Abstract
PURPOSE OF REVIEW
Obesity is a major worldwide health problem. Excess weight gain is the most significant, preventable, cause of increased blood pressure (BP) in patients with essential hypertension, and increases the risk for cardiovascular and renal diseases. Our goal is to review the mechanisms that link obesity with hypertension, with special emphasis on the role of leptin and the brain melanocortin system in driving sympathetic activation in obesity.
RECENT FINDINGS
Despite increased interest in obesity as a major risk for developing hypertension, the precise mechanisms linking excess weight gain with increases in BP are still elusive. Current evidence suggests that increased sympathetic nervous system (SNS) activity contribute to impaired renal-pressure natriuresis and sodium retention in obesity. Recent findings indicate that the adipocyte-derived hormone, leptin, activates brain centers that regulate SNS activity through a melanocortin system dependent pathway. The interaction of leptin and the brain melanocortin system represents an important area of research to further our understanding of the mechanisms leading to sympathetic activation in obesity.
SUMMARY
Sympathetic overactivity is an important link between excess weight gain and increased BP. Hormones/cytokines secreted by adipose tissue that interact with neural pathways (e.g. melanocortin system) appear to play a key role in contributing to sympathetic activation in obesity and represent potential new targets for therapies.
Keywords: Leptin, MC4R, melanocortin system, visceral fat, appetite
INTRODUCTION
In the past 25-35 years we have witnessed an unprecedented increase in the prevalence of obesity in developing as well as industrialized countries where over 1 billion people are estimated to be obese or overweight [1]. In the U.S. approximately 35.7% of adults are obese with body mass index (BMI) greater than 30 and more that 2/3 of the population is overweight, accounting for more than $150 billion/year in medical costs. [2**] Even more alarming is the increasing numbers of overweight and obese children and adolescents which has tripled since 1980; recent estimates indicate that 18.4% of 4-year-olds are obese, and even higher rates of obesity are observed in Black and Native American children as well as in children of Hispanic origin [3].
Obesity shifts the distribution of BP towards higher values
Being overweight or obese markedly increases the risk for cardiovascular disease via several mechanisms, including diabetes, dyslipidemia, atherosclerosis, renal disease, and hypertension [4, 5]. For instance, risk estimates from population studies suggest that excess weight gain may contribute to as much as 80% to 90% of the risk for diabetes and other metabolic disorders and up to 65% to 75% of the risk for essential hypertension [4]. Although the prevalence of hypertension is higher in obese than in lean populations, not all obese individuals, when assessed by standard clinical criteria, are found to be hypertensive. However, excess weight gain shifts the distribution of BP towards higher values. Thus, obese subjects not classified as being hypertensive would have lower BP at a lower body weight [5, 6]. This concept is supported by the nearly linear relationship between body mass index (BMI) and BP, by the fact that excess weight gain predicts future development of hypertension [7], and that weight loss helps prevent the development of hypertension and reduces BP in most hypertensive individuals [8].
Mechanisms of Increased BP in Obesity: Important Role of the Sympathetic Nervous System (SNS)
Despite the established importance of obesity as a major cause of essential hypertension the mechanisms by which excess weight gain elevates BP are not fully understood. Obesity related hypertension is associated with sodium retention and impaired renal-pressure natriuresis (rightward shift in the pressure-natriuresis curve toward hypertensive levels) [7, 9]. This appears to be mediated, at least in part, by activation of the renin-angiotensin-aldosterone system (RAAS), physical compression of the kidneys by surrounding visceral fat and increased renal sinus fat, and increased sympathetic nervous system (SNS) activity [7]. Although these 3 main factors are closely interrelated and act in concert to impair renal-pressure natriuresis and raise BP in obese individuals, in this review we will focus on the role of increased SNS activity.
Obesity is Associated with Tissue-Specific Activation of the Sympathetic Nervous System
Excess weight gain, especially when accompanied by increased visceral adiposity, is associated with increased SNS activity which has been shown to participate in the development of hypertension in obese humans as well as in animal models of diet-induced obesity [10, 11]. The rise in SNS activity in diet-induced obesity appears to develop as early as one week after exposure to high fat diets [12] and even modest weight gain in nonobese subjects is associated with increased SNS activity [10**]. Chronic pharmacological blockade of adrenergic receptors also causes greater reductions in BP in obese compared to lean hypertensive subjects [13]. However, the rise in SNS activity in obesity is modest and occurs only in certain organs and tissues instead of generalized whole-body sympathetic activation. For example, in obese subjects SNS activity in skeletal muscle and the kidneys is elevated whereas cardiac sympathetic activity is minimally increased, or even reduced, due to baroreflex inhibition [14]. Moreover, increased renal SNS activity leading to sodium retention and impaired pressure-natriuresis is the primary mechanism for elevated BP with excess weight gain rather than peripheral vasoconstriction caused by massive widespread SNS activation [6].
In addition to being tissue specific, increased SNS activity in obesity also appears to vary according to ethnicity and body fat distribution with visceral obesity eliciting greater SNS activation than subcutaneous obesity [6]. The reasons for this stronger association between visceral obesity and SNS activation when compared to abdominal or lower body obesity have not been widely studied, and in most human studies muscle SNS activity has been evaluated rather than renal SNS activity. Thus, additional studies are needed to examine the influence of body fat distribution and differential regulation of SNS activity, and its close association with increased BP in obesity.
Several factors have been proposed to contribute to increased SNS activity in obesity including impaired baroreflex sensitivity, angiotensin II, hyperinsulinemia, sleep apnea, hypoghrelinemia, and hypoadiponectemia. The role of these factors in obesity-induced SNS activation and hypertension has been previously reviewed [15] and in this brief review we focus mainly on two other factors that appear to be key players in linking excess weight gain with increased SNS activity: 1) hyperleptinemia and 2) activation of the brain pro-opiomelanocortin (POMC) neurons and melanocortin-4 receptors (MC4R).
Leptin Regulates Body Weight Homeostasis and Increases SNS Activity and BP
Leptin, a peptide hormone secreted by adipocytes in proportion to body fat mass, crosses the blood-brain barrier via a saturable transport system to activate its receptors in various regions of the central nervous system (CNS), especially in the hypothalamus and brainstem, to reduce appetite and increase energy expenditure [16]. Leptin deficiency or leptin receptor (LR) mutations that prevent normal activation of its intracellular signaling events lead to morbid early-onset obesity in humans and experimental animals models [17]. Despite marked obesity and many other characteristics of what has been termed “metabolic syndrome” including hyperinsulinemia, dyslipidemia, hyperglycemia, visceral adiposity, and insulin resistance, individuals with leptin gene mutations are not hypertensive and do not exhibit increased SNS activity as would be anticipated based on their increased adiposity; in fact they exhibit postural hypotension and attenuated RAAS to upright posture [18]. Further evidence supporting a role for leptin in contributing to increased SNS activity and hypertension in obesity derives from studies showing that acute leptin injections, either peripherally or directly into the brain ventricles, raise renal SNS activity[19]. Moreover, chronic infusion of leptin in lean rodents to achieve plasma levels that mimic those observed in obesity causes gradual elevation in BP over several days that can be completely abolished by chronic α and β adrenergic receptor blockade [20]; and similar to diet-induced obesity, the gradual rise in BP during chronic leptin infusion suggests slow-acting mechanisms, including modest renal SNS activation to promote sodium retention, rather than massive widespread SNS activation to promote peripheral vasoconstriction. Collectively, these observations suggest that hyperleptinemia may be an important link between obesity, SNS activation and hypertension.
Obesity May Be Associated with “Selective” Leptin Resistance
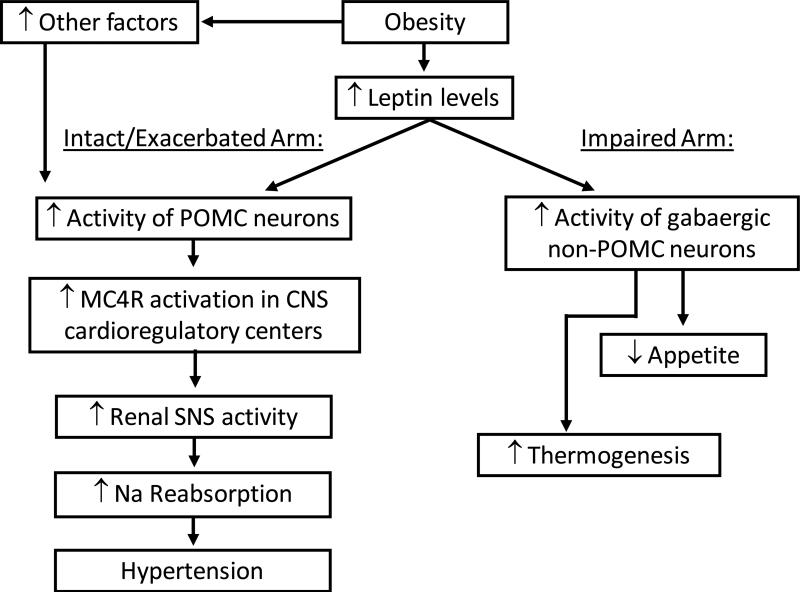
Another intriguing aspect of leptin-induced elevation in BP in non-obese animals is that it occurs concomitantly with marked reductions in appetite and significant weight loss which would normally be associated with decreased BP. Thus, in the absence of these metabolic actions of leptin to promote weight loss and reduce appetite the effects of leptin on SNS activity and on BP may be exacerbated. In fact, it is believed this scenario occurs in obesity where “selective” resistance to the appetite and metabolic actions of leptin may develop whereas leptin's cardiovascular effects remain unaltered or even enhanced (Figure 1). The fact that most obese individuals have high circulating leptin levels and continue to ingest excess calories corroborates the notion that obesity is associated with resistance to the anorexic effects of leptin; but the observation that intracerebroventricular leptin administration causes normal or even greater renal SNS activation in obese animals fed high-fat diet when compared to lean controls [21**] while its ability to acutely reduce food intake is impaired [19] is consistent with the hypothesis that obesity causes selective resistance to the metabolic effects of leptin instead of complete resistance to its many physiological actions. The mechanisms by which excess weight gain leads to “selective” leptin resistance, however, are still unclear.
Figure 1.
Schematic representation of the cardiovascular and metabolic effects of the leptin-MC4R axis in obesity. SNS indicates sympathetic nervous system; Na indicates sodium; POMC indicates proopiomelanocortin; MC4R indicates melanocortin 4 receptor; CNS indicates central nervous system.
Site specific activation of LRs and/or differential activation of intracellular signaling events may contribute to selective leptin resistance in obesity (Figure 1)
LRs have a wide distribution throughout the brain and most previous studies aimed at identifying the specific sites responsible for the effects of leptin on appetite regulation, using genetic approaches, have almost invariably failed to recapitulate the obesity observed in animals with whole-brain deletion of LRs. Vong and Colleagues [22*], however, recently showed that deleting LRs in gabaergic neurons recapitulates most of the obese phenotype observed in leptin deficiency including severe early onset obesity, hyperphagia, hyperglycemia and hyperinsulinemia; but since gabaergic neurons are numerous and widely distributed is still unclear which neuronal types or brain sites are most important in mediating the effects of leptin on appetite and body weight homeostasis. In addition, whether the obesity observed in mice with LRs deleted in gabaergic neurons is accompanied by elevations in BP is unknown.
The hypothalamus and the brainstem have received intense attention as potential sites mediating the actions of leptin on SNS activity and BP regulation. For instance, deletion of LRs in the arcuate nucleus of the hypothalamus markedly reduced the acute effects of leptin to increase renal SNS activity and attenuated the rise in BP induced by high fat feeing [23]. It has been hypothesized that each of the 3 main intracellular signaling pathways stimulated by LR activation may exert differential control of leptin's many physiological actions. LR activation triggers Janus tyrosine kinase 2 (Jak 2) leading to phosphorylation of specific tyrosine residues along the C-terminal domain of the LR that activates 3 distinct signaling events[11]: (1) latent signal transducers and activators of transcription 3 (STAT3) which regulates transcription of leptin target genes; (2) insulin receptor substrate 2 (IRS2) which activates phosphatidylinositol 3-kinase (PI3K); and (3) Src homology protein 2 (SHP2) that activates mitogen activates protein kinase (MAPK). Although CNS deletion of each of these signaling pathways results in varying degrees of obesity, neuron-specific deletion of the STAT3 pathway appears to be the only one capable of mimicking the obesity and hyperphagia found in leptin deficient animals[24]. Deletion of SHP2 or IRS2 pathways in the entire brain or in forebrain neurons causes only mild obesity and modest hyperphagia and may be more important in mediating other metabolic actions of leptin such as increased energy expenditure and improved glucose regulation [11]. However, the individual contribution of STAT3, IRS2 and SHP2 pathways to the effects of leptin on SNS activity and BP regulation have not been clearly defined and only acute pharmacological studies have been reported.
The CNS Melanocortin System Mediates Leptin Effects on SNS Activity and BP
Although the precise intracellular events and brain sites by which leptin regulates energy balance and BP are not fully understood there is strong evidence that leptin requires activation of the brain melanocortin system to exert most of its effects on RSNA and BP. Mice with MC4R deficiency are hyperphagic and obese, and exhibit most characteristics of metabolic syndrome that are also observed in leptin deficiency such as visceral adiposity, hyperinsulinemia and insulin resistance, but these mice are not hypertensive despite marked hyperleptinemia [25]; deletion of LRs in POMC neurons, which are particularly abundant in the arcuate nucleus of the hypothalamus, also completely abolished the chronic effects of leptin to raise BP [26*]; and mutations in the POMC or MC4R genes also lead to severe early-onset obesity and pronounce dysregulation of appetite in humans[27**]. However, it appears that other factors besides leptin contribute to body weight regulation by activation of POMC neurons as genetic deletion of LR in POMC neurons causes only mild obesity and does not completely abrogate the chronic anorexic effects of leptin [26], suggesting that POMC neurons may play a key role in leptin's ability to selectively regulate RSNA and BP independent of its anorexic effects. However, deletion of MC4R does markedly attenuate the effects of leptin to reduce appetite in mice [28].
MC4R are not only important for food intake and body weight regulation but may be a key component linking obesity and hyperleptinemia with increased SNS activity and hypertension. Obese MC4R deficient mice are normotensive when compared to lean wild-type controls and they are resistant to the pressor effects of chronic leptin administration [28], and activation of MC4R using synthetic agonists increases RSNA activity and BP [11]. The importance of the brain melanocortin system, particularly the MC4R, in contributing to obesity-induced elevations in RSNA and BP is also supported by studies in humans with MC4R mutations. These subjects have reduced 24-hour norepinephrine excretion, reduced diastolic and systolic BPs, and lower prevalence of hypertension than obese individuals with normal MC4R function [29**]. Also, pharmacological activation of MC4R in humans markedly elevates BP, similar to the responses observed in rodents [29**, 11]. Therefore, in humans as well as in rodents, chronic MC4R stimulation raises BP and the presence of a functional POMC-MC4R pathway appears to be necessary for obesity and hyperleptinemia to increase SNS activity and BP.
In addition to mediating the effects of leptin, activation of MC4R may exert a more fundamental role in modulating SNS activity in response to other stimuli. For example, pharmacological antagonism of MC4R markedly reduced BP in spontaneous hypertensive rats (SHR) (an experimental model of hypertension with increased SNS activity) to the same extent achieved by adrenergic receptor blockade [30]. The effects of various other peptides that induce SNS activation and elevate BP are also prevented by MC4R antagonism [31, 32]. In addition, MC4R blockade caused greater BP reduction in obese compared to lean Zucker rats demonstrating a role for MC4R in regulating SNS activity and BP even in models that lack normal leptin actions[33*].
Factors in Obesity That May Exacerbate the Pressor Actions of the Leptin-MC4R Axis
Despite clear evidence that leptin and MC4R are important for excess weight gain to increase RSNA and BP, the rise in BP observed during chronic administration of leptin or MC4R agonists is modest. One factor that may offset part of the hypertensive effects of leptin-MC4R activation is the weight loss that often occurs when leptin or MC4R agonists are administered. Another potential explanation for the modest hypertensive effects of leptin and MC4R agonists in lean animals is that obesity may be associated with other factors that enhance the chronic pressor actions of the leptin-MC4R axis. Endothelial dysfunction, which often develops in obesity and may lead to reduced nitric oxide availability, markedly augments the impact of leptin or MC4R activation on BP. For example, administration of the nitric oxide synthase inhibitor, L-NAME markedly amplified the chronic hypertensive effects of leptin as well as of a MC4R agonist in rats [34, 35].
In a recent study, Hilzendeger et al. suggested that leptin may also interact with the brain RAAS to regulate SNS activity and BP [36]. Obesity is also associated with reduced levels of factors believed to act in the CNS to reduce SNS and blunt the pressor effects of leptin and MC4R activation including ghrelin and adiponectin [37]. However, the role of hypoghrelinemia, hypoadiponectemia and increased angiotensin II levels in obesity among other factors in contributing to enhanced cardiovascular responses to leptin or MC4R activation, has to our knowledge, not been examined and remains unclear.
CONCLUSIONS
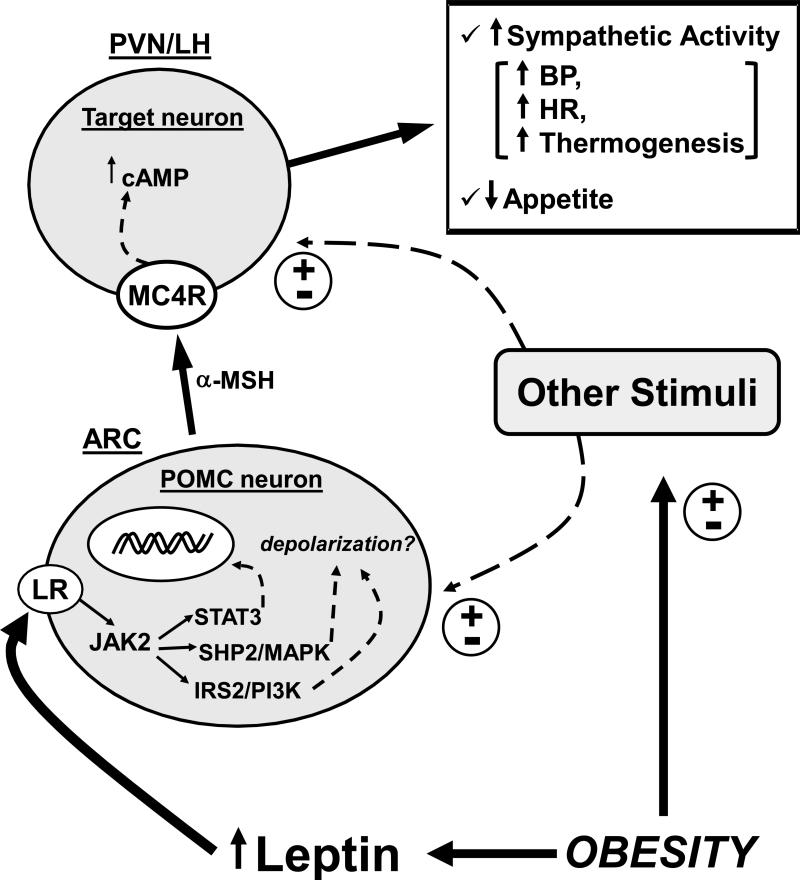
Obesity is a major cause of hypertension and cardiovascular diseases worldwide. Excess weight gain is associated with SNS activation that contributes to renal sodium retention and impaired pressure natriuresis. Increased circulating leptin levels and activation of the brain melanocortin system, in particular MC4R, are key factors in linking obesity, increased RSNA and elevated BP (Figure 2). Recent studies suggest that the leptin-MC4R axis exert differential control of appetite, metabolic and cardiovascular functions. Unraveling the site specific actions of LR and MC4R activation and the contributions of the different intracellular signaling pathways activated by the LR in controlling appetite, metabolic functions and SNS activity is critical for the development of better anti-obesity drugs with minimal cardiovascular and renal side-effects.
Figure 2.
Schematic representation of the interaction between obesity, leptin and the brain melanocortin system. LR indicates leptin receptor; MC4R indicates melanocortin 4 receptor; POMC indicates proopiomelanocortin protein; α-MSH indicates α-melanocyte stimulating hormone; cAMP indicates cyclic adenosine monophosphate; BP indicates blood pressure; HR indicates heart rate; PVN indicates paraventricular nucleus of the hypothalamus; LH indicates lateral hypothalamus; ARC indicates arcuate nucleus of the hypothalamus; (±) indicates stimulation or attenuation. Note: although this schematic representation highlights the importance of POMC neurons locates in the ARC and projecting to MC4R containing neurons in the PVN and lateral hypothalamic area, MC4R are expressed in many other important nuclei in the hypothalamus and other forebrain regions as well as in the brainstem where POMC neurons have also been found. The role of brainstem POMC neurons as well as of MC4R containing neurons in this region in regulating appetite and cardiovascular function, however, are still unclear.
KEY POINTS.
Excess weight gain is a major preventable cause of human essential hypertension;
Renal sympathetic overactivity contributes to sodium retention and elevated BP in obesity;
Hyperleptinemia and activation of the brain melanocortin system (MC4R) play a key role in linking obesity, sympathetic activation and elevated BP in obesity;
ACKNOWLEDGMENT
The authors’ research was supported by a grant from the National Heart, Lung and Blood Institute (P01 HL 51971) and by American Heart Association Scientist Development Grants to Alexandre A. da Silva and Jussara M. do Carmo.
REFERENCE LIST
- 1.Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006;12:62–6. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- 2**.Department of Health and Human Services – Center for disease Control and Prevention Obesity and overweight trends in the U.S. 2011 Available at: http://www.cdc.gov/nccdphp/dnpa/obesity/trend/index.htm. [This website maintained by the Department of Health and Human Services contains resourceful and updated information on obesity and overweight trends in U.S. adults and children, and also provides many other statistics related to the prevalence of obesity in the United States.]
- 3.Anderson SE, Whitaker RC. Prevalence of obesity among US preschool children in different racial and ethnic groups. Arch Pediatr Adolesc Med. 2009;163:344–8. doi: 10.1001/archpediatrics.2009.18. [DOI] [PubMed] [Google Scholar]
- 4.Wilson PWF, D'Agostino RB, Sullivan L, et al. Overweight and obesity as determinants of cardiovascular risk - the Framingham experience. Arch Intern Med. 2002;162:1867–72. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 5.Hall JE, Crook ED, Jones DW, et al. Mechanisms of obesity-associated cardiovascular and renal disease. Am J Med Sci. 2002;324:127–37. doi: 10.1097/00000441-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Davy KP, Hall JE. Obesity and hypertension: two epidemics or one? Am J Physiol Regul Integr comp Physiol. 2004;286:R803–13. doi: 10.1152/ajpregu.00707.2003. [DOI] [PubMed] [Google Scholar]
- 7.Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- 8.Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 9.Guyton AC. The surprising kidney-fluid mechanism for pressure control: Its infinite gain! Hypertension. 1990;16:425–30. doi: 10.1161/01.hyp.16.6.725. [DOI] [PubMed] [Google Scholar]
- 10.Davy KP, Orr JS. Sympathetic nervous system behavior in human obesity. Neurosci Biobehav Rev. 2009;33:116–124. doi: 10.1016/j.neubiorev.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall JE, da Silva AA, do Carmo JM, et al. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285:17271–6. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Armitage JA, Burke SL, Prior LJ, et al. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension. 2012;60:163–71. doi: 10.1161/HYPERTENSIONAHA.111.190413. [This study highlights the rapidity with which weight gain promoted by high fat feeding raises renal sympathetic activity using chronically implanted electrodes and the measurements were performed in awake animals.] [DOI] [PubMed] [Google Scholar]
- 13.Wofford MR, Anderson DC, Brown CA, et al. Antihypertensive effect of alpha and beta adrenergic blockade in obese and lean hypertensive subjects. Am J Hypertens. 2001;14:694–8. doi: 10.1016/s0895-7061(01)01293-6. [DOI] [PubMed] [Google Scholar]
- 14.Straznicky NE, Eikelis N, Lambert EA, et al. Mediators of sympathetic activation in metabolic syndrome obesity. Curr Hypertens Rep. 2008;10:440–7. doi: 10.1007/s11906-008-0083-1. [DOI] [PubMed] [Google Scholar]
- 15.da Silva AA, do Carmo J, Dubinion J, et al. The role of the sympathetic nervous system in obesity-related hypertension. Curr Hypertens Rep. 2009;11:206–11. doi: 10.1007/s11906-009-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz MW, Woods SC, Porte D, Jr, et al. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 17.Farooqi S, O'Rahilly S. Genetics of obesity in humans. Endocr Rev. 2006;27:710–8. doi: 10.1210/er.2006-0040. [DOI] [PubMed] [Google Scholar]
- 18.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;10:3686–95. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 19.Kalil GZ, Haynes WG. Sympathetic nervous system in obesity-related hypertension: mechanisms and clinical implications. Hypertens Res. 2012;35:4–16. doi: 10.1038/hr.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlyle M, Jones OB, Kuo JJ, et al. Chronic cardiovascular and renal actions of leptin-role of adrenergic activity. Hypertension. 2002;39:496–501. doi: 10.1161/hy0202.104398. [DOI] [PubMed] [Google Scholar]
- 21**.Prior LJ, Eikelis N, Armitage JA, et al. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2010;55:862–8. doi: 10.1161/HYPERTENSIONAHA.109.141119. [This study shows, using chronically instrumented awake animals, that weight gain by high fat feeding induces greater sensitivity to leptin's action to elevate renal sympathetic activity when compared to control standard fed animals.] [DOI] [PubMed] [Google Scholar]
- 22*.Vong L, Ye C, Yang Z, et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–54. doi: 10.1016/j.neuron.2011.05.028. [This is one of the few studies where deletion of leptin receptors in a particular set of neurons has been able to mimic most of the obese phenotype observed in whole-body or whole brain deletion of leptin receptors.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harlan SM, Morgan DA, Agassandian K, et al. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res. 2011;108:808–12. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao Q, Wolfgang MJ, Neschen S. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci USA. 2004;101:4661–6. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tallam LS, Stec DE, Willis MA, et al. Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia, and hyperleptinemia. Hypertension. 2005;46:326–332. doi: 10.1161/01.HYP.0000175474.99326.bf. [DOI] [PubMed] [Google Scholar]
- 26*.do Carmo JM, da Silva AA, Cai Z, et al. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension. 2011;57:918–26. doi: 10.1161/HYPERTENSIONAHA.110.161349. [This study demonstrates the importance of leptin receptor activation in POMC neurons for leptin to elevate blood pressure and to improve glucose homeostasis, but not for leptin's ability to regulate appetite and body weight. Thus, supporting the concept that leptin's various physiologic actions can be differentially regulated.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinelli CE, Keogh JM, Greenfield JR, et al. Obesity due to melanocortin 4 receptor (MC4R) deficiency is associated with increased linear growth and final height, fasting hyperinsulinemia, and incompletely suppressed growth hormone secretion. J Clin Endocrinol Metab. 2011;96:E181–8. doi: 10.1210/jc.2010-1369. [DOI] [PubMed] [Google Scholar]
- 28.Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension. 2006;48:58–64. doi: 10.1161/01.HYP.0000227966.36744.d9. [DOI] [PubMed] [Google Scholar]
- 29**.Greenfield JR. Melanocortin signaling and the regulation of blood pressure in human obesity. J Neuroendocrinol. 2011;23:186–93. doi: 10.1111/j.1365-2826.2010.02088.x. [This study reviews the recent findings on blood pressure regulation in humans with melanocortin-4 receptor deficiency, and highlights the fact that obese humans who lack melanocortin-4 receptors have reduced diastolic and systolic blood pressures compared to obese controls.] [DOI] [PubMed] [Google Scholar]
- 30.da Silva AA, do Carmo JM, Kanyicska B, et al. Endogenous melanocortin system activity contributes to the elevated arterial pressure in spontaneously hypertensive rats. Hypertension. 2008;51:884–890. doi: 10.1161/HYPERTENSIONAHA.107.100636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yosten GL, Samson WK. Nesfatin-1 exerts cardiovascular actions in brain: possible interaction with the central melanocortin system. Am J Physiol Regul Integr Comp Physiol. 2009;297:R330–6. doi: 10.1152/ajpregu.90867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yosten GL, Pate AT, Samson WK. Neuronostatin acts in brain to biphasically increase mean arterial pressure through sympatho-activation followed by vasopressin secretion: the role of melanocortin receptors. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1194–9. doi: 10.1152/ajpregu.00849.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.do Carmo JM, da Silva AA, Rushing JS, Hall JE. Activation of the central melanocortin system contributes to the increased arterial pressure in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2012;302:R561–7. doi: 10.1152/ajpregu.00392.2011. [The findings from this study indicate that other factors activate the central melanocortin system even in the absence of functional leptin receptors, and highlights the importance of the melanocortin system, in special the MC4R, in contributing to the elevated blood pressure in obese Zucker rats.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuo JJ, Jones OB, Hall JE. Inhibition of NO synthesis enhances chronic cardiovascular and renal actions of leptin. Hypertension. 2001;37:670–6. doi: 10.1161/01.hyp.37.2.670. [DOI] [PubMed] [Google Scholar]
- 35.do Carmo JM, Bassi M, da Silva AA, Hall JE. Systemic but not central nervous system nitric oxide synthase inhibition exacerbates the hypertensive effects of chronic melanocortin-3/4 receptor activation. Hypertension. 2011;57:428–34. doi: 10.1161/HYPERTENSIONAHA.110.163931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hilzendeger AM, Morgan DA, Brooks L, et al. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2012;303:H197–206. doi: 10.1152/ajpheart.00974.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bassi M, do Carmo JM, Hall JE, da Silva AA. Chronic effects of centrally administered adiponectin on appetite, metabolism and blood pressure regulation in normotensive and hypertensive rats. Peptides. 2012;37:1–5. doi: 10.1016/j.peptides.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]