Abstract
Objective: We aimed to assess the role of zoledronic acid (ZOL) on the risk of fracture and bone mineral density (BMD) in women with osteoporosis.
Methods: A double-blind and placebo-controlled design was taken in our study. 327 patients who received an intravenous 5-mg infusion zoledronic acid at day 0, at 12 months were enrolled in treatment group, and the remaining 333 patients who received placebo at the same time of the treatment group were included as control group. The incidence of fracture and BMD in the femoral neck and total hip were assessed.
Results: ZOL group had lower incidence of fracture at any clinical fracture, clinical vertebral fracture, non-vertebral fracture and hip fracture compared with placebo group at the time of one year and three years. We found that the BMD were significantly increased at femoral neck and total hip in ZOL group at the time of one year and three years follow-up when compared with placebo group (P<0.05). The adverse events in the ZOL within three days of drug infusion were significantly higher than the control group, but we did not find significant difference in the serious adverse effect between the two groups.
Conclusions: Zoledronic acid (ZOL) could be used as a safe and effective method for female with osteoporosis.
Key Words: Bone mineral density, Fracture, Osteoporosis, Zoledronic acid
INTRODUCTION
Osteoporosis is a skeletal disease that is characterized by compromised bone strength predisposing a person to an increased risk of fracture, and is common in elderly postmenopausal women.1 According to the World Health Organization (WHO) data, osteoporosis affects approximately 75 million person in Europe, the US, and Japan, and 9 million new fractures were caused by osteoporosis2 Osteoporosis-related fractures are associated with significant morbidity, increased mortality and enormous financial costs.3
Teriparatide, calcitonin, alendronate and stontim ranelate have proved to be the standard treatment of osteoporosis.4-6 Previous clinical studies indicated that nitrogen-containing bisphosphonates can inhibit bone resorption, keep bone mass and decrease the risk of osteoporosis-related fractures fractures7,8 Oral bisphosphonates have been shown to increase bone mineral density (BMD).9,10 Zoledronic acid (ZOL) is an intravenous, aminobisphosphonate with a high affinity for mineralized bone, which could increase patients’ compliance with bisphosphonate therapy and thus improve the clinical outcome. ZOL 5mg has been reported to decrease the risk of fracture and increase the bone mineral density among postmenopausal osteoporosis in several developed countries.11,12 However, there were few studies on the effectiveness and safety of intravenous ZOL in Chinese postmenopausal osteoporosis women. Therefore, we aimed to assess the role of ZOL on the risk of fracture and BMD in women with osteoporosis.
METHODS
Study population: A double-blind and placebo-controlled design was taken in our study. A total of 660 female patients who were diagnosed with osteoporosis were included from The First Affiliated Hospital of Xinxiang Medical College and the Second People’s Hospital of Ji’nan between January 2009 and May 2012. Patients with secondary osteoporosis or other diseases which were known to affect bone metabolism were excluded. Patients taking anabolic steroids, sodium fluoride, and parathyroid or growth hormone within 6 months were also excluded. Patients who had malignant neoplasm, serum calcium more than 11.0 mg/dl, or untreated hypocalcemia were also excluded. All patients signed the informed consent.
Techniques: 660 female patients were randomly divided into two groups. 327 patients who received an intravenous 5-mg infusion zoledronic acid at day 0, at 12 months were included in treatment group, and the remaining 333 patients who received placebo (Activated Vitamin D3, 0.25 mg) at the same time of the treatment group were included as control group. All patients were supplemented with 600-1500 mg elemental calcium and 400-1200 IU vitamin D every day. Patients were followed up for two years with telephone interviews and clinic visits at 12 and 36 months.
Fracture and BMD measurement: All the fractures were assessed by the Genet semi-quantitative method.13 Clinical fracture reports were obtained from the routine examination by radiologic or surgical procedure report or a copy of the radiograph. BMD in the femoral neck and total hip was measured by Hologic Dual Energy X-ray Absorptiometry (Hologic, Waltham, MA, USA) at 12 and 36 months.
Safety assessment: All adverse events and serious adverse events were recorded by physical examination and regular measurement of vital signs, hematologic, blood chemical and urinary values. Adverse events were assessed and categorized by the Medical Dictionary for Regulatory Activities.14 The most common adverse events were reported within three days of infusion 5-mg infusion zoledronic acid.
Statistical analysis: Efficacy and safety parameters were compared between ZOL and control groups by t-test for continuous variables or chi-square test for categorical variables. The incidence of fractures was expressed as percentage. The BMD change was evaluated as the mean percentage change from baseline. All statistical analyses were conducted by SPSS 11.0 software (SPSS, Chicago, IL), P value<0.05 was regarded as statistically significant and all tests were two-sides.
RESULTS
Among 660 patients, 327 patients were randomized in zoledronic acid group and 333 patients in placebo group. The clinical characteristics are shown in Table-I. The average age in ZOL and placebo groups was 54.6±7.3 years and 55.3±7.5 years, respectively. The BMI, femoral neck bone mineral density, vertebral fractures before treatment, and T score at femoral neck did not show significant difference between zoledronic acid and placebo groups.
Table-I.
Characteristics of included patients
| Items | Zoledronic acid | % | Placebo | % | Statistical value | P value |
|---|---|---|---|---|---|---|
| Age (years) | 54.6±7.3 | 55.3±7.5 | 0.44 | 0.77 | ||
| Body mass index(kg/m 2 ) | 24.2±0.5 | 24.3±0.5 | 0.11 | 0.06 | ||
| Femoral neck bone mineral density, g/cm 2 | 0.56±0.14 | 0.55±0.15 | 0.58 | 0.29 | ||
| Vertebral fractures before treatment | ||||||
| Yes | 184 | 56.3 | 182 | 54.7 | ||
| No | 143 | 43.7 | 151 | 45.3 | 0.08 | 0.77 |
| T score at femoral neck | ||||||
| ≤-2.5 | 151 | 46.3 | 152 | 45.7 | ||
| -2.5 to -1.5 | 168 | 51.3 | 172 | 51.6 | ||
| ≥-1.5 | 8 | 2.4 | 9 | 2.7 | 0.11 | 0.95 |
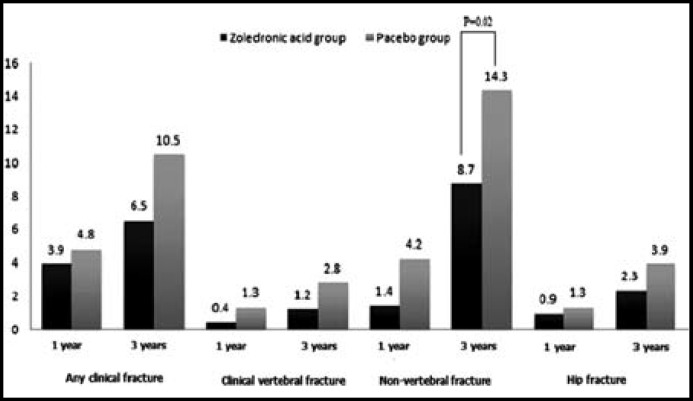
Fig.1 shows the incidence of fractures at the time of 12 months and 36 months. We found ZOL group had lower incidence of fracture at any clinical fracture, clinical vertebral fracture, non-vertebral fracture and hip fracture compared with placebo group. However, only the incidence of non-vertebral fracture in the ZOL group was significantly lower than the ZOL group (14.3% vs 8.7%, P<0.05), and a reduction of 46% in the risk of fracture when compared with placebo group (OR=0.55, 95% CI=0.33-0.93).
Fig.1.
Incidence of fractures at the time of 12 months and 24 months
Table-II shows the percentage change in Bone Mineral Density between the two groups. We found that the BMD was significantly increased at femoral neck and total hip in ZOL group at the time of one year and three years follow-up when compared with placebo group (P<0.05). The mean difference of femoral neck BMD percentage change of ZOL versus placebo was 1.81(1.54-2.26) at one year follow-up, and was 3.65(3.31-4.04) at 3 years follow-up. Meanwhile, the mean difference of total hip BMD was 2.12(1.78-2.45) at one year follow-up, and was 4.26(3.80-4.81) at three years follow-up.
Table-II.
Percentage change in Bone Mineral Density in the two groups
| Bone Mineral Density | Relative treatment differences (95% CI) 1 | P value |
|---|---|---|
| Femoral neck BMD | ||
| 1 year | 1.81(1.54-2.26) | <0.05 |
| 3 years | 3.65(3.31-4.04) | <0.05 |
| Total hip BMD | ||
| 1 year | 2.12(1.78-2.45) | <0.05 |
| 3 years | 4.26(3.80-4.81) | <0.05 |
1. The mean percentage difference of Bone Mineral Density of zoledronic acid group versus placebo
The incidence of adverse evidence of in the ZOL group was 84.2%, and 81.6% in the placebo group. There was no significant difference in the adverse events between the two groups. The adverse events in the ZOL within three days of drug infusion were significantly higher than the control group (42.3% versus 20.7%). The common adverse events in the two groups were back pain, urinary tract infection, hypertension, nasopharyngitis, arthralgia, pyrexia, myalgia, headache and influenza-like symptoms. Three patients in the ZOL treatment group and one patient in the control group showed serious cardiac symptoms, and no significant difference was found between them.
DISCUSSION
In the present study, intravenous 5-mg infusion zoledronic acid for 12 months significantly increased the BMD at femoral neck and total hip, and decreased the risk of any clinical, clinical vertebral and non-vertebral as well as hip fracture in postmenopausal women.
Our study found that intravenous 5-mg infusion zoledronic acid at the baseline and at 12 months could significantly the risk of non-vertebral fracture among females who were diagnosed with osteoporosis. Previous several studies indicated that oral risedronate treatment could reduce the risk of vertebral and nonvertebral fractures.15,16 Reginster indicated that the oral bisphosphonate could reduce the risk of new vertebral fracture by 49% over 3 years when compared with placebo control, and the risk of non-vertebral fractures was reduced by 33% compared with placebo control.15 Harris reported that oral risedronate could decrease the risk of new vertebral fractures and non-vertebral fractures by 41% and 39%, respectively.16 Our results are in line with the previous studies.
In our study, we found that the BMD was significantly increased at femoral neck and total hip in ZOL group over three years when compared with placebo group. Previous experimental study indicated that zoledronic acid administration could increase bone mineral density in the trabecular bone compared with control group.9 A previous clinical HORIZON trail with 107 patients indicated that intravenous zoledronate therapy significantly increased BMD of lumbar spine over 3 years.10 Another clinical trial conducted in China reported that zoledronic acid administration once a year could increase BMD and reduce the serum bone turnover metabolism.17 Boonen et al reported a single 15-minute infusion of zoledronic acid (5 mg) is associated with a significant improvement in BMD, and was not association with serious adverse events when compared with placebo-controlled trial.18 However, another study with 7765 postmenopausal osteoporosis women indicated that intravenous 5-mg infusion zoledronic acid did not increased the femoral neck BMD compared with control.19 The possible discrepancy of the results may be induced by different backgrounds of cases, sample size, sample size and etc. Therefore, the effect of ZOL on women with osteoporosis should be confirmed in further large sample size studies.
Our study showed that the adverse events in the ZOL within three days of drug infusion were significantly higher than the control group, and the main reason of the higher risk of adverse effect in the ZOL group was the first-dose acute-phase reaction. The most common adverse events of the first-dose acute-phase reaction were back pain, urinary tract infection, hypertension, nasopharyngitis, arthralgia, pyrexia, myalgia, headache and influenza-like symptoms. However, we did not find significant difference in the serious adverse effect between the two groups, which indicates ZOL is a safe and efficacy treatment for women with osteoporosis.
In conclusion, the present three -year follow-up study indicated that intravenous 5-mg infusion zoledronic acid at the baseline and at 12 months could significantly the risk of non-vertebral fracture among females who were diagnosed with osteoporosis, and increase the BMD of femoral neck and total hip over three years when compared with placebo group. In additional, this treatment did not have serious drug-related adverse effects, and intravenous 5-mg infusion ZOL could be used as a safe and effective method for female with osteoporosis.
ACKNOWLEDGEMENT
We thank the First Affiliated Hospital of Xinxiang Medical College and Second People’s Hospital of Ji’nan for their help and assistance in this studuy.
Conflict of interest: All authors declare no conflict of interest.
Authors Contribution:
Ma Chao and Dong Yuzhen contributed to the study design, conduction, and paper writing. Qin Hua, Zhou Yingfeng, Wan Guang, Shi Shufen, Wang Wei and Tan Haifeng contributed in conducting the study conduction.
References
- 1.National Institutes of Health Consensus Development Panel on Osteoporosis Prevention. Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795. [Google Scholar]
- 2.Eastell R, Black DM, Boonen S, Adami S, Felsenberg D, Lippuner K, et al. Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. J Clin Endocrinol Metab. 2009;94:3215–3225. doi: 10.1210/jc.2008-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 4.Nshimyumukiza L, Durand A, Gagnon M, Douville X, Morin S, Lindsay C, et al. An economic evaluation: simulation of the cost-effectiveness and cost-utility of universal prevention strategies against osteoporosis-related fractures. J Bone Miner Res. 2013;28:383–394. doi: 10.1002/jbmr.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viswanathan HN, Curtis JR, Yu J, White J, Stolshek BS, Merinar C, et al. Direct healthcare costs of osteoporosis-related fractures in managed care patients receiving pharmacological osteoporosis therapy. Appl Health Econ Health Policy. 2012;10(3):163–173. doi: 10.2165/11598590-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Hwang JS, Tu ST, Yang TS, Chen JF, Wang CJ, Tsai KS. Teriparatide vs calcitonin in the treatment of Asian postmenopausal women with established osteoporosis. Osteoporos Int. 2006;17:373–378. doi: 10.1007/s00198-005-2002-5. [DOI] [PubMed] [Google Scholar]
- 7.Yen ML, Yen BL, Jang MH, Hsu SH, Cheng WC, Tsai KS. Effects of alendronate on osteopenic postmenopausal Chinese women. Bone. 2000;27:681–685. doi: 10.1016/s8756-3282(00)00384-7. [DOI] [PubMed] [Google Scholar]
- 8.Hwang JS, Chen JF, Yang TS, Wu DJ, Tsai KS, Ho C, et al. The effects of strontium ranelate in Asian women with postmenopausal osteoporosis. Calcif Tissue Int. 2008;83(5):308–314. doi: 10.1007/s00223-008-9180-z. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto M, Hotokezaka H, Sirisoontorn I, Nakano T, Arita K, Tanaka M, et al. The effect of bone morphometric changes on orthodontic tooth movement in an osteoporotic animal model. Angle Orthod. 2013;83(5):766–773. doi: 10.2319/111312-869.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Popp AW, Guler S, Lamy O, Senn C, Buffat H, Perrelet R, Hans D, Lippuner K. Effects of zoledronate versus placebo on spine bone mineral density and microarchitecture assessed by the trabecular bone score in postmenopausal women with osteoporosis: a three-year study. J Bone Miner Res. 2013;28:449–454. doi: 10.1002/jbmr.1775. [DOI] [PubMed] [Google Scholar]
- 11.Jacques RM, Boonen S, Cosman F, Reid IR, Bauer DC, Black DM, et al. Relationship of changes in total hip bone mineral density to vertebral and nonvertebral fracture risk in women with postmenopausal osteoporosis treated with once-yearly zoledronic acid 5 mg: the HORIZON-Pivotal Fracture Trial (PFT) J Bone Miner Res. 2012;27:1627–1634. doi: 10.1002/jbmr.1644. [DOI] [PubMed] [Google Scholar]
- 12.Black DM, Reid IR, Boonen S, Bucci-Rechtweg C, Cauley JA, Cosman F, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT) J Bone Miner Res. 2012;27(2):243–254. doi: 10.1002/jbmr.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genant HK, Wu CY, van Kuijk C. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 14.Cramer JA, Amonkar MM, Hebborn A. Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin. 2005;21:1453–1460. doi: 10.1185/030079905X61875. [DOI] [PubMed] [Google Scholar]
- 15.Reginster J, Minne HW, Sorensen OH. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- 16.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 17.Xu L, Deng H, Zhi X, Zhang W, Wang X, Wu W. Therapeutic effect of zoledronic acid on primary ostsoporosis in elderly patients. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32(9):1287–1289. [PubMed] [Google Scholar]
- 18.Boonen S, Black DM, Colon-Emeric CS, Eastell R, Magaziner JS, Eriksen EF, et al. Efficacy and safety of a once-yearly intravenous zoledronic acid 5 mg for fracture prevention in elderly postmenopausal women with osteoporosis aged 75 and older. J Am Geriatr Soc. 2010;58(2):292–299. doi: 10.1111/j.1532-5415.2009.02673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eastell R, Black DM, Boonen S. Effect of once-yearly zoledronic acid five milligrams on fracture risk and change in femoral neck bone mineral density. J Clin Endocrinol Metab. 2009;94(9):3215–3225. doi: 10.1210/jc.2008-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]