Abstract
Context
The reporting of funding support and conflict of interest has not been examined in the supportive/palliative oncology literature.
Objectives
We examined the frequency of funding and conflict of interest reporting and various study characteristics associated with such reporting.
Methods
We systematically searched MEDLINE PubMed, PsycInfo, EMBASE, ISI Web of Science, and CINAHL for original studies related to palliative care and cancer in the first six months of 2004 and 2009. For each article, we reviewed the study design, research topic, journal type, and reporting of funding and conflict of interest.
Results
Three hundred forty-four (41%) and 504 (59%) of 848 articles were from 2004 and 2009, respectively. Five hundred two of 848 (59%) studies reported no funding sources, whereas 216 (26%), 70 (8%), 34 (4%), and 26 (3%) reported one, two, three, and four or more sources, respectively. Key funding sources included governmental agencies (n = 182/848, 21%), philanthropic foundations (n = 163/848, 19%), university departments (n = 76/848, 9%), and industry (n = 27/848, 3%). Conflict of interest was not reported in 436 of 848 (51%) studies, and only 94 of 848 (11%) explicitly stated no conflict of interest. Other than extramural funding, conflict of interest reporting of any kind was extremely rare (mostly less than 1%). Conflict of interest reporting increased between 2004 and 2009 (39% vs. 55%, P < 0.001). Both funding and conflict of interest reporting were associated with prospective studies, larger sample sizes, nontherapeutic studies, North American authors, and publication in palliative care/oncology journals (P ≤ 0.008 for all comparisons).
Conclusion
A majority of supportive/palliative oncology studies did not report funding sources and conflict of interest, raising the need for standardization.
Keywords: Neoplasms, palliative care, literature, funding, conflict of interest
Introduction
The literature related to palliative care has been growing over the past few decades, providing an increased evidence base for clinical practice and innovations.1 Competing financial interests among authors, institutions, reviewers, and editors, such as research funding and honoraria, could represent an important source of bias for both the conduction of studies and interpretation of the literature.2–4 Authors with major roles in study design and execution are more likely to have industry financial ties compared with those without such roles,5 and industry-sponsored studies are more likely to be interpreted as positive.6 Appropriate disclosure of all financial relationships that could be perceived as potential conflicts of interest represents an important step toward upholding public trust and scientific integrity.7
In an attempt to standardize conflict of interest reporting, the International Committee of Medical Journal Editors (ICMJE) developed a disclosure form in 2009, which was subse-quently revised in 2010 and adopted by all ICMJE journals.8 Several studies have examined the quality of reporting of funding sources and conflict of interest in the medical literature;9,10 however, none focused on the supportive/palliative oncology publications. We identified only one study on sources of palliative care funding11 and no studies on conflict of interest reporting. A better understanding of the extent of research funding and conflict of interest reporting could provide the basis for standardization, which could help to minimize bias when interpreting the literature. Insights into existing funding mechanisms also would allow us to identify areas where further support is needed. In this study, we examined the reporting of funding sources and conflict of interest in the supportive/palliative oncology literature and various study characteristics associated with such reporting.
Methods
Palliative Oncology Literature
The Institutional Review Board at M. D. Anderson Cancer Center provided approval to proceed without the need for full committee review. Details on how we identified and characterized the supportive/palliative oncology literature have been published.12–14 Briefly, inclusion criteria were 1) original studies in MEDLINE PubMed, PsycInfo, EMBASE, ISI Web of Science, and/or CINAHL related to both palliative care and oncology, 2) published in the first six months of 2004 or 2009, and 3) English language. Two reviewers manually examined each abstract for relevance to supportive/palliative oncology. We then obtained full electronic copies of all unique publications identified and abstracted various study characteristics, including the year of publication, study design, therapeutic nature, sample size, country of origin of corresponding author, and journal type. The research design was decided based on the primary objective.
Funding and Conflict of Interest Reporting
One reviewer (A. R.), blinded to literature characteristics, reviewed each of the included manuscripts for funding sources and conflict of interest reporting, paying particular attention to the author contributions and acknowledgment sections. Funding sources were classified as pharmaceutical companies, government agencies, philanthropic foundations, university/departmental, others, and not reported. When the category for a particular source of funding was not clearly identifiable,we searched the Internet to obtain further information.
Conflict of interest information was collected based on the form for disclosure of potential conflicts of interest established by ICMJE, which included two major categories of conflict of interest.15 The first category is related to financial interest relevant to the work under consideration for publication, including 1) all third-party grants (excluding departmental/ institutional funding), 2) consulting fee or honorarium, 3) support for travel to meetings for the study or other purposes, 4) fees for participation in review activities, such as data monitoring boards, statistical analysis, end point committees, and the like,5)payment for writing and reviewing the manuscript, 6) provision of writing assistance, medicines, equipment, or administrative support, and 7) other. The second category included any relevant financial activities outside the submitted work during the 36 months before submission and included 1) board membership, 2) consultancy, 3) employment, 4) expert testimony, 5) grants/grants pending, 6) payment for lectures including service on speakers’ bureaus, 7) payment for manuscript preparation, 8) patents (planned, pending, or issued), 9) royalties, 10) payment for development of educational presentations, 11) stock/stock options, 12) travel/accommodations/meeting expenses unrelated to activities listed, and 13) other. In this study, conflict of interest reporting was considered to be present if the article either reported any conflict of interest listed above or explicitly stated no conflict of interest.
To examine various journals’ policies regarding funding and conflict of interest reporting, we summarized the authors’ instructions from the websites of the 10 journals that published the highest number of supportive/palliative oncology studies (search date: March 2, 2011). These journals were identified in a previous analysis by our group.12
Statistical Analysis
We summarized the publication characteristics using frequencies, percentages, medians, and interquartile ranges. We compared the presence and absence of reporting by journal characteristics using the Chi-squared test and Fisher’s exact test for categorical variables (e.g., year and journal type) and the Mann-Whitney test for continuous variables (e.g., sample size). A two-sided P-value less than 0.05 was considered to be statistically significant. The STATA special edition software version 10.0 (StataCorp LP, College Station, TX) was used for statistical analysis.
Results
Literature Characteristics
We identified 848 supportive/palliative oncology original articles. The literature characteristics have been reported in detail previously.12 Three hundred forty-four (41%) and 504 (59%) articles were from 2004 and 2009, respectively. Three hundred ninety-eight (48%) articles were published in palliative care journals, 132 (16%) in oncology journals, and 308 (36%) in other journals.
Funding Sources Reporting
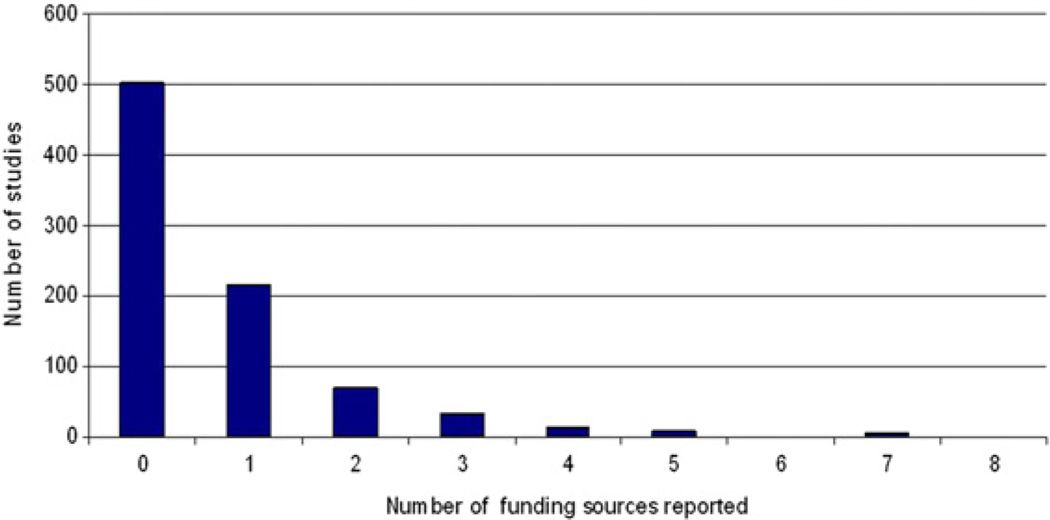
Five hundred two (59%) studies did not report any funding sources (Table 1). Fig. 1 shows the number of funding sources reported. Key funding sources included government agencies, philanthropic foundations, and university departments (Table 1). Industry sponsorship was only reported in 27 (3%) studies.
Table 1.
Reported Funding Sources for Supportive/Palliative Oncology Publications (n = 848)
| Reported Funding Sources | n (%)a |
|---|---|
| Not reported | 502 (59) |
| Pharmaceutical companies | 27 (3) |
| Government agencies | 182 (21) |
| Philanthropic foundations | 163 (19) |
| University/department | 76 (9) |
| Others | 4 (0.5) |
The total is greater than 100% because some publications reported more than one source of funding.
Fig. 1.
Number of funding sources for supportive/palliative oncology publications (n = 848).
Table 2 shows the reporting of funding by literature characteristic. We found no significant increase in funding reporting between 2004 and 2009. Funding sponsors were reported in 107 of 429 (25%) case series, 56 of 95 (59%) qualitative studies, 89 of 149 (60%) cross-sectional studies, and 27 of 47 (57%) randomized controlled trials. The presence of funding reporting was associated with prospective studies, nontherapeutic studies, studies with larger sample sizes, North American and Australian authors, and publication in palliative care/oncology journals.
Table 2.
Reporting of Funding by Publication Characteristics
| Funding Reported |
Funding Not Reported |
||
|---|---|---|---|
| Publication Characteristics | n = 346 (%)a | n = 502 (%)a | P-valueb |
| Year | |||
| 2004 | 136 (40) | 208 (60) | 0.54 |
| 2009 | 210 (42) | 294 (58) | |
| Research design | |||
| Case series | 107 (25) | 322 (75) | <0.001 |
| Cohort | 28 (39) | 44 (61) | |
| Cross-sectional | 89 (60) | 60 (40) | |
| Qualitative | 56 (59) | 39 (41) | |
| Randomized controlled trials | 27 (57) | 20 (43) | |
| Other | 39 (70) | 17 (30) | |
| Type | |||
| Retrospective | 69 (20) | 281 (80) | <0.001 |
| Prospective | 277 (56) | 221 (44) | |
| Therapeutic | |||
| No | 282 (53) | 253 (47) | <0.001 |
| Yes | 64 (20) | 249 (80) | |
| Sample size, median (interquartile range) | 114 (41–345) | 34 (6–106) | <0.001 |
| Continent | |||
| Africa | 3 (43) | 4 (57) | <0.001 |
| Asia | 47 (33) | 96 (67) | |
| Australia | 24 (48) | 26 (52) | |
| Europe | 119 (35) | 221 (65) | |
| Latin America | 1 (13) | 7 (87) | |
| North America | 152 (51) | 148 (49) | |
| Journal type | |||
| Palliative care | 198 (50) | 200 (50) | <0.001 |
| Oncology | 53 (44) | 79 (56) | |
| Others | 85 (28) | 223 (72) |
Unless otherwise specified.
Comparisons between studies that reported funding and those that did not report funding were made by the Chi-squared test and Fisher’s exact test (if cell value ≤5) for categorical variables and the Mann-Whitney test for continuous variables.
Conflict of Interest Reporting
The extent of conflict of interest reporting based on the ICMJE criteria is shown in Table 3. Avast majority of studies did not provide any information on conflict of interest. Only 94 (11%) studies specifically stated no conflict of interest related to the study, and 90 (11%) studies declared no conflict of interest outside the study. Other than extramural funding, conflict of interest reporting of any kind was extremely rare (mostly less than 1%, Table 3).
Table 3.
Reported Conflicts of Interest for Supportive/Palliative Oncology Publications
| Number |
|
|---|---|
| Reported Conflicts of Interest | n = 848 (%)a |
| Conflict of interest related to study | |
| Not reportedb | 436 (51) |
| Explicitly declared no conflict of interest | 94 (11) |
| Extramural funding | 318 (38) |
| Consulting fee or honorarium | 2 (0.2) |
| Support for travel to meetings for the study or other purposes | 0 (0) |
| Fees for participation in review activities, such as data monitoring boards, statistical analysis, end point committees, and the like | 1 (0.1) |
| Payment for writing or reviewing the manuscript | 1 (0.1) |
| Provision of writing assistance, medicines, equipment, or administrative support | 7 (0.8) |
| Others | 0 (0) |
| Conflict of interest outside the study | |
| Not reported | 743 (88) |
| Explicitly declared no conflict of interest | 90 (11) |
| Board membership | 10 (1.2) |
| Consultancy | 5 (0.6) |
| Employment | 6 (0.7) |
| Expert testimony | 0 (0) |
| Grants/grants pending | 2 (0.2) |
| Payment for lectures including service on speakers’ bureaus | 1 (0.1) |
| Payment for manuscript preparation | 0 (0) |
| Patents (planned, pending, or issued) | 0 (0) |
| Royalties | 0 (0) |
| Payment for development of educational presentations | 1 (0.1) |
| Stock/stock options | 4 (0.5) |
| Travel/accommodations/meeting expenses unrelated to activities listed | 0 (0) |
| Others | 1 (0.1) |
The total is greater than 100% because some publications reported more than one source of conflict of interest.
Studies were considered to have not reported conflict of interest if they neither stated “no conflict of interest” nor reported any conflict of interests listed by the International Committee of Medical Journal Editors (ICMJE). The ICMJE included extramural funding as one of the potential sources of conflict of interest. If funding reporting was considered separately from conflict of interest reporting, the number of manuscripts without any conflict of interest reporting increases to 745 (88%).
We found that the proportion of studies that reported conflict of interest (including the declaration of no conflict of interest) increased between 2004 and 2009 (Table 4). The presence of reporting of conflict of interest was associated with prospective studies, noncase series study design, nontherapeutic studies, studies with larger sample size, North American authors, and publication in palliative care/oncology journals.
Table 4.
Reporting of Funding and Conflict of Interest by Supportive/Palliative Oncology Publication Characteristics
| Conflict of Interest Related to Study |
Conflict of Interest Not Related to Study |
|||||
|---|---|---|---|---|---|---|
| Reported |
Not Reported |
Reported |
Not Reported |
|||
| Publication Characteristics | n =412 (%)a | n = 436 (%)a | P-value | n= 105 (%)a | n =743 (%)a | P-valueb |
| Year | ||||||
| 2004 | 134 (39) | 210 (61) | <0.001 | 17 (5) | 327 (95) | <0.001 |
| 2009 | 278 (55) | 226 (45) | 88 (17) | 416 (83) | ||
| Research design | ||||||
| Case series | 155 (36) | 274 (64) | <0.001 | 27 (6) | 402 (94) | <0.001 |
| Cohort | 38 (53) | 34 (47) | 13 (18) | 59 (82) | ||
| Cross-sectional | 89 (60) | 60 (40) | 29 (20) | 120 (80) | ||
| Other | 40 (71) | 16 (29) | 16 (28) | 41 (72) | ||
| Qualitative | 59 (62) | 36 (38) | 12 (13) | 83 (87) | ||
| Randomized controlled trials | 31 (66) | 16 (34) | 9 (19) | 38 (81) | ||
| Type | ||||||
| Retrospective | 106 (30) | 244 (70) | <0.001 | 36 (10) | 314 (90) | 0.12 |
| Prospective | 306 (61) | 192 (39) | 69 (14) | 429 (86) | ||
| Therapeutic | ||||||
| No | 300 (56) | 235 (44) | <0.001 | 78 (15) | 457 (85) | 0.011 |
| Yes | 112 (36) | 201 (64) | 27 (9) | 286 (91) | ||
| Sample size, median (interquartile range) | 100 (32–306) | 35 (6–106) | <0.001 | 124 (64–588) | 50 (13–158) | <0.001 |
| Continent | ||||||
| Africa | 3 (43) | 4 (57) | 0.006 | 1 (14) | 6 (84) | <0.001 |
| Asia | 62 (43) | 81 (57) | 10 (7) | 133 (93) | ||
| Australia | 27 (54) | 23 (46) | 3 (6) | 47 (94) | ||
| Europe | 145 (43) | 195 (57) | 30 (9) | 310 (91) | ||
| Latin America | 4 (50) | 4 (50) | 0 (0) | 8 (100) | ||
| North America | 171 (57) | 129 (43) | 61 (20) | 239 (80) | ||
| Journal type | ||||||
| Palliative care | 206 (52) | 192 (48) | <0.001 | 35 (9) | 363 (91) | 0.006 |
| Oncology | 85 (60) | 57 (40) | 26 (18) | 116 (82) | ||
| Others | 121 (39) | 187 (61) | 44 (14) | 264 (86) | ||
Unless otherwise specified.
Comparisons between studies that reported funding and those that did not report funding were made by the Chi-squared test and Fisher’s exact test (if cell value ≤5) for categorical variables and the Mann-Whitney test for continuous variables.
Journal Policies
We identified significant heterogeneity in both the requirement and format of funding and conflict of interest reporting among various journals (Table 5). Four of the 10 journals examined did not provide any explicit instructions on funding reporting, and two of the journals did not mandate any conflict of interest reporting. Only four journals published explicit statements when the authors declare no conflict of interest. None adopted the ICMJE conflict of interest reporting form.
Table 5.
Funding and Conflict of Interest Reporting Policy for 10 Supportive/Palliative Oncology Journals
| Supportive/Palliative Oncology Journals |
Instructions to Report Funding | Instructions to Report Conflict of Interest |
Statement If No Conflict of Interest |
Format of Reporting |
|---|---|---|---|---|
| American Journal of Hospice and Palliative Medicine | Disclose “all funding sources supporting the work” | Disclose “any commercial or financial association that might pose a conflict of interest in connection with their submitted article” | No | No standardized form |
| International Journal of Palliative Nursing | No explicit instructions | Disclose “any conflicts of interest at the end of your article. These are any possible interests, financial or otherwise, which may embarrass the author or the journal if highlighted at a later date.” | No | No standardized form |
| Journal of Clinical Oncology | Disclose “financial research support received from commercial entities” | Disclose “financial interests or relationships involving the authors” | Yes | Journal-specific, non-ICJME disclosure form |
| Journal of the National Cancer Institute | Disclose “details of all funding sources for the work in question” | Disclose “1) any financial interest in or arrangement with a company whose product was used in a study or is referred to in a review, opinion piece, or letter, 2) any financial interest in or arrangement with a competing company, and 3) any other financial connections …” | No | No standardized form |
| Journal of Pain and Symptom Management | Disclose “all sources of funding and/or sponsorship” | Disclose “1) any financial or personal relationships with individuals, organizations, or companies that might be perceived to bias the work and 2) the role of the funding source/ sponsor in study design or in the collection, analysis, interpretation, or presentation of the information” | Yes | No standardized form |
| Journal of Palliative Care | No explicit instructions | No explicit instructions | No | No standardized form |
| Journal of Palliative Medicine | No explicit instructions | Disclose “any commercial associations that might create a conflict of interest in connection with submitted manuscripts” | Yes | No standardized form |
| Palliative and Supportive Care | No explicit instructions | No explicit instructions | No | No standardized form |
| Palliative Medicine | Disclose “their funding in a consistent fashion under a separate heading” | Disclose “any financial relationship that all authors of the article has with any sponsoring organization and the for-profit interests the organization represents and with any for-profit product discussed or implied in the text of the article” | Yes | Standardized statements for reporting |
| Supportive Care in Cancer | Disclose “all funding sources supporting the work and all institutional or corporate affiliations” | Disclose “any real or apparent conflict(s) of interest that may have a direct bearing on the subject matter of the article” | No | Journal-specific, non-ICJME disclosure form |
ICMJE = International Committee of Medical Journal Editors.
Discussion
In this systematic review of the supportive/ palliative oncology literature, we identified significant inadequacies in the reporting of funding sources and conflict of interest, raising concerns about publication standards and scientific integrity. Few studies are supported by government and philanthropic organizations, and even fewer studies reported industry sponsorship or any conflict of interest. Urgent measures from journal editors and authors are required to improve the standard of conflict of interest reporting.
Most supportive/palliative oncology studies (59%) did not report research funding. Scientific research demands significant resources in regard to personnel time, materials, and institutional infrastructure. Importantly, the lack of funding reporting does not necessarily mean lack of funding. For instance, only 57% of randomized controlled trials reported funding when all of them undoubtedly required significant resources. Without proper documentation, it is not clear whether the cost of research was subsidized by patients, institutions, researchers, industry, or other third parties. Because funding support represents a significant source of bias in study design, conduction, and interpretation,6,16–18 it is critical that its reporting be mandated by journals universally. Both the Consolidated Standards of Reporting Trials and Preferred Reporting Items for Systematic Reviews and Meta-Analyses criteria, which were, respectively, developed to assess the quality of reporting of randomized controlled trials and systematic reviews, have included funding reporting on their checklists.19–21
We found that less than half of all supportive/palliative oncology studies reported funding sources. This represents a significantly lower proportion compared with other disciplines, such as primary care, oncology, internal medicine/neurology, and psychiatry, in which 60%–80% reported funding.5,22–24 Our finding that supportive/palliative oncology research appears to be underfunded raises serious concerns because high-quality studies, such as randomized controlled trials, often require substantial investment of research dollars. This is supported by our observation that funding reporting is associated with prospective trials and larger sample sizes.14 Thus, the lack of funding could significantly impede vertical development in the field of supportive/palliative oncology. Contributions from government and philanthropic organizations are particularly important to minimize the potential conflict of interest from industry sponsorship and maximize the quality of study design through a competitive peer-review process.25
Industry involvement is a double-edged sword. On the one hand, industry involvement can potentially stimulate innovations and new therapeutics; on the other hand, industry involvement is always a perceived source of conflict of interest and has consistently been shown to be associated with biased study design and findings.26–28 Few published studies in supportive/palliative oncology reported industry ties, which is in stark contrast to other oncology articles, in which up to 52% reported pharmaceutical company support.5,29 The relative lack of industry involvement may have contributed to the limited growth of pharmacological treatments in supportive/palliative oncology.
In addition to grants, there are many other potential financial conflicts of interest highlighted by the ICMJE, such as consulting fees, board memberships, and stock ownerships. We found that reporting of such competing interests is rare. The frequency of financial ties to funding sources exceeds 33% for senior journal authors in leading medical journals,30 raising doubts regarding the apparent lack of conflict of interest in the supportive/palliative oncology literature. Further research is needed to clarify whether this is a result of lack of competing interest or lack of proper reporting.
More than half of the studies did not report any conflict of interest. This lack of reporting is related to one of the three possibilities: 1) no conflict of interest was present, and no explicit statement was published; 2) conflict of interest was present but was not reported by authors unintentionally; or 3) conflict of interest was present but was not reported by authors intentionally. Without any clear statements on conflict of interest, readers cannot distinguish among these possibilities. A simple solution to this problem would be a universal requirement from journals for authors to declare their conflicts of interest and explicitly state “no conflict of interest” when applicable.31 It is the responsibility of journal editors to establish high-quality reporting of funding sources and conflicts of interest by standardizing what needs to be reported and publishing such statements consistently. At the same time, authors are obliged to provide balanced and scientifically rigorous interpretations of their work, adhere to journal publication policy, and honestly report any conflict of interest. The ICMJE piloted a form for reporting of financial ties, although this has been variably adopted by various journals. None of the 10 journals that publish the highest number of supportive/palliative oncology studies currently use this form (Table 5). Further studies are required to identify the barriers to conflict of interest reporting.
This study has several limitations. First, we only documented funding sources and conflict of interest as reported by authors and did not verify the accuracy of reporting. Second, our literature search was restricted to palliative cancer care studies and only to those published in the first six months of 2004 and 2009. This sample may not be representative of the greater supportive/palliative oncology literature. Third, the five-year interval may not be long enough to notice any change in frequency. Finally, our study period (2004 and 2009) predated the release of the ICMJE disclosure form. Rather than examining the adherence to ICMJE standards, we used this form as a template to comprehensively examine the various domains of conflict of interest reporting.
In summary, we found significant deficiencies in the reporting of funding sources and conflict of interest in supportive/palliative oncology literature, highlighting the need for journals to mandate standardized reporting. Only about 40% of studies reported funding support, highlighting the need for injection of funding to optimize cancer care. Urgent measures are required from policy makers and funding agencies to support unbiased supportive/palliative oncology research, and further efforts from journal editors and authors are needed to uphold the standards of conflict of interest reporting.
Acknowledgments
Eduardo Bruera is supported in part by the National Institutes of Health grants RO1NR010162-01A1, RO1CA122292-01, and RO1CA124481-01. David Hui is supported in part by a startup grant from M. D. Anderson Cancer Center.
Footnotes
Disclosures
The authors report no relevant financial conflicts of interest.
References
- 1.Tieman J, Sladek R, Currow D. Changes in the quantity and level of evidence of palliative and hospice care literature: the last century. J Clin Oncol. 2008;26:5679–5683. doi: 10.1200/JCO.2008.17.6230. [DOI] [PubMed] [Google Scholar]
- 2.McCrary SV, Anderson CB, Jakovljevic J, et al. A national survey of policies on disclosure of conflicts of interest in biomedical research. N Engl J Med. 2000;343:1621–1626. doi: 10.1056/NEJM200011303432207. [DOI] [PubMed] [Google Scholar]
- 3.Lathyris DN, Patsopoulos NA, Salanti G, Ioannidis JP. Industry sponsorship and selection of comparators in randomized clinical trials. Eur J Clin Invest. 2010;40:172–182. doi: 10.1111/j.1365-2362.2009.02240.x. [DOI] [PubMed] [Google Scholar]
- 4.Smith R. Conflicts of interest: how money clouds objectivity. J R Soc Med. 2006;99:292–297. doi: 10.1258/jrsm.99.6.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rose SL, Krzyzanowska MK, Joffe S. Relationships between authorship contributions and authors’ industry financial ties among oncology clinical trials. J Clin Oncol. 2010;28:1316–1321. doi: 10.1200/JCO.2008.21.6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA. 2003;289:454–465. doi: 10.1001/jama.289.4.454. [DOI] [PubMed] [Google Scholar]
- 7.Irwin RS. The role of conflict of interest in reporting of scientific information. Chest. 2009;136:253–259. doi: 10.1378/chest.09-0890. [DOI] [PubMed] [Google Scholar]
- 8.Drazen JM, de Leeuw PW, Laine C, et al. Toward more uniform conflict disclosuresd-the updated ICMJE conflict of interest reporting form. N Engl J Med. 2010;363:188–189. doi: 10.1056/NEJMe1006030. [DOI] [PubMed] [Google Scholar]
- 9.Bhargava N, Qureshi J, Vakil N. Funding source and conflict of interest disclosures by authors and editors in gastroenterology specialty journals. Am J Gastroenterol. 2007;102:1146–1150. doi: 10.1111/j.1572-0241.2007.01268.x. [DOI] [PubMed] [Google Scholar]
- 10.Birkhahn RH, Van Deusen SK, Okpara OI, et al. Funding and publishing trends of original research by emergency medicine investigators over the past decade. Acad Emerg Med. 2006;13:95–101. doi: 10.1197/j.aem.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Gelfman LP, Morrison RS. Research funding for palliative medicine. J Palliat Med. 2008;11:36–43. doi: 10.1089/jpm.2006.0231. [DOI] [PubMed] [Google Scholar]
- 12.Hui D, Parsons HA, Damani S, et al. Quantity, design, and scope of the palliative oncology literature. Oncologist. 2011;16:694–703. doi: 10.1634/theoncologist.2010-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hui D, Mori M, Parsons H, et al. Lack of definitions for common terminologies in the supportive and palliative oncology literature. J Pain Symptom Manage. 2012;43:582–592. doi: 10.1016/j.jpainsymman.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hui D, Arthur J, Dalal S, Bruera E. Quality of the supportive and palliative oncology literature: a focused analysis on randomized controlled trials. Support Care Cancer. 2011 doi: 10.1007/s00520-011-1275-9. [DOI] [PubMed] [Google Scholar]
- 15.International Committee of Medical Journal Editors (ICMJE) [Accessed April 15, 2011];ICMJE uniform disclosure form for potential conflicts of interest. Available from http://www.icmje.org/ [Google Scholar]
- 16.Hui D, Bruera E. Breakthrough pain in cancer patients: the need for evidence. Eur J Palliat Care. 2010;17:58–67. [Google Scholar]
- 17.Hui D, Bruera E. Transdermal fentanyl: not ready for front line. J Palliat Care. 2009;25:300. author reply 301. [PubMed] [Google Scholar]
- 18.Bruera E, Hui D. Spinal analgesia: where is the evidence? Support Care Cancer. 2010;18:1237. doi: 10.1007/s00520-010-0881-2. [DOI] [PubMed] [Google Scholar]
- 19.The CONSORT Group. [Accessed May 7, 2011];CONSORT checklist. 2010 Available from http://www.consort-statement.org/home/ [Google Scholar]
- 20.Auperin A, Pignon JP, Poynard T. Review article: critical review of meta-analyses of randomized clinical trials in hepatogastroenterology. Aliment Pharmacol Ther. 1997;11:215–225. doi: 10.1046/j.1365-2036.1997.131302000.x. [DOI] [PubMed] [Google Scholar]
- 21.PRISMA. [Accessed May 9, 2011];PRISMA checklist. Available from http://www.prisma-statement.org/statement.htm. [Google Scholar]
- 22.Stein MD, Rubenstein L, Wachtel TJ. Who pays for published research? JAMA. 1993;269:781–782. [PubMed] [Google Scholar]
- 23.Ruffin MT, Sheets KJ. Primary care research funding sources. J Fam Pract. 1992;35:281–287. [PubMed] [Google Scholar]
- 24.Cleary M, Walter G, Hunt G. The quest to fund research: playing research lotto. Australas Psychiatry. 2006;14:323–326. doi: 10.1080/j.1440-1665.2006.02286.x. [DOI] [PubMed] [Google Scholar]
- 25.Curtis JR, Morrison RS. The future of funding for palliative care research: suggestions for our field. J Palliat Med. 2009;12:26–28. doi: 10.1089/jpm.2009.9691. [DOI] [PubMed] [Google Scholar]
- 26.Perlis CS, Harwood M, Perlis RH. Extent and impact of industry sponsorship conflicts of interest in dermatology research. J Am Acad Dermatol. 2005;52:967–971. doi: 10.1016/j.jaad.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Garattini L, Koleva D, Casadei G. Modeling in pharmacoeconomic studies: funding sources and outcomes. Int J Technol Assess Health Care. 2010;26:330–333. doi: 10.1017/S0266462310000322. [DOI] [PubMed] [Google Scholar]
- 28.Warner TD, Gluck JP. What do we really know about conflicts of interest in biomedical research? Psychopharmacology (Berl) 2003;171:36–46. doi: 10.1007/s00213-003-1657-x. [DOI] [PubMed] [Google Scholar]
- 29.Booth CM, Cescon DW, Wang L, Tannock IF, Krzyzanowska MK. Evolution of the randomized controlled trial in oncology over three decades. J Clin Oncol. 2008;26:5458–5464. doi: 10.1200/JCO.2008.16.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krimsky S, Rothenberg LS, Stott P, Kyle G. Scientific journals and their authors’financial interests:a pilot study. Psychother Psychosom. 1998;67:194–201. doi: 10.1159/000012281. [DOI] [PubMed] [Google Scholar]
- 31.Krimsky S, Rothenberg LS. Financial interest and its disclosure in scientific publications. JAMA. 1998;280:225–226. doi: 10.1001/jama.280.3.225. [DOI] [PubMed] [Google Scholar]