Abstract
Using a double immunofluorescence procedure, we report the discovery of a novel group of fibrous astrocytes that co-express epithelial sodium channel (ENaC) γ-subunit protein along with glial acidic fibrillary protein (GFAP). These cells are concentrated along the borders of the sensory circumventricular organs (CVOs), embedded in the white matter (e.g., optic nerve/chiasm, anterior commissure, corpus callosum, pyramidal tract) and are components of the pia mater. In the CVOs, a compact collection of ENaC γ-immunoreactive glial fibers form the lamina terminalis immediately rostral to the organum vasculosum of the lamina terminalis (OVLT). Astrocyte processes can be traced into the median preoptic nucleus – a region implicated in regulation of sodium homeostasis. In the subfornical organ (SFO), ENaC γ-GFAP astrocytes lie in its lateral border, but not in the ventromedial core. In the AP, a dense ENaC γ-GFAP glial fibers form the interface between the AP and nucleus tractus solitarius; this area is termed the subpostremal region. Antibodies against the ENaC α- or β-subunit proteins do not immunostain these regions. In contrast, the antibodies against the ENaC γ-subunit protein react weakly with neuronal cell bodies in the CVOs. Besides affecting glial-neural functions in the CVOs, the astrocytes found in the white matter may affect saltatory nerve conduction, serving as a sodium buffer. The ENaC γ-expressing astrocytes of the ventral medulla send processes into the raphe pallidus which intermingle with the serotoninergic (5-HT) neurons found in this region as well as with the other nearby 5-HT neurons distributed along ventral medullary surface.
Keywords: circumventricular organ, epithelial sodium channel, ENaC, glial acidic fibrillary protein, saltatory nerve conduction, ventral medullary surface
1. Introduction
Astrocytes play a critical role in brain function by providing a functional interface between neurons and capillaries (Gourine and Kasparov, 2011), and they modulate numerous brain systems via what has been termed ‘glial-neuronal vascular units’ (Kirischuk et al., 2012). These units regulate the ionic and metabolic conditions of the brain environment (Magistretti, 2006), and this is accomplished by the release of a host of chemicals from astrocytes which include ATP/adenosine, glutamate, D-serine, and others which are now catalogued as ‘gliotransmitters’ (Haydon and Carmignoto, 2006).
From a neural systems perspective, the most compelling data suggesting that gliotransmitters modulate CNS functions has been the demonstration that astrocytes found on the ventral surface of the medulla oblongata function as central chemoreceptors. Small changes in the partial pressure of CO2 or pH trigger the release ATP from these electrically nonexcitable cells which activates the local central chemorespiratory neurons of the ventral medulla, and in turn, these neurons induce changes in the central respiratory network that affects rate and volume of breathing (Gourine et al., 2010).
Astrocyte excitability is largely dependent on increases in internal cytosolic concentration of calcium, but often overlooked is the fact that increases in the cytosolic Na+ concentration – [Na+]i is also an important factor that affects the regulation of synaptic transmission (Kirischuk et al., 2012). While much of the research on astroglia has focused on Ca2+ metabolism, there is a substantial literature supporting the idea that sodium channels and its related transporters play an important function of astrocytes (Kirischuk et al., 2012). In fact, one of the early discoveries in this area demonstrated the Müller glial cells of the retina express epithelial sodium channels (ENaCs) (Brockway et al., 2002). Thus, these workers provided the first anatomical evidence that astrocytes express the ENaC α-subunit, and also found that the inward Na+ current recorded from these cells could be blocked by amiloride.
During a study of the patterns of c-Fos activation of ENaC-expressing neurons in the sensory circumventricular organs (CVOs) (Miller, 2013), we observed a unique group of astrocytes that lie in the border zones of the CVOs. These CVO areas were intensely immunostained by antibodies directed against the ENaC α-subunit antibody protein. In addition, the ENaC-expressing astrocytes of the pia mater were also strongly immunostained as well. The latter group of astrocytes sent fibrous processes into regions of the brainstem implicated in cardiovascular and respiratory functions. The present study describes the location of these ENaC γ-subunit expressing astrocytes and briefly discusses their potential role in Na+ functions that occur in the brain.
ENaCs (Scnn1) are amiloride-sensitive, non-voltage dependent sodium channels that conduct Na+ across the apical membrane of cells in salt-reabsorbing epithelia, such as in the distal nephron, airways, and distal colon. To date, the bulk of the research done on ENaCs has focused on the kidney and airways (Kashlan and Kleyman, 2012), but ENaCs are also present in the brain (Waldmann et al., 1995, Amin et al., 2005, Giraldez et al., 2007, Teruyama et al., 2012, Miller, 2013). ENaCs are expressed in astrocytes, ependymal cells of the choroid plexus, endothelial cells, and neurons in the brain (Amin et al., 2005), and due to this widespread expression, it is likely that these channels may affect a range of functions. Here, we describe a novel group of ENaC γ-subunit expressing astrocytes that have a highly specific distribution in the brain.
2. Experimental procedures
2.1 Animals and surgical procedure
The animal experiments described here were reviewed and approved by the Washington University School of Medicine Institutional Animal Care and Use Committee and followed NIH guidelines. Adult Sprague-Dawley rats (wt= 250–300 gm, male and female, Charles River Laboratories, Wilmington, MA, USA) were provided with free access to tap water and standard rat chow (Pico Lab rodent #20, containing 0.33% sodium; Lab-Diet, Richmond, IN). They were housed in a room with an automated lighting system: 12/12 hour light-dark schedule (lights on at 5:30 AM; lights off at 5:30 PM) and with a controlled temperature of 23° C.
Between 7–10 AM, the rats were anesthetized with 3.5 % chloral hydrate (1 ml/100gm body weight; intraperitoneal injection; Sigma, St. Louis, MO), and perfused through the heart with 0.9% saline, followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH=7.4). The brains were removed and stored in fixative for 3 days-2 weeks. The brains from these animals were used for immunohistochemistry. Sections were cut in the transverse or sagittal plane at 50 µm on a freezing microtome.
2.2. Immunohistochemisty
Free floating sections were processed by the indirect ABC immunofluorescence procedure given below. All solutions were made in a 5% donkey serum in a 0.1M sodium phosphate buffer (pH = 7.4) containing 0.01% sodium azide. The detergent Triton-X 100 was omitted to prevent the solubilization of ENaCs. Histochemical reactions were carried out on a rotary shaker at room temperature. The immunoreactive reaction products were colorized with diaminobenzidine (Sigma) or Cy2 or Cy3 streptavidin (Jackson ImmunoReserach, West Grove, PA).
2.3 Primary Antibodies
Antibodies to the rat ENaC-α subunit, ENaC- β subunit, and ENaC- γ subunit were purchased from StressMarq (Victoria, BC, Canada) and used to immunostain rat brain sections through the AP, SFO, and OVLT. These antibodies were originally made by Dr. Mark Knepper (NIH); they are highly specific as demonstrated in Western blots which show single bands between 75–82 kDa. These bands were blocked when the respective antibody was preadsorbed with the immunizing peptide (Masilamani et al., 1999, Hager et al., 2001). The same antibodies were used in our recent study on systemic sodium induced changes in the CVOs (Miller, 2013)
The ENaC α-subunit (Scnn1a) antibody (1;1000; SPC-403D) was produced against a synthetic peptide from the N-terminus of the ENaC α-subunit (amino acids 46–68, NP_113736; # 3560-2). Immunostaining of ENaC α- positive cell groups in the brainstem (viz., hypoglossal and dorsal motor nuclei) was blocked when this antibody was preadsorbed with the ENaC α-subunit peptide with the following sequence: LGKGDKREEQGLGPEPSAPRQPTC-COOH (500µg/ml; Thermo Fisher Scientific, Rockford, IL). The ENaC β-subunit antibody (1:250; SPC-404D) was produced against the C-terminal tail of rat ENaC β-subunit (amino acids 617–638; # 3755-2). Similarly, immunostaining of brainstem motor nucleus was blocked when this antibody was preadsorbed with the ENaC β-subunit peptide CNYDSLRLQPLDTMESDSEVEAI-COOH (500µg/ml; Thermo Fisher Scientific, Rockford, IL). The ENaC γ-subunit antibody (1:750; SPC-405D) was produced against the C-terminal tail of rat ENaC γ-subunit (AA629-650; # L550). Immunostaining in the subpostremal region of the NTS was blocked when this antibody was preadsorbed with the ENaC γ-subunit peptide CNTLRLDRAFSSQLTDTQLTNEL-COOH (500µg/ml; Thermo Fisher Scientific, Rockford, IL. No immunostaining was present in brainstem tissues when the primary antibodies had been omitted from this immunohistochemical staining procedure.
Three mouse monoclonal antibodies were used. One was made against glial fibrillary acidic protein monoclonal (1:1000; GFAP, MU020-UC; clone GA-5; Biogenex, Fremont CA, the second was directed against tryptophan hydroxylase – the enzyme involved in the synthesis of serotonin (1:4000; MAB 5278, Millipore), and the third was directed against NeuN which is a generic marker for neurons (1:500; MAB377; Millipore). The specificity of all three of these antibodies has been described in previous studies that are listed in the Journal of Comparative Neurology database: http://onlinelibrary.wiley.com/journal/10.1002/%28ISSN%291096-9861/homepage/jcn_antibody_database.htm.
2.4 Immunohistochemical Staining Procedures
DAB procedure
Free-floating sections were incubated overnight in rabbit antibodies that were made against either to ENaC α-subunit, ENaC β-subunit, or ENaC γ-subunit at concentrations described above. The sections were reacted at room temperature and gently agitated on a rotary shaker. All solutions were made up in 5% donkey serum in 0.1 M sodium phosphate (pH = 7.4, washed in potassium phosphate buffered saline (KPBS; 0.01 M, pH = 7.4). The sections were transferred to a biotinylated donkey anti-rabbit (1:250; Jackson ImmunoResearch, West Grove, PA) solution for 2.5 h, washed in KPBS, treated for 1 h in the avidin–biotin complex (ABC, Vectastain kit, Vector Labs, Burlingame, CA) solution for 2 h, washed in KPBS, and colorized in diaminobenzidine (DAB) solution (D-4418, Sigma, St. Louis). A single DAB tablet was dissolved in 20–25 ml of distilled water containing one tablet of urea. The sections were reacted for 15 min in this solution, washed three times in KPBS, mounted on gelatinized slides, and air dried. The sections were dehydrated and coverslipped directly without any counterstaining. Control sections were also reacted without primary antibodies and this resulted in no staining in the brain tissues.
Double indirect immunofluorescence procedure
Additional sections were immunostained by a double indirect immunofluorescence procedure. Free-floating sections were incubated in the ENaC γ-subunit antibody overnight, washed in KPBS, transferred to a solution of biotinylated donkey anti-rabbit (1:250; Jackson) for 2.5 h, washed in KPBS, transferred to the ABC complex (Vectastain kit, Vector Labs, Burlingame, CA) for 2 h, washed in KPBS, and transferred to Cy3-streptavidin (1:250; Jackson) for 3 h. The sections were transferred to a solution of mouse monoclonal antibodies against either GFAP, tryptophan hydroxylase, or NeuN (see above) overnight. The following morning, the sections were washed in KBPS buffer and transferred to a solution of biotinylated donkey anti-mouse (1:250; Jackson) for 2.5 h, washed in KPBS, transferred to the ABC complex (Vectastain kit, Vector Labs, Burlingame, CA) for 2 h, washed in KPBS, and transferred to Cy2-streptavidin (1:250; Jackson) for 3 h. The sections were washed in buffer, and then mounted on gelatin-coated glass slides. After drying, slides were coverslipped using a fade-retardant glycerol mounting solution containing sodium azide and n-propyl gallate, and secured around the edges with fingernail polish.
2.5 Digital images
Brightfield images were taken on a Nikon microscope using a CCD camera with Nikon ACT-1 software (v2.62). Image cropping, resizing and adjustments in brightness, contrast, sharpness, and color balance were performed using Adobe Photoshop CS3 (San Jose, CA).
Confocal immunofluorescence images were obtained with an Olympus Fluoview FV500b laser-scanning microscope using either 20× (NA 1.17) or 40× (NA 1.35) oil objective lens in steps of 0.621 or 0.311 µM, respectively, through the tissue section. Image resolution was 1024×1024 pixels. One pixel in the X-Y plane was the minimum unit of resolution which covered an area roughly 0.6 µM ×0.6 µM. The z-frames were collapsed to a two-dimensional image. Photomontages were constructed, and adjustments in brightness and contrast were made using the Adobe Photoshop program. Manipulations of the confocal stacks, z-frame projections, and pseudocoloration were performed using MetaMorph software (Molecular Devices, Sunnyvale, CA). Cytoarchitectonic boundaries were added to the photoimages with the aid of the Adobe Illustrator program.
3. Results
3.1 General description of the ENaC-immunoreactive neurons and astrocytes in the circumventricular organs (CVOs), white matter, and pia mater
ENaC-expressing neurons are present in the sensory CVOs (Amin et al., 2005, Miller, 2013). As shown by our laboratory, the location of the ENaC α-immunoreactive neurons closely correlates with data gathered from in situ hybridization studies (Miller, 2013). The immunostaining pattern obtained with the ENaC α-subunit antibody was similar to the results found for the anti-sera that was generated against ENaC β-subunit. In contrast, the ENaC γ-immunostaining pattern was different, yielding very weak neuronal staining in the three sensory CVOs: AP, SFO, and OVLT. An example of the weak ENaC- γ immunoreactive neurons is presented in Figure 1. Unlike the immunostaining resulting from the antibodies directed against the ENaC α- and β-subunits, the ENaC γ-subunit resulted in robust staining of the astrocytes that border these three CVOs (Figs. 1–5). In addition, ENaC γ-immunoreactive astrocytes were also prominent in white matter, such as the optic chiasm (Figs. 1 and 2) and pyramidal tract (Fig.6) as well as in the pia mater (Figs. 1, 2, 4, 5, 6). The pia mater was also immunostained with antibodies directed against ENaC α-and β-subunits. ENaC γ-subunit immunoreactivity was colocalized in GFAP pial fibers (Figs. 2A and 5A). In addition, all three antibodies resulted in immunostaining of the ependymal lining of the brain, but, as expected, no GFAP immunoreactivity was found in this tissue.
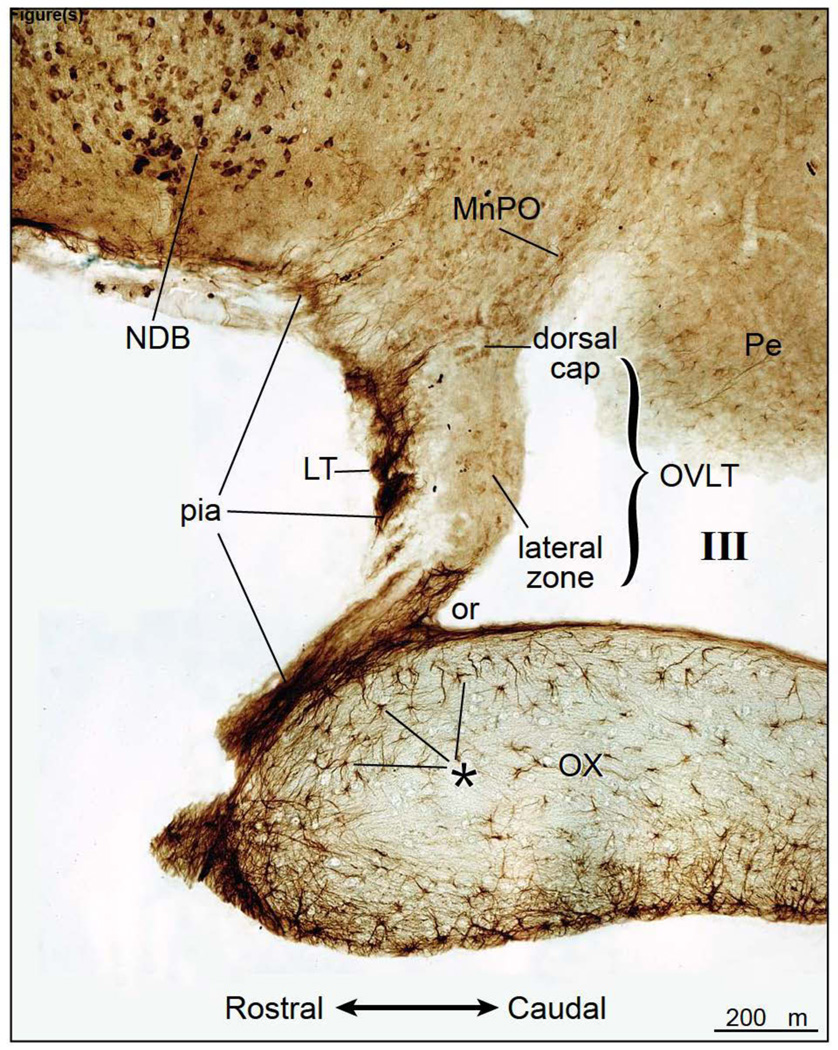
Figure 1.
Distribution of ENaC γ-subunit immunoreactivity as demonstrated in a parasagittal section of the rat forebrain. ENaC γ-subunit immunoreactivity was present in the lamina terminalis (LT), pia mater, and strongly expressed in the neurons of the nucleus of the diagonal band (NDB). Weak ENaC γ-expression was observed in the neurons of the dorsal cap and lateral zone of the organum vasculosum of the lamina terminalis (OVLT), median preoptic nucleus (MnPO), and periventricular hypothalamic region (Pe). In the optic chiasm (OX), very strong ENaC γ-expression was identified in astrocytes that lie in the border of this fiber bundle; this region is marked by asterisks (*). Note the absence of ENaC γ-subunit immunoreactive staining in the astrocytes in the brain proper. The ENaC γ-subunit expressing astrocytes are distributed in select brain regions, including in the zone between the NDB and LT. III, third ventricle; or, optic recess.
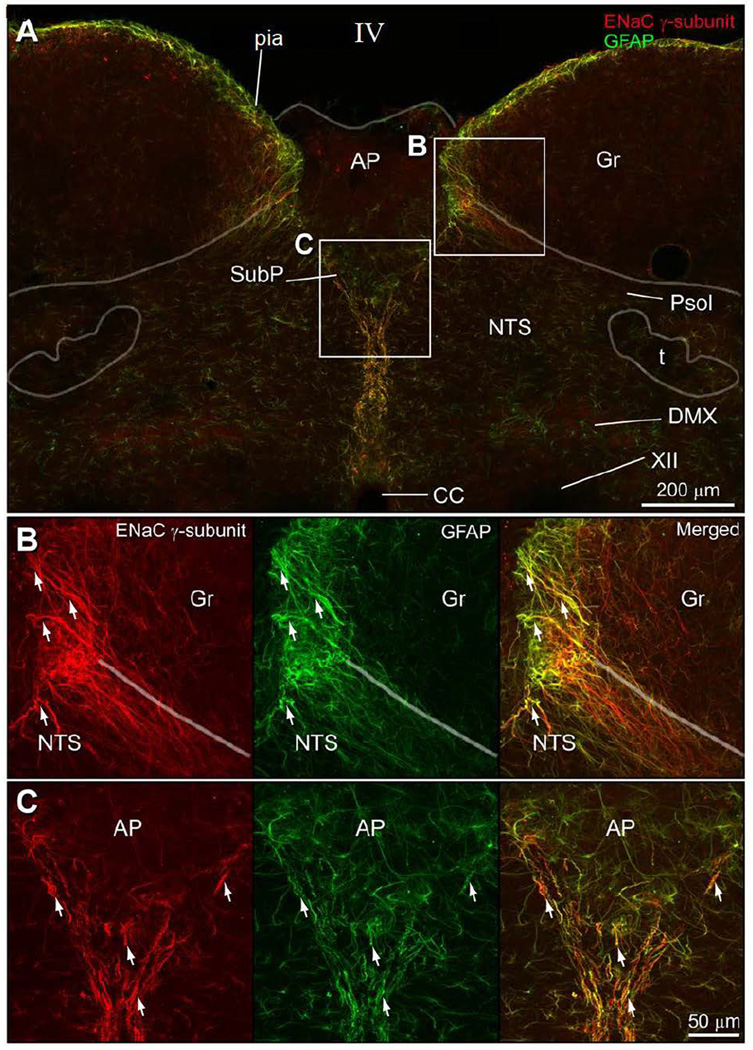
Figure 5.
Double immunofluorescence preparation to show that the pia mater is made up of glial fibers that co-contain ENaC γ-subunit and GFAP immunoreactivity. A. The ENaC γ-pia membrane surrounds the surface of the dorsal medulla, but it does not cover the dorsal surface of the area postrema (AP). One branch curves underneath the white matter associated with the gracile nucleus (Gr) and another branch travels ventral to nucleus tractus solitarius (NTS), ultimately defining the subpostremal NTS region (SubP). This plexus can be followed ventrally as midline raphe, dividing the NTS, and eventually surrounding the central canal. B. Dorsomedial part of the NTS co-contains immunoreactive ENaC γ-subunit and GFAP processes. C. The area ventral to the AP has glial processes that co-express ENaC γ-subunit and GFAP.
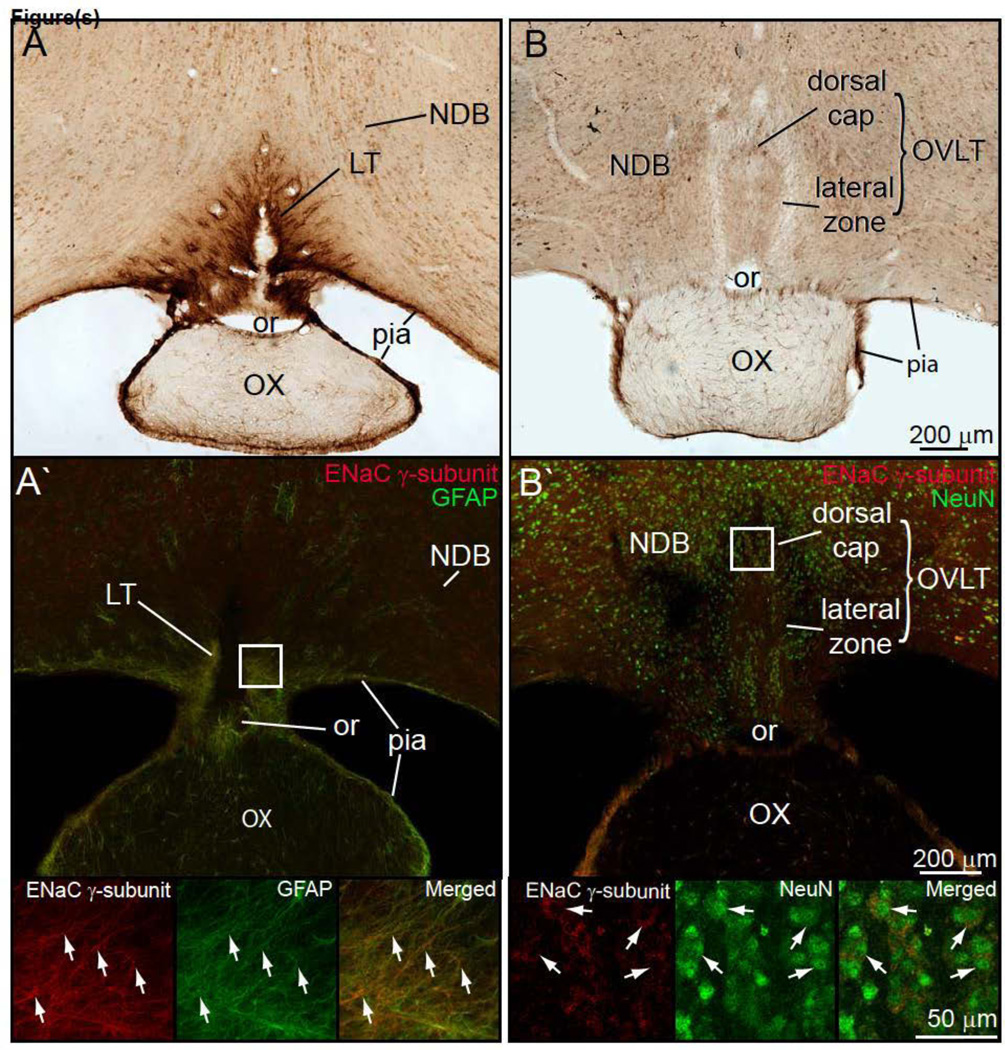
Figure 2.
A. Brightfield preparation of a transverse section through the forebrain showing dense ENaC γ-subunit expressing astrocyte plexus that forms the lamina terminalis (LT). This plexus is continuous with the pia mater that invests the ventral brain surface and surrounds the optic chiasm (OX). The ENaC γ-positive glial fibers outline the optic recess (or) which lies in the most rostral part of the third ventricle. A' Double immunofluorescence preparation to demonstrate co-expression of glial fibrillary acidic protein (GFAP) and ENaC γ-subunit protein is seen in the pia. Lower part of this figure shows enlarged photoimages to show overlap ENaC γ-subunit protein and GFAP. B. ENaC γ-subunit expressing neurons were present in the dorsal cap and lateral zone of the OVLT. Dorsal cap OVLT neurons were more ENaC γ-subunit immunoreactive than the lateral zone OVLT neurons. B'. Double-immunostained preparation to show the ENaC γ-subunit immunoreactive in the lateral zone OVLT neurons also express NeuN – a neuronal nuclear marker. Similar results were found for the dorsal cap OVLT neurons.
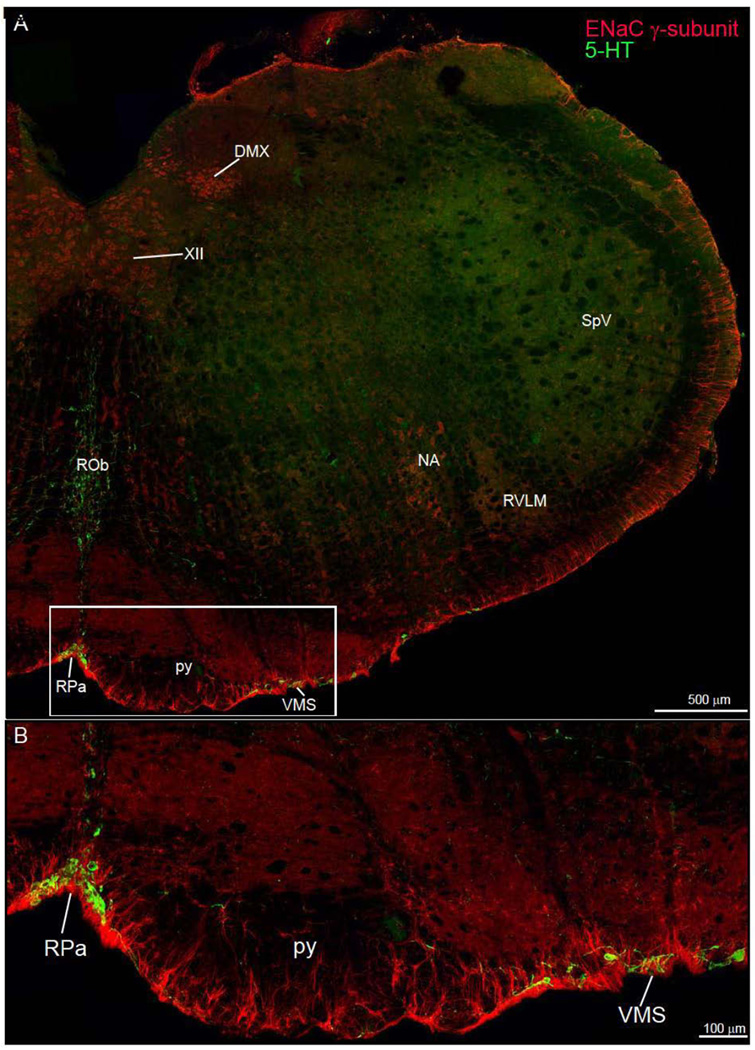
Figure 6.
Low power photoimage of the medulla oblongata. This double immunofluorescence preparation shows the distribution of ENaC γ-subunit and 5-HT immunoreactivity. A. The pia matter strong expresses the ENaC γ-subunit protein. The insert (rectangle) shows ENaC γ-expressing astrocytes of the ventral medulla intermingle with the 5-HT neurons of the raphe pallidus (RPa) and ventral medullary surface (VMS). B. Higher power photoimage of the ventral medullary surface showing the overlap of ENaC γ-immunoreactive astrocytes and 5-HT neurons. Other abbreviations: NA, nucleus ambiguus; py, pyramidal tract; ROb, raphe obscurus nucleus; RVLM, rostral ventrolateral medulla; SpV, spinal trigeminal nucleus.
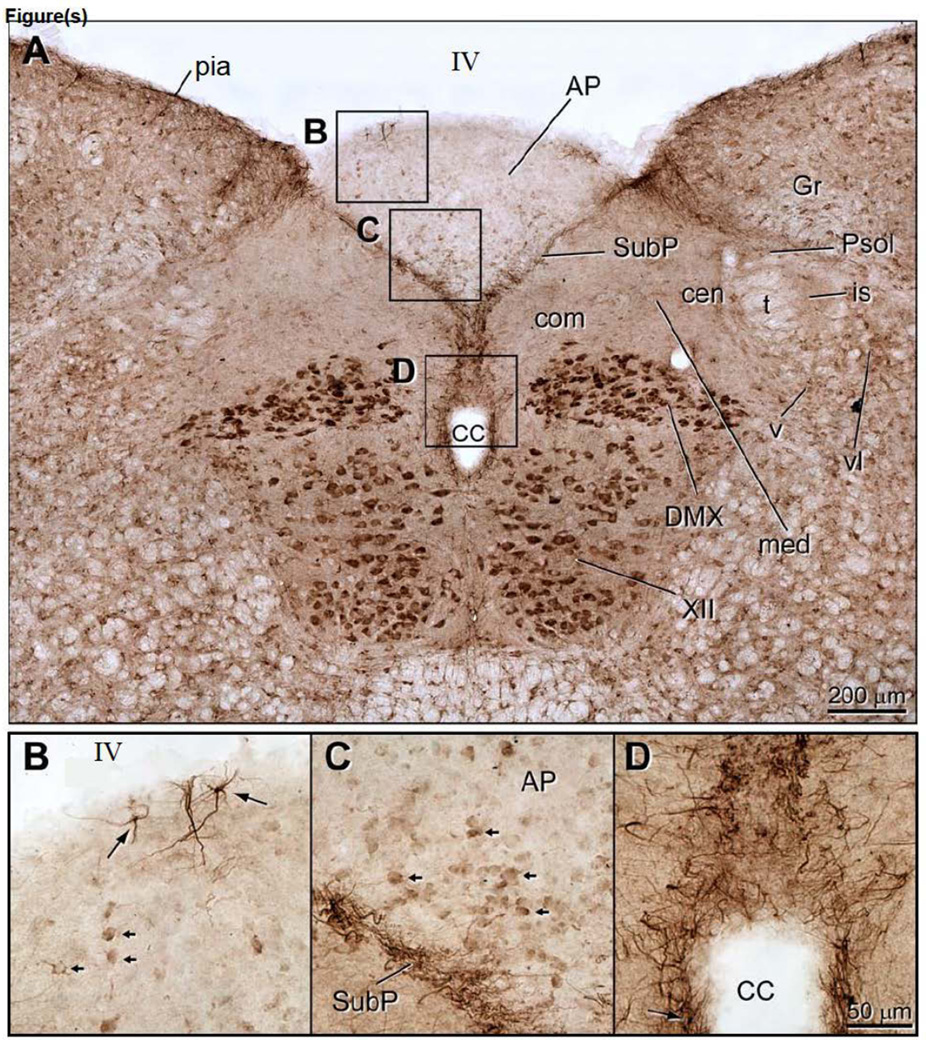
Figure 4.
Transverse section of the dorsomedial medulla to show the distribution of ENaC γ-immunoreactivity in neurons and astrocytes. A. The most intense ENaC γ immunostaining was seen in the neurons in the dorsal motor vagal (DMX) and hypoglossal (XII) nuclei. In addition, the subpostrema region (SubP), which lies along the ventral border of the area postrema (AP), was intensely stained as well. The SubP forms the dorsomedial limit of the nucleus tractus solitarius (NTS), and continues ventrally to surround the central canal (Dallaporta et al.). This glial plexus is also continuous with the pia mater that surrounds the brainstem, except at the dorsal surface of the AP. B. ENaC γ-expressing astrocytes in the dorsal AP that send their processes towards the IVth ventricular surface (arrows). Note the small arrows that indicate weakly ENaC γ-expressing AP neurons. C. ENaC γ-immunoreactive AP neurons that a clustered along the SubP border (small arrows). Very few ENaC γ-expressing glial processes extend into the AP proper. D. ENaC γ-immunoreactive glial fibers form a dense plexus in midline that surrounds the central canal. Note that these glial cells send processes into ependymal cell layer. Other abbreviations: IV, fourth ventricle; cen, central nucleus of the NTS; com, commissural nucleus; Gr, gracile nucleus; is, interstitial subnucleus of the NTS; med, medial subnucleus of the NTS; Psol, parasolitary nucleus; t, solitary tract; v, ventral subnucleus of the NTS; vl, ventrolateral subnucleus of the NTS.
3.2 ENaC γ-subunit immunoreactive astroglia associated with the circumventricular organs
Fig. 1 presents a parasagittal section through the ventral forebrain which includes optic chiasm and OVLT region. Intense ENaC γ-subunit immunoreactivity was found in the lamina terminalis, and was continuous with the pia mater which wraps around the optic chiasm. This plexus could be traced along the ventral surface of the brain and was present in the region immediately ventral to the nucleus of the diagonal band (Fig. 1). Note there were a few sites where the ENaC γ-immunoreactive pial plexus appeared to be discontinuous, presumably an artifact due to the histological processing. This dense glial plexus enwrapped the outer surface of the optic chiasm, and was continuous with the dorsal surface of the optic chiasm. Some ENaC γ-immunoreactive glial fibers penetrate the brain dorsal to the OVLT, where they can be traced into the median preoptic nucleus. Almost none of these fibers penetrate the OVLT or other nearby brain sites. In addition, individual astrocytes that were immunostained by the ENaC γ-antibody were clearly demonstrated in the optic chasm; these astrocytes were not immunostained by the ENaC α- and β-antibodies (data not shown).
Fig. 2A shows a photoimage of the transverse section of the ventral forebrain. This shows the ENaC γ-subunit immunostained glial fibers in the lamina terminalis and their continuation into the pia mater of the base of the brain. These fibers continue ventrally to encompass the optic chiasm. Fig. 2A' is a double-color immunofluorescence preparation to document that the ENaC γ-subunit immunostained glial fibers also contain GFAP immunoreactivity. Fig. 2B shows ENaC γ-subunit immunoreactive neurons in the dorsal cap and lateral zone of the OVLT. Fig. 2B' is a double-color immunofluorescence preparation that shows ENaC γ-neurons in dorsal cap of the OVLT; these cells co-contain ENaC γ-subunit and NeuN immunoreactivity, and thus, are neurons.
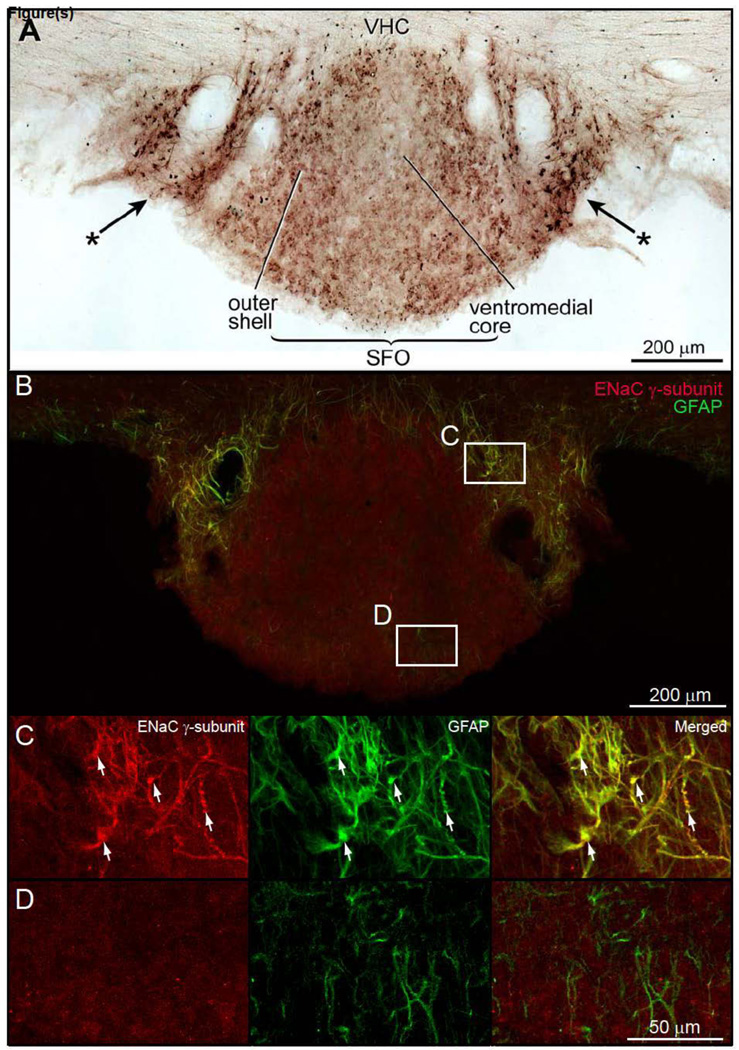
ENaC γ- immunoreactive neurons were seen in the ventromedial core and outer shell of the SFO (Fig. 3A). A collection of darkly stained glial cells were present in the lateral border of the SFO (Fig. 3A). These astrocytes surround the paired blood vessels that lie in the peripheral edge of the SFO (Fig. 3A). Double-immunofluorescence preparations were used to show the ENaC γ-subunit was co-contained in GFAP astrocytes (Figs. 3B and 3C). Note that in the core of the SFO, only astrocytes that express GFAP were found (Figs. 3B and 3D).
Figure 3.
Brightfield preparation of a transverse section through the subfornical organ (SFO) to demonstrate ENaC γ-immunoreactive neurons and astrocytes. A. Very weak ENaC γ-expressing neurons were seen in both the ventromedial core and outer shell of the SFO. Intensely immunostained ENaC γ-positive astroglia were present on the lateral borders of the SFO (*→). B. Double immunofluorescence preparation to show ENaC γ-expressing astrocytes in the lateral border of the SFO. The expression of ENaC γ-subunit in the ventromedial core and outer shell of the SFO is much lower than the astrocytes in the border zone. In this particular preparation, ENaC γ-subunit immunoreactivity in neurons was below detectable levels. Other preparations (data not shown) show ENaC γ-subunit and NeuN co-localization, indicating that SFO neurons express low levels of ENaC γ-subunit. C. In the SFO border zone, astrocytes co-express ENaC γ-subunit protein and GFAP. D. GFAP immunoreactive astrocytes in the core of the SFO do not co-contain ENaC γ-subunit immunoreactivity.
ENaC γ-subunit immunoreactive staining of the dorsal medulla is shown in Figure 4. Robust cell body immunostaing was present in the dorsal vagal and hypoglossal nuclei (Fig. 4A), which was similar to the immunostaining patterns seen with ENaC α-subunit antibody (Miller, 2013). In addition, immunostaining with the ENaC β-subunit antibody was similar to that obtained with ENaC α-subunit antibody (data not presented).
The principal difference was the AP was very weakly immunostained with the ENaC γ-antibody as compared to the results of the other two antibodies (data not presented). On close inspection, weakly immunostained AP neurons are clearly evident (small arrows in Figs 4 B&C). Also note that strong ENaC γ-subunit immunoreactivity was seen in the pia mater, and was absent over the ventricular surface of the AP. The pial fibers could be traced into the medulla where they arched around the medial part of the gracile nucleus, and then, split into two bundles. One bundle outlined the dorsal edge of the NTS, and the other continued into the subpostremal NTS area (Fig. 3A). This glial plexus could be followed ventrally in the midline and separated the commissural NTS nuclei, ultimately encompassing the central canal (Figs 3A and 3D). Double immunofluorescence preparations revealed the same organization, and as shown in Fig. 5A the GFAP immunoreactive fibers were distributed in the same manner. These fibers also co-expressed ENaC γ-subunit immunoreactivity (Figs. 5B & C).
4. Discussion
This study demonstrates a unique type of astrocyte which co-expresses ENaC γ-subunit and GFAP. These cells were found in three brain sites: 1) border zones of the sensory CVOs which include the AP, SFO, and OVLT, 2) white matter (e.g., optic chiasm, anterior commissure, corpus callosum, pyramidal tract, and lateral funciulus), and 3) pia mater. Ependymal cells, in contrast, express all three types of ENaC subunits: α, β, and γ, which is in agreement with earlier findings (Amin et al., 2005).
ENaCs are made up of three protein subunits: α, β, and γ (Loffing and Schild, 2005), and a fourth protein, the δ-subunit, that has an amino acid sequence similar to the α-subunit, has not yet been identified in the rat but is present in humans (Ji et al., 2012). Unlike the GFAP astrocytes that form the pia mater which contain all three subunits, the astrocytes lie in the border zones of the CVOs only express the ENaC γ-subunit. The antibodies directed against the α- and β- subunit may not have been sensitive enough to reveal the presence of these proteins, but we do not think this is case since we examined in situ hybridization preparations designed to identify the ENaC β- and γ-subunits mRNA in the AP, SFO, and OVLT and did not find labeled cells in these border zones of the respective CVOs (unpublished data).
The presence of only the ENaC γ-subunit protein in the astrocytes of localized in the CVO border zone is quite unusual. Whether the ENaC γ-subunits form homogeneous channels or combine with other proteins, such as other members of the ENaC/degenerin family is not known. Also, the exact localization of these proteins needs further study, especially to establish they that are present on the surface of the cell membrane of the astrocytes. Voltage-gated sodium channels are present in some types of astrocytes (Sontheimer et al., 1992), and now, it would be of considerable interest to learn whether these cells also express ENaCs in order to develop a more complete picture of the electrophysiological properties of glial cells.
It is conceivable that the ENaC γ-expressing astrocytes we have described here, each subserve different functions. For example, the astrocytes associated with the circumventricular organs may function in a special capacity as a sodium sensor of the cerebrospinal fluid. The astrocytes found the in white matter, probably are involved in sodium functions related to saltatory nerve conduction where they contribute to the ionic homeostasis found at nodes of Ranvier (Waxman and Black, 1984). The ENaC γ-expressing astrocytes associated with the 5-HT neurons of the ventral medulla are likely to be involved in respiratory functions.
The sensory CVOs are made up of a heterogeneous group of cells, which include neurons, astrocytes, tanyocytes, microglia, and endothelial cells. Large number of different channels and receptors are present in the CVOs as revealed by microarray analyses (Hindmarch et al., 2008, Hindmarch et al., 2011). These studies, however, need further refinement and thus, it will be important in the future to perform single-cell analyses of each of channels, exchangers, receptors, and transporters found at these sites.
It has been reported that astrocytes in the SFO and OVLT express Nax channels (Scn7a) and function as sodium sensors (Watanabe et al., 2006), but more recent studies have failed to confirm this finding (Nehme et al., 2012). Nax channels are, however, present in the ependymal cells and neurons of the SFO and OVLT, and not in the AP (Nehme et al., 2012). Whether Nax channels are present in the ENaC γ-expressing GFAP astrocytes has not yet been explored.
GFAP astrocytes localized in the pia mater form a distinctive outline of the medulla oblongata that can be traced into the subpostremal region, thus outlining the ventral part of the AP (Figs 4 & 5). This region has been noted in previous studies (Wang et al., 2006, Willis et al., 2007, Maolood and Meister, 2009). Here we found these glial fibers also co-express ENaC γ-subunit, but earlier work established this region contains other proteins. For example, the imidazonine receptor protein was localized in AP, SFO and OVLT (Ruggiero et al., 1998). Leptin receptors have are densely concentrated in the GFAP astrocytes that make up the subpostremal NTS and glial raphe regions (Dallaporta et al., 2009). Endothelin (ET-1/ET-3) specific antibodies intensely react with the outer shell of the SFO and the lamina terminalis which defines the rostralmost part of the OVLT; data regarding the AP were not presented (Gebke et al., 2000).
5. Conclusions
Astrocytes that co-express the ENaC γ-subunit and GFAP have been identified in the border zones of the sensory CVOs, along the ventral medulla, and within major fiber bundles of the brain. The functional roles these cells play in sodium homeostasis remain to be examined.
Research Highlights.
ENaC γ-subunit protein and GFAP are co-localized in select groups of astrocytes
ENaC γ-expressing astrocytes lie in the border zone of the circumventricular organs
ENaC γ-expressing astrocytes lie in the white matter
ENaC γ-expressing astrocytes are found in the pia mater
Acknowledgements
We thank Xay Van Nguyen and Michelle Wang for technical assistance, Marcy Hartstein for the computer graphics, and Dennis Oakley of the Bakewell Neuroimaging Laboratory at Washington University Medical School for aid with the confocal images. This study was supported by National Institute of Heart, Lung, and Blood of the NIH, Grant #: HL-25449 (ADL), Bakewell Imaging Center Fund, and National Institutes of Health, Grant #: NS057105, Neuroscience Blueprint Core Grant.
Abbreviations
- III
third ventricle
- IV
fourth ventricle
- XII
hypoglossal nucleus
- AP
area postrema
- CC
central canal
- com
commissural subnucleus of the NTS
- CVO
circumventricular organ
- DMX
dorsal motor nucleus of vagus nerve
- ENaC
epithelial sodium channel
- GFAP
glial fibrillary acidic protein
- Gr
gracile nucleus
- is
interstitial subnucleus of the NTS
- LT
lamina terminalis
- med
medial subnucleus of the NTS
- MnPO
median preoptic nucleus
- NDB
nucleus of the diagonal band
- NeuN
neuronal nuclear marker
- NTS
nucleus tractus solitarius
- or
optic recess
- NA
nucleus ambiguus
- OVLT
organum vasculosum of the lamina terminalis
- OX
optic chiasm
- Pe
periventricular hypothalamus
- Psol
parasolitary subnucleus of the NTS
- py
pyramidal tract
- ROb
raphe obscurus nucleus
- RPa
raphe pallidus nucleus
- RVLM
rostral ventrolateral medulla
- SFO
subfornical organ
- SpV
Spinal trigeminal nucleus
- SubP
subpostremal region of the NTS
- t
solitary tract
- v
ventral subnucleus of the NTS
- VHC
ventral hippocampal commissure
- vl
Ventrolateral subnucleus of the NTS
- VMS
ventral medullary surface
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts.
Ethics Statement
This work was carried out in accordance with NIH guidelines regarding animal experiments, and was approved by the Washington University School of Medicine Institutional Animal Care and Use Committee.
Literature Cited
- Amin MS, Wang HW, Reza E, Whitman SC, Tuana BS, Leenen FH. Distribution of epithelial sodium channels and mineralocorticoid receptors in cardiovascular regulatory centers in rat brain. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1787–R1797. doi: 10.1152/ajpregu.00063.2005. [DOI] [PubMed] [Google Scholar]
- Brockway LM, Zhou ZH, Bubien JK, Jovov B, Benos DJ, Keyser KT. Rabbit retinal neurons and glia express a variety of ENaC/DEG subunits. Am J Physiol Cell Physiol. 2002;283:C126–C134. doi: 10.1152/ajpcell.00457.2001. [DOI] [PubMed] [Google Scholar]
- Dallaporta M, Pecchi E, Pio J, Jean A, Horner KC, Troadec JD. Expression of leptin receptor by glial cells of the nucleus tractus solitarius: possible involvement in energy homeostasis. J Neuroendocrinol. 2009;21:57–67. doi: 10.1111/j.1365-2826.2008.01799.x. [DOI] [PubMed] [Google Scholar]
- Gebke E, Muller AR, Pehl U, Gerstberger R. Astrocytes in sensory circumventricular organs of the rat brain express functional binding sites for endothelin. Neuroscience. 2000;97:371–381. doi: 10.1016/s0306-4522(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Giraldez T, Afonso-Oramas D, Cruz-Muros I, Garcia-Marin V, Pagel P, Gonzalez-Hernandez T, Alvarez de la Rosa D. Cloning and functional expression of a new epithelial sodium channel delta subunit isoform differentially expressed in neurons of the human and monkey telencephalon. J Neurochem. 2007;102:1304–1315. doi: 10.1111/j.1471-4159.2007.04622.x. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasparov S. Astrocytes as brain interoceptors. Exp Physiol. 2011;96:411–416. doi: 10.1113/expphysiol.2010.053165. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager H, Kwon TH, Vinnikova AK, Masilamani S, Brooks HL, Frokiaer J, Knepper MA, Nielsen S. Immunocytochemical and immunoelectron microscopic localization of alpha-, beta-, and gamma-ENaC in rat kidney. Am J Physiol Renal Physiol. 2001;280:F1093–F1106. doi: 10.1152/ajprenal.2001.280.6.F1093. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Hindmarch C, Fry M, Yao ST, Smith PM, Murphy D, Ferguson AV. Microarray analysis of the transcriptome of the subfornical organ in the rat: regulation by fluid and food deprivation. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1914–R1920. doi: 10.1152/ajpregu.90560.2008. [DOI] [PubMed] [Google Scholar]
- Hindmarch CC, Fry M, Smith PM, Yao ST, Hazell GG, Lolait SJ, Paton JF, Ferguson AV, Murphy D. The transcriptome of the medullary area postrema: the thirsty rat, the hungry rat and the hypertensive rat. Exp Physiol. 2011;96:495–504. doi: 10.1113/expphysiol.2010.056515. [DOI] [PubMed] [Google Scholar]
- Ji HL, Zhao RZ, Chen ZX, Shetty S, Idell S, Matalon S. delta ENaC: a novel divergent amiloride-inhibitable sodium channel. Am J Physiol Lung Cell Mol Physiol. 2012;303:L1013–L1026. doi: 10.1152/ajplung.00206.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashlan OB, Kleyman TR. Epithelial Na(+) channel regulation by cytoplasmic and extracellular factors. Exp Cell Res. 2012;318:1011–1019. doi: 10.1016/j.yexcr.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirischuk S, Parpura V, Verkhratsky A. Sodium dynamics: another key to astroglial excitability? Trends Neurosci. 2012;35:497–506. doi: 10.1016/j.tins.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Loffing J, Schild L. Functional domains of the epithelial sodium channel. J Am Soc Nephrol. 2005;16:3175–3181. doi: 10.1681/ASN.2005050456. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- Maolood N, Meister B. Protein components of the blood-brain barrier (BBB) in the brainstem area postrema-nucleus tractus solitarius region. J Chem Neuroanat. 2009;37:182–195. doi: 10.1016/j.jchemneu.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest. 1999;104:R19–R23. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RL, Wang MH, Gray PA, Salkfoff LB, Loewy AD. ENaC-expressing neurons in the sensory circumventricular organs become c-Fos activated following systemic sodium changes. Am J Physiol Regul Integr Comp Physiol. 2013 doi: 10.1152/ajpregu.00242.2013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehme B, Henry M, Mouginot D, Drolet G. The Expression Pattern of the Na(+) Sensor, Na(X) in the Hydromineral Homeostatic Network: A Comparative Study between the Rat and Mouse. Front Neuroanat. 2012;6:26. doi: 10.3389/fnana.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero DA, Regunathan S, Wang H, Milner TA, Reis DJ. Immunocytochemical localization of an imidazoline receptor protein in the central nervous system. Brain Res. 1998;780:270–293. doi: 10.1016/s0006-8993(97)01203-1. [DOI] [PubMed] [Google Scholar]
- Sontheimer H, Black JA, Ransom BR, Waxman SG. Ion channels in spinal cord astrocytes in vitroITransient expression of high levels of Na+ and K+ channels. J Neurophysiol. 1992;68:985–1000. doi: 10.1152/jn.1992.68.4.985. [DOI] [PubMed] [Google Scholar]
- Teruyama R, Sakuraba M, Wilson LL, Wandrey NE, Armstrong WE. Epithelial Na(+) sodium channels in magnocellular cells of the rat supraoptic and paraventricular nuclei. Am J Physiol Endocrinol Metab. 2012;302:E273–E285. doi: 10.1152/ajpendo.00407.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Voilley N, Lazdunski M. Molecular cloning and functional expression of a novel amiloride-sensitive Na+ channel. J Biol Chem. 1995;270:27411–27414. doi: 10.1074/jbc.270.46.27411. [DOI] [PubMed] [Google Scholar]
- Wang W, Li C, Nejsum LN, Li H, Kim SW, Kwon TH, Jonassen TE, Knepper MA, Thomsen K, Frokiaer J, Nielsen S. Biphasic effects of ANP infusion in conscious, euvolumic rats: roles of AQP2 and ENaC trafficking. Am J Physiol Renal Physiol. 2006;290:F530–F541. doi: 10.1152/ajprenal.00070.2005. [DOI] [PubMed] [Google Scholar]
- Watanabe E, Hiyama TY, Shimizu H, Kodama R, Hayashi N, Miyata S, Yanagawa Y, Obata K, Noda M. Sodium-level-sensitive sodium channel Na(x) is expressed in glial laminate processes in the sensory circumventricular organs. Am J Physiol Regul Integr Comp Physiol. 2006;290:R568–R576. doi: 10.1152/ajpregu.00618.2005. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Black JA. Freeze-fracture ultrastructure of the perinodal astrocyte and associated glial junctions. Brain Res. 1984;308:77–87. doi: 10.1016/0006-8993(84)90919-3. [DOI] [PubMed] [Google Scholar]
- Willis CL, Garwood CJ, Ray DE. A size selective vascular barrier in the rat area postrema formed by perivascular macrophages and the extracellular matrix. Neuroscience. 2007;150:498–509. doi: 10.1016/j.neuroscience.2007.09.023. [DOI] [PubMed] [Google Scholar]