Abstract
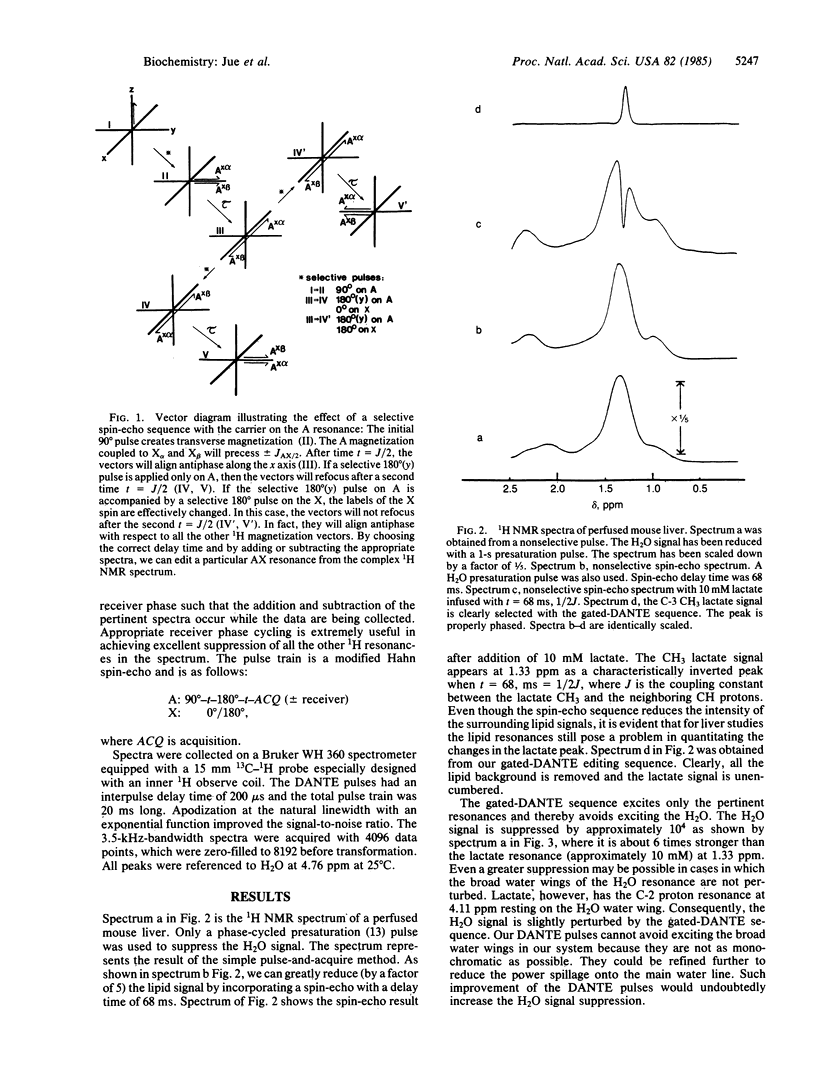
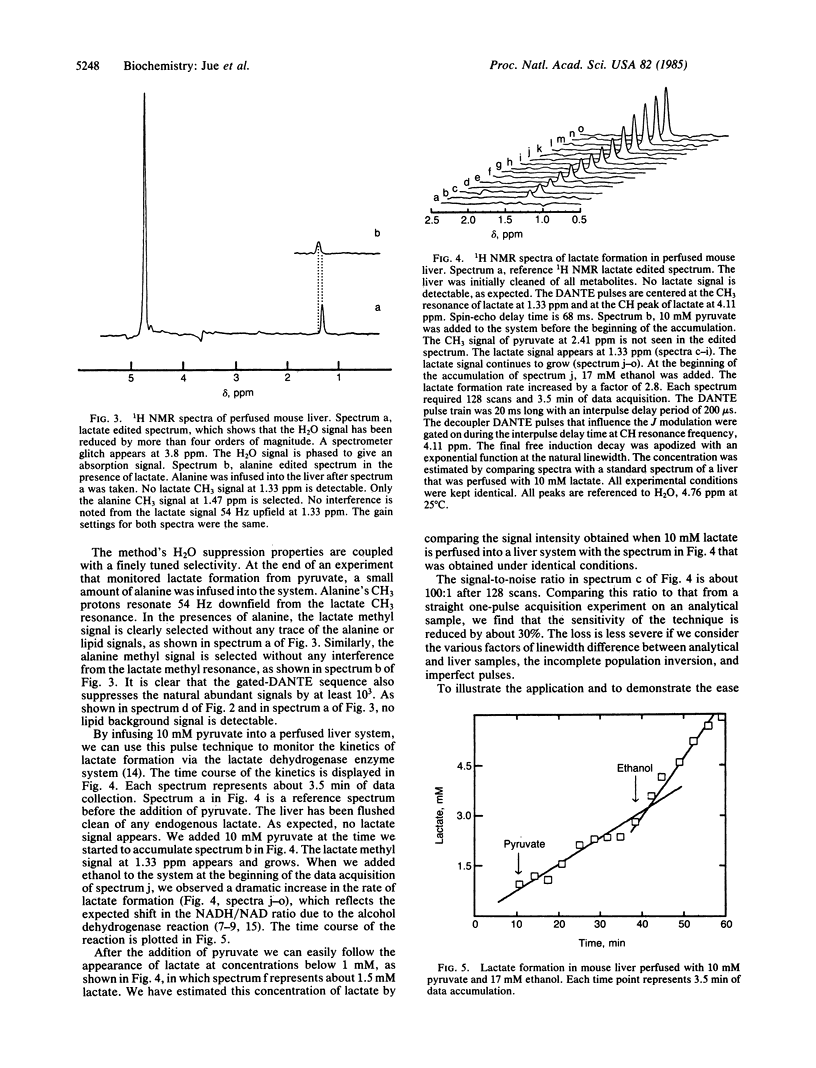
We have developed a 1H NMR technique to selectively edit the spectrum of perfused liver for specific resonances of metabolites that occur in low concentration. The method employs selective DANTE pulses, which avoid exciting the water signal and at the same time control the J modulation effect in the homonuclear spin-echo experiment. By difference spectroscopy, we have suppressed the background signals from lipids and water and have resolved the CH3 resonance of lactate at 1.33 ppm. Moreover, the technique is highly selective and allows us to select the CH3 resonance of alanine at 1.47 ppm in the presence of the CH3 resonance of lactate at 1.33 ppm, even though the latter was much larger before editing. We have applied this technique to study the metabolic effect of ethanol in perfused mouse liver and have observed that the rate of formation of lactate from pyruvate is increased by a factor of 2.8 when ethanol is added.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borgmann U., Moon T. W., Laidler K. J. Molecular kinetics of beef heart lactate dehydrogenase. Biochemistry. 1974 Dec 3;13(25):5152–5158. doi: 10.1021/bi00722a016. [DOI] [PubMed] [Google Scholar]
- Campbell I. D., Dobson C. M. The application of high resolution nuclear magnetic resonance to biological systems. Methods Biochem Anal. 1979;25:1–133. doi: 10.1002/9780470110454.ch1. [DOI] [PubMed] [Google Scholar]
- Campbell I. D., Dobson C. M., Williams R. J., Wright P. E. Pulse methods for the simplification of protein NMR spectra. FEBS Lett. 1975 Sep 1;57(1):96–99. doi: 10.1016/0014-5793(75)80160-8. [DOI] [PubMed] [Google Scholar]
- FROSANDER O. A., RAEIHAE N., SALASPURO M., MAEENPAEAE P. INFLUENCE OF ETHANOL ON THE LIVER METABOLISM OF FED AND STARVED RATS. Biochem J. 1965 Jan;94:259–265. doi: 10.1042/bj0940259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington H. P., Avison M. J., Shulman R. G. 1H homonuclear editing of rat brain using semiselective pulses. Proc Natl Acad Sci U S A. 1985 May;82(10):3115–3118. doi: 10.1073/pnas.82.10.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Freedland R. A., Hems R., Stubbs M. Inhibition of hepatic gluconeogenesis by ethanol. Biochem J. 1969 Mar;112(1):117–124. doi: 10.1042/bj1120117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. The effects of ethanol on the metabolic activities of the liver. Adv Enzyme Regul. 1968;6:467–480. doi: 10.1016/0065-2571(68)90029-0. [DOI] [PubMed] [Google Scholar]
- Salaspuro M. P., Mäenpä P. H. Influence of ethanol on the metabolism of perfused normal, fatty and cirrhotic rat livers. Biochem J. 1966 Sep;100(3):768–778. doi: 10.1042/bj1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K., Petein M., Maidan R., Michurski S., Cohn J. N., From A. H. High resolution proton NMR studies of perfused rat hearts. FEBS Lett. 1984 Feb 13;167(1):73–78. doi: 10.1016/0014-5793(84)80835-2. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Scholz R., Browning E. T., Thurman R. G., Fukami M. H. Metabolic effects of ethanol in perfused rat liver. J Biol Chem. 1969 Sep 25;244(18):5044–5054. [PubMed] [Google Scholar]


