAP regimen would improve the ORR to at least 75%. The study had 95% power, with a 5% Type I error rate, to detect an effective treatment if the true success rate was at least 75% versus the null hypothesis that it was at most 50%. A patient was considered evaluable for response if they were eligible and received any treatment. A minimum of 17 and a maximum of 50 patients were required to evaluate the decision criteria. At least 10 of the first 17 evaluable patients needed to have a success as defined above at the interim analysis to warrant continuation of accrual. At the final analysis, at least 31 successes in the first 50 evaluable patients were necessary for R-DHAP to be considered a promising treatment in this patient population. Assuming that the number of responses was binomially distributed, 95% confidence intervals for the true response rate were calculated according to the approach of Duffy and Santner [16].
OS, TTP and EFS were evaluated for all patients as well as stratified by transplant status in responders. OS time was defined as the number of days from registration date to the date of death or last follow-up. Time to progression is the number of days from registration to disease progression, or last follow-up. Patients who had not progressed and went on to receive other chemotherapy or SCT after R-DHAP were censored at the date subsequent treatment was initiated. Patients dying without formal assessment of disease progression were considered to have had disease progression at the time of death unless there was documented evidence that no progression had occurred before death. EFS was defined as the number of days from registration to disease progression, initiation of chemotherapy other than RDHAP, or death. Since the intent was that patients would go to SCT after R-DHAP, SCT was not considered an event and patients who had not progressed at the time of SCT were censored on the date of SCT. Duration of response (DR) was defined as the number of days from the first date of a documented response (CR, PR, CRu) to the date of progression. If patients received some other treatment regimen without actually having tumour progression they were censored on the date subsequent treatment was initiated. Patients who did not receive additional treatment or SCT were censored on the date of their last evaluation. The distributions of time-to-event endpoints were estimated using the Kaplan–Meier method [17]. For the purposes of classification, diffuse large cell, follicular grade III, mantle cell and high-grade Burkitt NHL were considered aggressive NHL and small lymphocytic lymphoma, follicular grades I and II, and marginal zone NHL were considered indolent NHL.
Results
Baseline characteristics
Fifty-eight patients were enrolled in this Phase II study between October 29, 2000 and June 20, 2003 (Table I). One patient was declared ineligible because the tumour was CD20 negative. Five eligible patients could not be assigned an exact disease type on central pathology review and no additional slides were available. The pathologists at the sites that enrolled these patients read the tissue as indolent NHL in four of the cases and aggressive (large cell) in the other case.
Table I.
Patient characteristics of the 57 eligible patients.
| Characteristic | N (%)⋆ |
|---|---|
| Age, years | |
| Median (Range) | 63 (45, 83) |
| Male sex | 38 (67%) |
| Bulky disease | 19 (33%) |
| Tumor stage at study entry | |
| 1 | 4 (7%) |
| 2 | 11 (19%) |
| 3 | 11 (19%) |
| 4 | 31 (54%) |
| Elevated baseline lactate dehydrogenase | 21 (37%) |
| Performance status | |
| 0 | 29 (51%) |
| 1 | 24 (42%) |
| 2 | 4 (7%) |
| Previous radiation therapy | |
| Yes | 13 (23%) |
| Previous rituximab | |
| Yes | 19 (33%) |
| Number of prior chemotherapies | |
| Mean | 2.2 |
| Median (min, max) | 2 (1, 7) |
| Number of extranodal sites | |
| 2 or more | 11 (19%) |
| B Symptoms | |
| Yes | 11 (19%) |
| IPI Score | |
| 0 | 4 (7%) |
| 1 | 14 (25%) |
| 2 | 20 (35%) |
| 3 | 16 (28%) |
| 4 | 3 (5%) |
| Disease types | |
| Small lymphocytic | 3 (5%) |
| Mantle cell | 4 (7%) |
| Follicular | 12 (21%)⋆⋆ |
| Diffuse large cell | 31 (54%) |
| Nodal marginal zone | 1 (2%) |
| High grade, Burkitt-like | 1 (2%) |
| Unclassifiable† | 5 (9%) |
Unless otherwise noted.
2 grade 1, 8 grade 2, and 2 grade 3.
Unclassifiable on central pathology review; however, the local pathologist reading was indolent NHL in four of the cases and aggressive (large cell) in one case.
Clinical responses
All 57 eligible patients received one cycle of treatment; 84% (48/57) of patients received cycle two (Table II). Forty-seven patients (82%, 95% CI: 71%–91%) had a tumour response with 33% (19/57) CR/CRu and 49% (28/57) PR. When the analysis was restricted to the 38 patients with aggressive NHL, the ORR was 82% (95% CI: 66%–92%) with 42% CR/CRu and 40% PR. Thirty-six (77%) of the 47 patients who responded achieved the response after only one cycle; the other 11 patients required 2 cycles of R-DHAP. Nine patients experienced progression during treatment – three during cycle one and six on cycle two (including 3 of the patients who responded on cycle one). The response rates were similar between groups for patients who did not receive prior rituximab (82%) and patients who did receive prior rituximab (84%). Patients who had not received prior rituximab were more likely to have a CR/CRu (39% vs. 21%; p = not significant).
Table II.
Maximal response to R-DHAP by disease type.
| Overall response rate (%) | Complete remission rate (%) | Partial remission rate (%) | |
|---|---|---|---|
| All patients (n=57) | 82 | 33 | 49 |
| Aggressive (n=38) | 82 | 42 | 40 |
| Indolent (n=14) | 86 | 14 | 71 |
| Unclassifiable (n=5) | 80 | 20 | 60 |
Patients with chemosensitive disease were allowed (but not mandated) to proceed to SCT after R-DHAP (Table III). Of the 57 eligible patients, 26% (15/57) proceeded to SCT after R-DHAP; two additional patients eventually received a SCT after additional salvage therapy.
Table III.
Patient characteristics of responders by transplant status.
| Characteristic | Responders who received a transplant directly after RDHAP (n=15) | Responders who did not receive a transplant (n=31) |
|---|---|---|
| Age, years | ||
| Mean±S.D. | 60.5±9.8 | 65.4±9.0 |
| Median (Min, Max) | 61 (45, 75) | 64 (48, 83) |
| Bulky Disease | 5 (33%) | 9 (29%) |
| Number of prior chemotherapies | ||
| Mean | 2.1 | 2.4 |
| Median (Min, Max) | 2 (1, 5) | 2 (1, 7) |
| IPI Score | ||
| 0 | 1 (7%) | 2 (6%) |
| 1 | 4 (27%) | 10 (32%) |
| 2 | 6 (40%) | 7 (23%) |
| 3 | 4 (27%) | 10 (32%) |
| 4 | 0 (0%) | 2 (6%) |
| Disease type | ||
| Small lymphocytic | 0 (0%) | 3 (10%) |
| Mantle Cell | 2 (13%) | 2 (6%) |
| Follicular | 1 (7%) | 9 (29%) |
| Diffuse large cell | 9 (60%) | 14 (45%) |
| Nodal Marginal Zone | 0 (0%) | 1 (3%) |
| High Grade, Burkitt-like | 0 (0%) | 1 (3%) |
| Unclassifiable | 3 (20%) | 1 (3%) |
Survival outcomes
The median TTP for all eligible patients was 10.3 months (95% CI: 5.3–14.0), the median EFS was 5.3 months (95% CI: 3.9–11.0), and the median OS was 30.5 months (95% CI: 17.8–60.6) (Figure 1). The median DR for the 47 responders was 10.5 months (95% CI: 5.3–16.8). As of March 2007, 37% (21/57) patients were alive and 21% (12/57) were progression-free. The median follow-up time for living patients was 54 months (range, 43–63). Eight of the 17 patients (47%) who received a SCT remain alive, with a median follow-up of 56 months.
Figure 1.
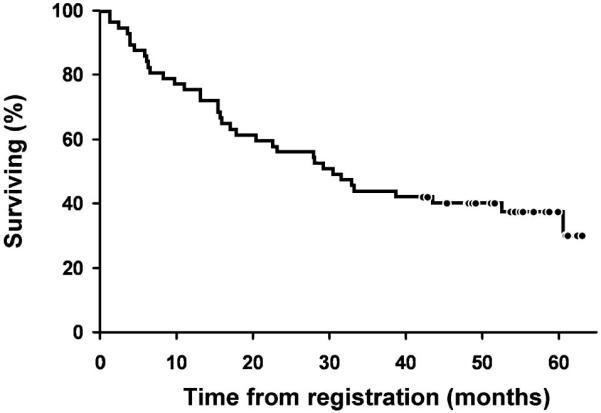
OS of all 57 eligible patients.
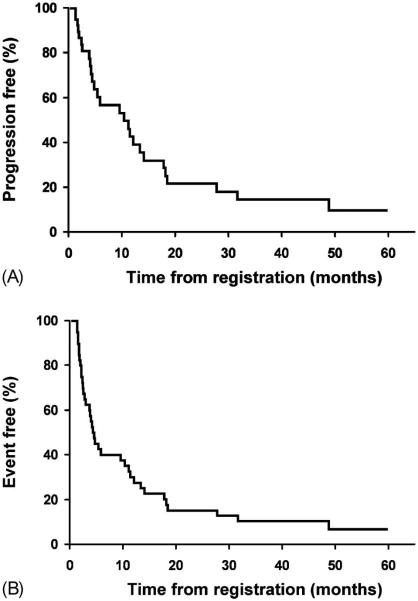
After receiving R-DHAP, 70% (40/57) of patients did not proceed to SCT (Figure 2). The median TTP, EFS and OS for these patients were 10.3 months (95% CI: 4.7–14.0), 4.4 months (95% CI: 2.9–10.3) and 27.9 months (95% CI: 15.4–52.5), respectively. Of these 40 patients, 31 were responders to RDHAP (Table III). The median DR, TTP, EFS and OS for these patients who achieved a response were 10.5 months (95% CI: 4.7–16.8), 12.1 months (95% CI: 5.8–18.4), 5.8 months (95% CI: 4.1–13.3) and 30.5 months (95% CI: 15.9-not reached), respectively.
Figure 2.
Time to progression (A) and event free survival (B) of the 40 patients treated with RDHAP that did not proceed to transplant.
For the 15 patients who achieved a response to RDHAP and proceeded directly to SCT, the median OS was not reached (95% CI: 29.2-not reached). The 3-year survival rate was 60% (95% CI: 40–91%). The median DR, TTP and EFS could not be determined since all patients were censored at time of SCT.
Toxicity and tolerability
The majority of patients received two cycles of RDHAP (84%, 48/57) and 79%, (45/57) completed cycle 2 per protocol. Three patients did not complete cycle 2 as planned due to adverse reactions (1), disease progression (1) and death on study (1). Nine additional patients went off study after cycle 1 due to: adverse reactions (6), refusal (1), alternate treatment (1) and other medical problems (1). Eighteen patients (32%) experienced a total of 19 dose delays. Eleven delays were due to haematologic adverse events and eight delays were due to other reasons. Of the 48 patients who received two cycles of DHAP, only three patients experienced a dose reduction on cycle two.
A toxicity was defined as an adverse event at least possibly related to treatment (possibly, probably, or definitely related). Thirteen patients had a maximum of grade 3 toxicity, 42 patients had a maximum of grade 4 and 1 patient had grade 5. For non-haematologic toxicities, 27 patients had a maximum of grade 3, six patients had a maximum of grade 4 and 1 patient had a grade 5. The grade 5 toxicity was death due to septicemia related to pancytopenia, which resulted from an accidental overdose of cytosine arabinoside. A second death from a cardiac arrhythmia was considered unrelated to treatment.
Commonly observed grade 3 or 4 toxicities (Table IV) were thrombocytopenia (91%) and neutropenia (79%). Febrile neutropenia occurred in 23% of patients and in 12% (13/105) of treatment cycles. Five events of nephrotoxicity occurred in 7% (4/57) of patients – four patients had grade 3 creatinine and one patient had renal failure grade 4. The patient with the overdose of cytosine arabinoside experienced a grade 3 and a grade 4 nephrotoxicity and died on study during cycle 2.
Table IV.
Grade 3+ toxicity (adverse events considered at least possibly related to R-DHAP) was observed in 98% (56/57) of patients.
| Toxicity type | Grade 3 (%) | Grade 4 (%) | Grade 5 (%) |
|---|---|---|---|
| General | |||
| Fatigue | 6 (11) | 1 (2) | |
| Hematological | |||
| Anemia | 12 (21) | 1 (2) | |
| Neutropenia | 6 (11) | 39 (68) | |
| Thrombocytopenia | 30 (53) | 22 (39) | |
| Infection | |||
| Infection without neutropenia | 1 (2) | ||
| Infection with neutropenia | 2 (4) | 1 (2) | |
| Febrile neutropenia | 12 (21) | 1 (2) | |
| Gastrointestinal | |||
| Anorexia | 3 (5) | ||
| Nausea | 10 (18) | ||
| Vomiting | 6 (11) | 1 (2) | |
| Diarrhea | 3 (5) | 1 (2) | |
| Metabolic | |||
| Hypokalemia | 13 (23) | ||
| Hypomagnesemia | 1 (2) | 1 (2) | |
| Creatinine | 4 (7) | ||
| Renal failure | 2 (4) | ||
| Maximum overall toxicity grade | 13 (23) | 42 (25) | 1 (2) |
Maximal overall toxicity grade refers to the number of patients that had the respective grade toxicity across all toxicity types.
Discussion
The treatment of relapsed DLBCL is problematic because even though salvage regimens can be effective, they are often intensive, hospital-based and usually not curative by themselves. The reduction in morbidity and mortality with autologous SCT has enabled patients who are less than 75 years old who respond to salvage therapy to undergo SCT. A retrospective study has demonstrated that patients with aggressive NHL have a superior OS post-SCT if they received rituximab pre-SCT [18]. The goal of this phase II study was to improve the DHAP regimen by administering the rituximab concomitantly with the DHAP.
This trial demonstrated an ORR to RDHAP of 82% – slightly higher than the study goal of 75% and higher than the response rates found by others using traditional DHAP. Velasquez et al. [10] reported an ORR of 58% with 31% CR in 90 patients (median age, 55 years) with relapsed NHL. The largest trial to date of DHAP accrued 204 patients and found an ORR of 59% (120/204) with a 25% (51/204) CR rate [12]. It was limited to patients who were less than 60 years of age and most (87%) were in their first relapse. Oliveri et al. [8] modified the DHAP regimen for outpatient use and reported a ORR of 80% with 30% CR in 79 patients with relapsed NHL. This ORR is the highest reported for DHAP but the study group was young with a median age of only 49 years and the ORR varied by disease type. The ORR was 93% (29/31) with 35% (11/31) CR for low grade compared to 68% (20/28) with 28% CR for the high grade NHL. Josting et al. [19] treated 57 patients with two cycles of DHAP as part of a high-dose sequential pre-SCT strategy and reported a ORR of 72% with 9% CR. The median age was 43 years, substantially younger than the patients in our study.
Other groups have combined rituximab with DHAP. A recent study reported a 92% CR rate in 24 patients with previously untreated mantle cell NHL with RDHAP [20]. Mey et al. [21] performed a trial of RDHAP that focused only on patients with relapsed aggressive NHL and patients could not have received prior rituximab. The RDHAP regimen differed from this trial in that the rituximab was given d1 of each of a planned four cycles. The cisplatin was given over 4 days and there were dose reductions in the first cycle and for patients over age 60. They found an ORR of 63% with 32% of patients attaining a CR/CRu. The authors then reported separately an analysis of the results from 23 patients from the RDHAP trial with a matched control group of 23 patients treated with DHAP alone [9]. The ORR was 74% in both groups with a CR rate of 44% for RDHAP compared to 35% for DHAP. Other salvage regimens produce similar response rates [22]. Etoposide, methylprednisolone, cytosine arabinoside and cisplatin (ESHAP) produced an ORR of 67% with 37% CR in 122 patients with a variety of NHL disease types [23]. The miniBEAM regimen includes a lower dose of cytosine arabinoside and no platinum analogues. It has been extensively tested prior to SCT and produced a 37% ORR [24].
More recently the ICE regimen has come into common use as a salvage regimen. Moskowitz et al. [11] administered three cycles of the ICE regimen to 163 transplant-eligible patients and reported an ORR of 66% (108/163) with a 24% (39/163) rate of CR. This group has recently reported the results of a phase II study that added rituximab to ICE (RICE) in 34 patients [25]. They administered four doses of rituximab with three cycles of ICE and demonstrated an ORR of 78% with 53% CR. The ORR is similar to the results of RDHAP (82%) but the CR rate of 53% is substantially higher than the 33% found for RDHAP and the 27% found for ICE [25,26]. The RICE trial was different than this study of RDHAP in that it restricted eligibility to relapsed DLBCL, the patients received three cycles of RICE compared to two RDHAP, it was single-institution whereas RDHAP was cooperative group, and the median age was substantially younger at 45 years compared with 63 years for RDHAP.
RDHAP, like other intensive salvage programs has substantial toxicity and is not curative. Febrile neutropenia occurred in 23% of cases (12% of treatment cycles) which is similar to the 10% with DHAP [19] and higher than the 7.5% of cycles of RICE [25]. The evidence to date from the RICE and RDHAP phase II trials is that it is safe to add rituximab to these salvage regimens. In order to determine the relative merits of RICE and RDHAP a phase III trial is being conducted [27]. While this randomized trial is accruing, it will be important to explore methods to improve these salvage regimens by performing phase I/II trials that add agents with new mechanisms of action that have shown single-agent activity in relapsed DLBCL. In the meantime, in the non-protocol situation RDHAP and RICE are both acceptable salvage regimens.
Although the ORR to RDHAP in this trial was high, only 15 of the responders proceeded directly to SCT. This finding was unexpected since one of the eligibility criteria of the trial was that the patient be a candidate for a platinum-based salvage regimen. Patients who can endure programs like DHAP and have a tumour response are usually eligible for (and will tolerate) an autologous SCT. Since SCT was not a part of this study, we can only speculate on the low rate of SCT. The trial was conducted in the cooperative group setting rather than solely at a referral centre; therefore, this may reflect the actual overall use of SCT for NHL in the United States. The toxicity of the salvage regimens can also discourage some patients from proceeding to SCT. Finally, this trial was not limited to aggressive NHL and SCT is not considered the standard of care for indolent NHL.
Acknowledgements
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-15083; CA-35269, CA-35431, CA-60276, CA-35267, CA-35195, CA-37417, CA-52352, CA-35101, CA-35448, CA-35415, CA-63849 from the National Cancer Institute, Department of Heath and Human Services. Additional participating institutions include: Carle Cancer Center CCOP, Urbana, IL 61801 (Kendrith M. Rowland, Jr, M.D.); Meritcare Hospital CCOP, Fargo, ND 58122 (Preston D. Steen, M.D.); Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA 52403 (Martin Wiesenfeld, M.D.); Iowa Oncology Research Association, Des Moines, IA 50309 (Roscoe F. Morton, M.D.); Geisinger Clinic & Medial Center CCOP, Danville, PA 17822 (Albert M. Bernath, Jr, M.D.); Toledo Community Hospital Oncology Program CCOP, Toledo, OH 43623 (Paul L. Schaefer, M.D.); Missouri Valley Cancer Consortium, Omaha, NE 68106 (Gamini S. Soori, M.D.).
Footnotes
Publisher's Disclaimer: The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- 1.Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Ferme C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 2.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 3.Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 4.Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 5.Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma [see comments] N Engl J Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 6.Chen CI, Roitman D, Tsang R, Stewart AK, Keating A, Crump M. `Relative' chemotherapy sensitivity: the impact of number of salvage regimens prior to autologous stem cell transplant for relapsed and refractory aggressive non-Hodgkin's lymphoma. Bone Marrow Transplant. 2002;30:885–891. doi: 10.1038/sj.bmt.1703772. [DOI] [PubMed] [Google Scholar]
- 7.Josting A, Nogova L, Franklin J, Glossmann J-P, Eich HT, Sieber M, et al. Salvage radiotherapy in patients with relapsed and refractory Hodgkin's lymphoma: a retrospective analysis from the German Hodgkin Lymphoma Study Group. J Clin Oncol. 2005;23:1522–1529. doi: 10.1200/JCO.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Olivieri A, Brunori M, Capelli D, Montanari M, Massidda D, Gini G, et al. Salvage therapy with an outpatient DHAP schedule followed by PBSC transplantation in 79 lymphoma patients: an intention to mobilize and transplant analysis. Eur J Haematol. 2004;72:10–17. doi: 10.1046/j.0902-4441.2004.00171.x. [DOI] [PubMed] [Google Scholar]
- 9.Mey UJ, Olivieri A, Orlopp KS, Rabe C, Strehl JW, Gorschlueter M, et al. DHAP in combination with rituximab vs DHAP alone as salvage treatment for patients with relapsed or refractory diffuse large B-cell lymphoma: a matched-pair analysis. Leuk Lymphoma. 2006;47:2558–2566. doi: 10.1080/10428190600926572. [DOI] [PubMed] [Google Scholar]
- 10.Velasquez WS, Cabanillas F, Salvador P, McLaughlin P, Fridrik M, Tucker S, et al. Effective salvage therapy for lymphoma with cisplatin in combination with high-dose Ara-C and dexamethasone (DHAP) Blood. 1988;71:117–122. [PubMed] [Google Scholar]
- 11.Moskowitz C, Bertino J, Glassman J, Hedrick E, Hunte S, Coady-Lyons N, et al. Ifosphamide, carboplatin, and etoposide: a highly effective cytoreduction and peripheral-blood progenitor-cell mobilization regimen for transplant-eligible patients with non-Hodgkin's lymphoma. J Clin Oncol. 1999;17:3776–3785. doi: 10.1200/JCO.1999.17.12.3776. [DOI] [PubMed] [Google Scholar]
- 12.Blay J, Gomez F, Sebban C, Bachelot T, Biron P, Guglielmi C, et al. The International Prognostic Index correlates to survival in patients with aggressive lymphoma in relapse: analysis of the PARMA trial. Parma Group. Blood. 1998;92:3562–3568. [PubMed] [Google Scholar]
- 13.Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998;92:1927–1932. [PubMed] [Google Scholar]
- 14.Cheson B, Horning S, Coiffier B, Shipp M, Fisher R, Connors J, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphoma. J Clin Oncol. 1999;17:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 15.Simon R. Optimal two-stage designs for phase II clinical trials. Controlled Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 16.Duffy D, Santner T. Confidence intervals for a binomial parameter based on multistage tests. Biometrics. 1987;43:81–93. [Google Scholar]
- 17.Kaplan E, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Hoerr AL, Gao F, Hidalgo J, Tiwari D, Blum KA, Mathews V, et al. Effects of pretransplantation treatment with rituximab on outcomes of autologous stem-cell transplantation for non-Hodgkin's lymphoma. J Clin Oncol. 2004;22:4561–4566. doi: 10.1200/JCO.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 19.Josting A, Sieniawski M, Glossmann JP, Staak O, Nogova L, Peters N, et al. High-dose sequential chemotherapy followed by autologous stem cell transplantation in relapsed and refractory aggressive non-Hodgkin's lymphoma: results of a multicenter phase II study. Ann Oncol. 2005;16:1359–1365. doi: 10.1093/annonc/mdi248. [DOI] [PubMed] [Google Scholar]
- 20.de Guibert S, Jaccard A, Bernard M, Turlure P, Bordessoule D, Lamy T. Rituximab and DHAP followed by intensive therapy with autologous stem-cell transplantation as first-line therapy for mantle cell lymphoma. Haematologica. 2006;91:425–426. [PubMed] [Google Scholar]
- 21.Mey UJ, Orlopp KS, Flieger D, Strehl JW, Ho AD, Hensel M, et al. Dexamethasone, high-dose cytarabine, and cisplatin in combination with rituximab as salvage treatment for patients with relapsed or refractory aggressive non-Hodgkin's lymphoma. Cancer Invest. 2006;24:593–600. doi: 10.1080/07357900600814490. [DOI] [PubMed] [Google Scholar]
- 22.Salar A, Martino R, Perea G, Ribera JM, Lopez-Guillermo A, Guardia R, et al. High-dose infusional ifosfamide, etoposide plus methylprednisolone followed by dexamethasone, high-dose ara-C and cisplatinum and autologous stem cell transplantation for refractory or relapsed aggressive non-Hodgkin's lymphoma. Haematologica. 2002;87:1028–1035. [PubMed] [Google Scholar]
- 23.Velasquez WS, McLaughlin P, Tucker S, Hagemeister FB, Swan F, Rodriguez MA, et al. ESHAP – an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study [see comments] J Clin Oncol. 1994;12:1169– 1176. doi: 10.1200/JCO.1994.12.6.1169. [DOI] [PubMed] [Google Scholar]
- 24.Girouard C, Dufresne J, Imrie K, Stewart AK, Brandwein J, Prince HM, et al. Salvage chemotherapy with mini-BEAM for relapsed or refractory non-Hodgkin's lymphoma prior to autologous bone marrow transplantation. Ann Oncol. 1997;8:675–680. doi: 10.1023/a:1008294725992. [DOI] [PubMed] [Google Scholar]
- 25.Kewalramani T, Zelenetz AD, Nimer SD, Portlock C, Straus D, Noy A, et al. Rituximab and ICE (RICE) as second-line therapy prior to autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2004;103:3684–3688. doi: 10.1182/blood-2003-11-3911. [DOI] [PubMed] [Google Scholar]
- 26.Hamlin PA, Zelenetz AD, Kewalramani T, Qin J, Satagopan JM, Verbel D, et al. Age-adjusted International Prognostic Index predicts autologous stem cell transplantation outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2003;102:1989–1996. doi: 10.1182/blood-2002-12-3837. [DOI] [PubMed] [Google Scholar]
- 27.Hagberg H, Gisselbrecht C. Randomised phase III study of R-ICE versus R-DHAP in relapsed patients with CD20 diffuse large B-cell lymphoma (DLBCL) followed by high-dose therapy and a second randomisation to maintenance treatment with rituximab or not: an update of the CORAL study. Ann Oncol. 2006;17(Suppl 4):iv31–32. doi: 10.1093/annonc/mdj996. [DOI] [PubMed] [Google Scholar]