Abstract
Reactive species oxidatively modify numerous proteins including ion channels. Oxidative sensitivity of ion channels is often conferred by amino acids containing sulfur atoms, such as cysteine and methionine. Functional consequences of oxidative modification of methionine in hERG1 (human ether à go-go related gene 1), which encodes cardiac IKr channels, are unknown. Here we used chloramine-T (ChT), which preferentially oxidizes methionine, to examine the functional consequences of methionine oxidation of hERG channels stably expressed in a human embryonic kidney cell line (HEK 293) and native hERG channels in a human neuroblastoma cell line (SH-SY5Y). ChT (300 µM) significantly decreased whole-cell hERG current in both HEK 293 and SH-SY5Y cells. In HEK 293 cells, the effects of ChT on hERG current were time- and concentration-dependent, and were markedly attenuated in the presence of enzyme methionine sulfoxide reductase A that specifically repairs oxidized methionine. After treatment with ChT, the channel deactivation upon repolarization to −60 or −100 mV was significantly accelerated. The effect of ChT on channel activation kinetics was voltage-dependent; activation slowed during depolarization to +30 mV but accelerated during depolarization to 0 or −10 mV. In contrast, the reversal potential, inactivation kinetics, and voltage-dependence of steady-state inactivation remained unaltered. Our results demonstrate that the redox status of methionine is an important modulator of hERG channel.
Keywords: hERG, K-channel, chloramine-T, methionine, oxidation, methionine sulfoxide reductase
1. Introduction
Human ether à go-go related gene (hERG) K+ channels mediate the rapidly activating IKr current in cardiac myocytes and play a pivotal role in the termination of the cardiac action potential [1–4]. Defects in the hERG1 gene or pharmacological interventions that alter the hERG1 channel properties can give rise to inherited (long QT-syndrome 2) or acquired cardiac arrhythmias, respectively [3,5–7]. In addition to their importance in the heart, hERG channels are also important for neuronal function; the variants hERG2 and hERG3 in particular are expressed in neuronal tissue where they may take part in regulation of cellular excitability [8]. Both in a cardiac and a neuronal environment, hERG channels are exposed to a variety of reactive species (RS), such as reactive oxygen and nitrogen species, which are generated continuously as an inevitable consequence of normal aerobic cellular metabolism. In addition, some pathophysiological conditions may dramatically increase the exposure of the channels to RS. For example, reperfusion following ischemia in cardiac or cerebral tissues creates a burst of excess RS, which can adversely affect a variety of cell functions, including electrical excitability. Indeed, cardiac arrhythmia is a common complication of ischemia-reperfusion injury that creates excess RS [9–11].
RS readily modify cysteine residues in ion channel proteins, causing marked functional changes. For example, N-type “ball and chain” inactivation of selected mammalian K+ channels is altered by oxidation of conserved cysteine residues in the ball domain [12]. In addition, auxiliary β-subunits of voltage-dependent K+ channels also contain such RS-sensitive cysteine residues, resulting in redox-dependent inactivation of the α-β-subunit channel complexes [13]. Similarly, functional properties of hERG1 channels also have been suggested to be altered by RS [14–17], potentially in a cysteine- [15] or a histidine-dependent manner [17].
Cysteine is very susceptible to RS-mediated modification, but all other amino acids can be oxidized. Also readily oxidized is methionine (Met); addition of an oxygen atom to the sulfur moiety results in methionine sulfoxide (MetO) that exhibits physicochemical properties dramatically different from those of Met [18]. Protein oxidation at Met residues often results in loss of function [19]. Met oxidation is also relevant for the regulation of cellular excitability as suggested by previous studies on ion channel function. For example, oxidation of a critical Met residue in the N-terminal inactivation domain of the Drosophila ShC/B K+ channel disrupts its fast inactivation [20]. Oxidation of a single Met residue in the S6 segment near the channel pore also alters a distinct inactivation process, P/C-type inactivation, in ShB channels [21]. Another K+ channel robustly regulated by Met oxidation is the large-conductance Ca2+-activated K+ channel, in which oxidation of specific Met residues in the cytosolic C-terminal domain dramatically increases the channel activity [22, 23].
Unlike oxidation of other amino acid residues, oxidation of Met to MetO is reversed by the action of the enzyme methionine sulfoxide reductase (MSR) [24]. Because MetO occurs in two epimeric configurations (Met-S-O and Met-R-O), two specific MSR enzymes for their reduction exist: MSRA and MSRB, respectively [24]. These enzymes are physiologically coupled to the thioredoxin/thioredoxin reductase system (19) but in vitro the enzymes exhibit robust catalytic activity in the presence of dithiothreitol (DTT) [25]. Both types of MSRs are strongly expressed in kidney and liver, but there is also considerable expression in the cardiovascular system and the brain where MSRA may play a role in age-associated functional changes [26].
Despite the increasing evidence that oxidation of Met and its repair by MSRs modulate ion channel function and cellular electrical excitability, whether oxidation of Met has any functional consequence in hERG1 channels with fast P/C-type inactivation [27, 28] is not known. Thus we investigated if these K+ channels undergo functional changes in response to treatments that promote Met oxidation. We used chloramine-T (ChT) as a Met-preferring oxidant [20–24] and studied its effect on recombinant hERG1 channels expressed in mammalian cells with and without recombinant bovine MSRA in the cytosolic solution. We report here that Met oxidation potently inhibits hERG1 channels and that exogenous MSRA protects the channels against the oxidative damage.
2. Materials and Methods
2.1. Culture of HEK 293 Cells
The HEK 293 cells stably transfected with wild-type (WT) hERG1 cDNA (U04270) [29], have been described previously [30]. Cells were maintained at 37°C in Minimal Eagle Medium (MEM) supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, 2 mM Lglutamine, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, and 0.2 mg/ml geneticin (Invitrogen, Carlsbad, CA, USA) and passaged weekly to avoid >80% confluence. For electrophysiological recordings, the cells were harvested from the culture dish by trypsinization, washed twice with standard MEM, and maintained in culture medium at room temperature for use on the same day [31].
2.2. Culture of SH-SY5Y cells
The human neuroblastoma cell line SH-SY5Y was obtained from the German Collection of Microorganisms and Cell Cultures (DMSZ, Braunschweig, Germany). SH-SY5Y cells were maintained in 250-ml culture flaks (Greiner, Frickenhausen, Germany) in Dulbecco’s modified Eagle’s medium (GIBCOBRL, Karlsruhe, Germany) containing 10% fetal calf serum at 37°C in a humidified atmosphere with 5% CO2. Cells were passaged every 4–5 days. For electrophysiological recordings, the cells were harvested as described for HEK 293 cells.
2.3. Electrophysiology
hERG currents in HEK 293 cells were recorded at 37°C using the whole-cell patch-clamp technique (Axopatch 200A, MDC, Sunnyvale, CA, USA). Cells were voltage-clamped with pipettes fabricated from borosilicate glass capillaries (initial resistances of 1.5–2.7 MΩ). Series resistance compensation (75–80%) was used in all experiments. Currents were filtered at 2–5 kHz and digitized at 5–10 kHz. PCLAMP 8 software (MDC) was used to generate voltage-clamp protocols and to acquire data as described previously [31]. Giga seal was well maintained in our experiments with HEK 293 cells and leak current was small and stable. Therefore, no leak subtraction was performed. HEK 293 Cells with unstable seal or holding current were excluded. hERG current in SH-SY5Y cells was activated at 22°C with a 1-sec pulse to +50 mV at an interval of 10 sec. Since SH-SY5Y cells express various types of ion channels, the tail current at −120 mV was used to characterize the endogenous hERG current. For data obtained from SH-SY5Y cells (Fig. 2) a p/n leak correction procedure was applied
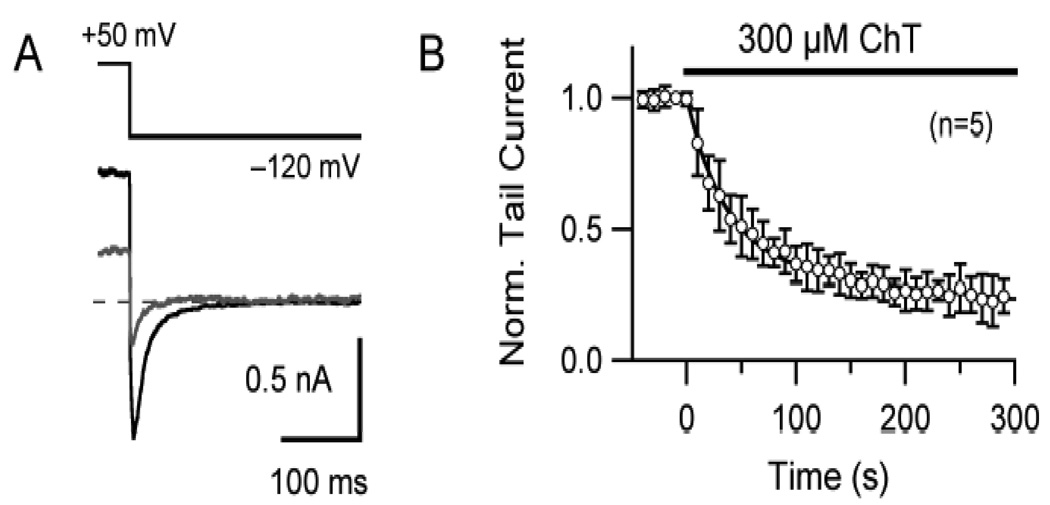
Fig. 2.
Effect of ChT on native hERG current in SH-SY5Y neuroblastoma cells. A, An example of superimposed current traces from a SH-SY5Y neuroblastoma cell before (black) and 300 sec after (grey) application of 300 µM ChT. hERG currents were elicited by 1-sec depolarization to +50 mV from a holding potential of −60 mV. The characteristic inward current was measured during the repolarization phase at −120 mV. B, Pooled data (n = 5) showing the time-dependent inhibition of hERG tail current. The tail current was normalized to the respective initial control value.
2.4. Solutions and Drugs
HEK 293 cells were superfused with a HEPES-buffered Tyrode solution containing (in mM): 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 5 glucose, 20 HEPES (pH = 7.4, adjusted with NaOH). The pipette solution for HEK 293 cells contained (in mM): 125 K aspartate, 20 KCl, 10 EGTA, 1 MgCl2, 5 MgATP, 5 HEPES (pH = 7.3, adjusted with KOH). For SH-SY5Y cells, the external solution contained (in mM): 110 NaCl, 40 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, pH 7.4 and the internal solution contained (in mM): 130 KCl, 2.56 MgCl2, 10 EGTA, 10 HEPES, pH 7.4. ChT (Sigma, St. Louis, MO. USA) was prepared fresh before each experiment and used within 5 hours. Cells were treated with ChT using two perfusion protocols: long perfusion procedure in which bath chamber was continuously perfused with ChT-containing solution for 4 to 5 min (Fig. 1) and short perfusion procedure in which the bath chamber was perfused for 1 min with ChT-solution followed by a wash-out period of 3 to 4 min (Figs. 3–8). In some experiments, the wash-out period lasted for more than 10 min. Recombinant bovine MSRA (bMSRA) was purified from E. coli as described previously [20, 22].
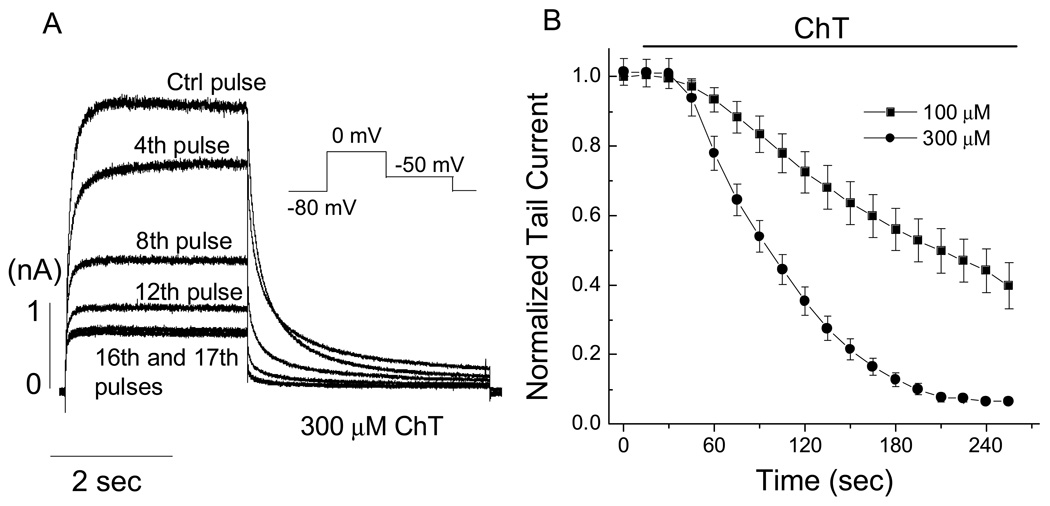
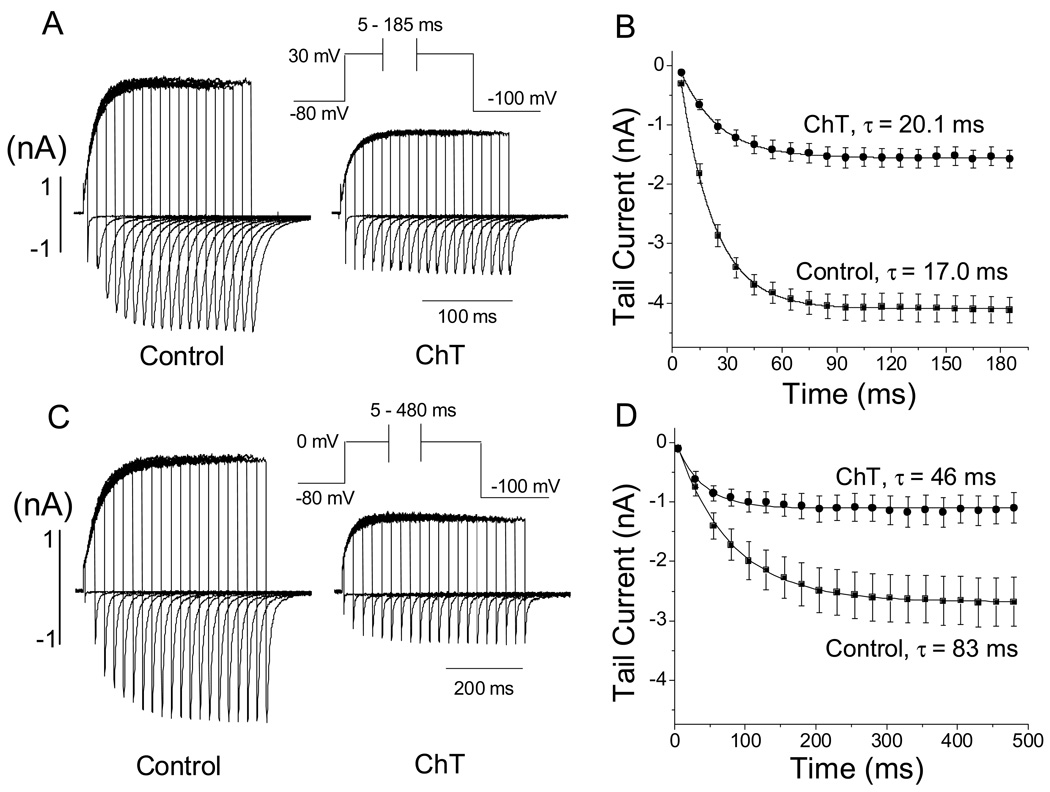
Fig. 1.
Time- and concentration-dependent effect of ChT on hERG current in HEK 293 cells. A, hERG activating and outward tail currents obtained in the absence and presence of ChT (300 µM). The hERG current was activated by applying a 3-s depolarization step to 0 mV from a holding potential of −80 mV and the outward tail current was recorded during a 4-s repolarization pulse at −50 mV (see inset in A). Pulses were applied once every 15 s during superfusion with ChT. B, pooled data (n = 6–7) showing the time- and concentration- dependent inhibition of hERG tail current. The tail currents were normalized to the respective initial value at time t = 0 s. Straight lines connect the data points.
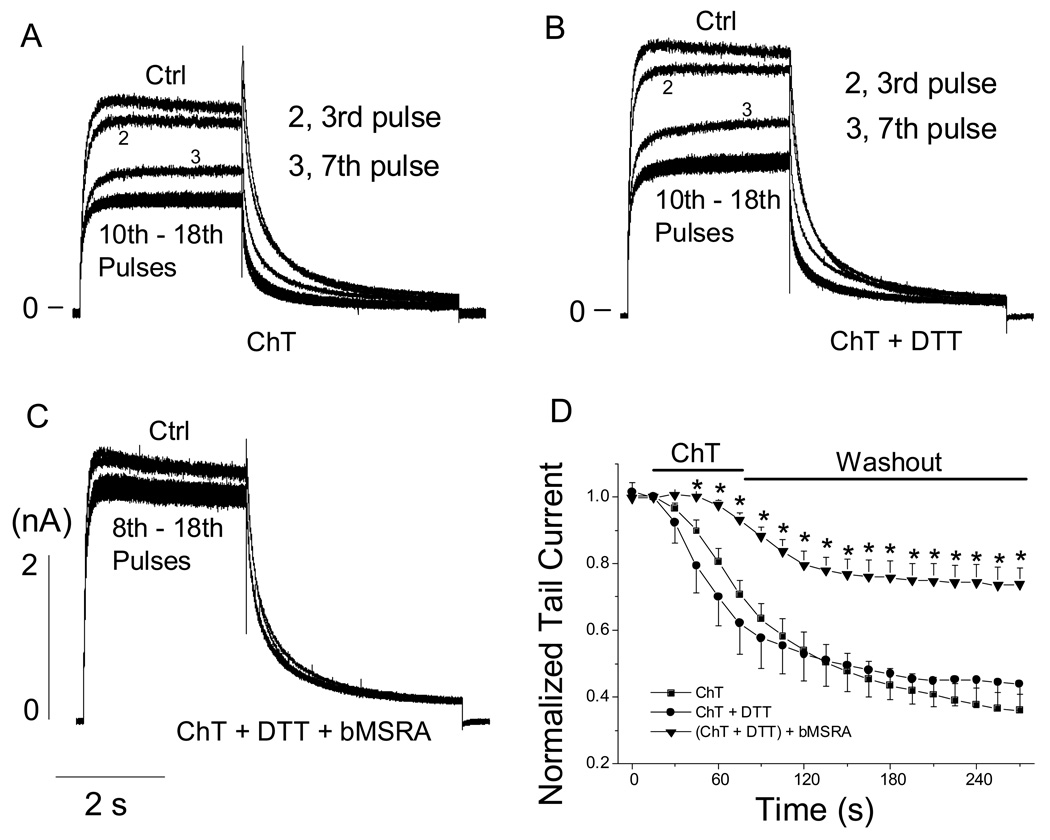
Fig. 3.
Effect of MSRA on ChT-mediated inhibition of hERG current in HEK 293 cells. In panels A, B, and C, hERG activating and tail currents were recorded in the absence and presence of ChT using the same voltage protocol as shown in Fig. 1A. Current recording was started at least 3 min after obtaining access to whole-cell configuration. ChT (300 µM) was perfused for 1 min and then washed out for 3 to 4 min. A, normal internal solution was used. B, DTT (4 mM) was present in the internal solution. C, bMSRA (15 µg/ml) and DTT (4 mM) were included in the internal solution. D, pooled data showing the time-dependent change of tail currents under the conditions shown in A-C. Tail currents were normalized to the respective initial current size at time t = 0 s. n = 5–10. * P < 0.05 vs (ChT + DTT).
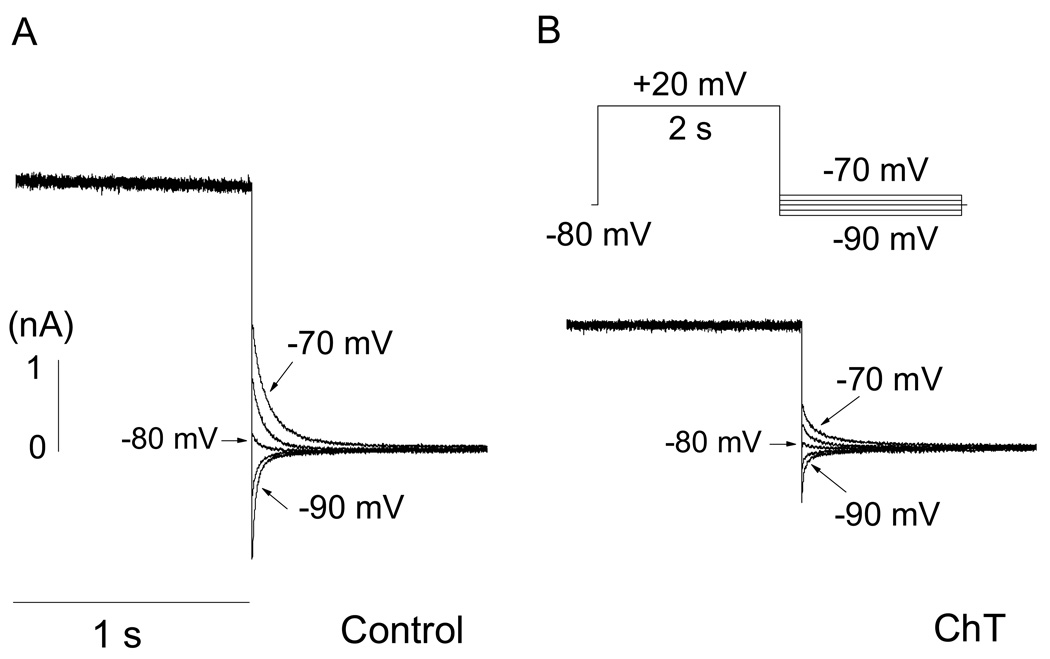
Fig. 8.
ChT does not alter the reversal potential of hERG current in HEK 293 cells. Cells were depolarized from a holding potential of −80 mV to +20 mV for 2 s followed by repolarizing in 5-mV increments to different voltages, which bracketed the reversal potential (see inset in B). A, Representative data obtained from a cell before treatment with ChT. B, Data from the same cell after treatment with ChT. Only part of the activating and tail currents is shown in both panels. The reversal potential was measured as the zero intercept of the linear fit to the instantaneous tail currents at voltages bracketing the reversal potential.
2.5. Data analysis
Data were digitized online with a DigiData 1200 interface (MDC) and stored for later analysis. The digitized data were analyzed with pCLAMP8 and ORIGIN software (OriginLab, Northampton, MA, USA). Results are expressed as mean ± S.E.M. Paired or unpaired Student’s t-test was used as appropriate to evaluate the statistical significance of differences between two group means, and ANOVA followed by Dunnett’s post-hoc test was used for multiple groups. Differences were considered statistically significant at P < 0.05.
3. Results
3.1. Effects of ChT on hERG K+ current
Depolarization to 0 mV from the holding potential of −80 mV activated hERG K+ current and repolarization to −50 mV elicited a slowly-decaying outward tail current in HEK 293 cells. Application of the oxidant ChT to the extracellular medium progressively decreased the amplitudes of tail current at −50 mV (Fig. 1). With 300 µM ChT, a near complete inhibition was obtained within 3 to 4 min perfusion and with 100 µM ChT, the currents decreased more slowly. These results demonstrate the effect of ChT on hERG current is time- and concentration-dependent. Extended treatment with ChT (long perfusion procedure, see Materials and Methods) essentially rendered the channel non-functional as evidenced by a near complete inhibition of hERG current in HEK 293 cells. ChT at 300 µM also showed similar inhibitory effect on native hERG current in SH-SY5Y neuroblastoma cells (Fig. 2).
ChT at concentrations of ≤10 mM preferentially oxidizes Met to MetO in various proteins and MSRA can at least partially repair MetO to Met in the presence of DTT [23,24]. If the inhibition of hERG current (Figs. 1, 2) involves Met oxidation, MSRA may antagonize the inhibitory effect of ChT. This prediction was tested by including purified recombinant bMSRA in the recording pipette. In this subset of experiments, hERG currents in HEK 293 cells were recorded using the same voltage protocol as described in Fig. 1, but ChT was applied to the cells using the short perfusion procedure (1 min wash-in followed by 3 to 4 min wash-out, see Materials and Methods). The catalytic cycle involving MSRA requires an electron source such as the reducing agent DTT. The presence of DTT (4 mM) in the recording pipette had no effect on the control hERG current (data not shown) and did not interfere with the strong inhibitory effect of ChT (300 µM) (Fig. 3A, B, D). However, when bMSRA (15 µg/ml) was present in the pipette solution in addition to DTT, the decline of hERG current by ChT was significantly slower and the extent of inhibition by the oxidant was considerably smaller (Fig. 3C, D). The observation that MSRA antagonized the inhibitory effect of ChT suggests that Met oxidation is at least partially responsible for the hERG current inhibition.
3.2. Effect of ChT on hERG channel activation
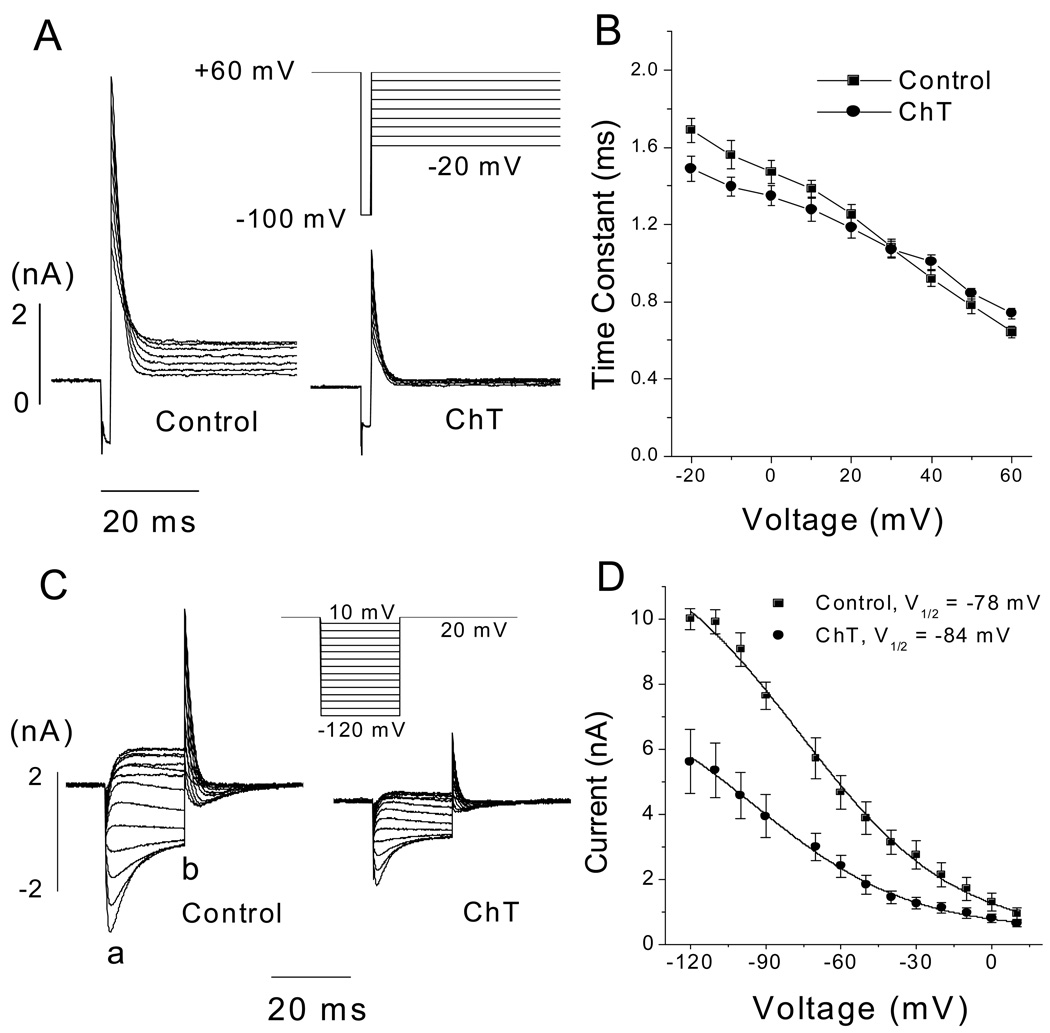
The activation time course of hERG current was examined by using an envelope of tails protocol [31], and was well described by a single exponential function both before and after treatment (short perfusion procedure) with ChT (Fig. 4). ChT application slightly increased the activation time constant (τ) of hERG current at +30 mV (17.0 ± 1.1 vs 20.1 ± 0.8 ms, n = 7, P = 0.014) (Fig. 4A, B). In contrast, the time constant of hERG current activation at 0 mV was decreased significantly by ChT treatment (τ = 46.2 ± 9.6 vs 83.4 ± 11.6 ms, n =6, P < 0.05) (Fig.4C, D). The time constant of hERG current activation at −10 mV was also decreased by ChT (112 ± 14 vs 196 ± 34 ms, n = 5, P < 0.05) (traces not shown). These results indicate that the effect of Met oxidation (induced by ChT) on hERG current activation kinetics is voltage-dependent, with activation slowed at high voltages such as +30 mV and accelerated at low voltages such as 0 or −10 mV.
Fig. 4.
Effect of ChT on hERG activation kinetics in HEK 293 cells. A, Activation time course of hERG current (at +30 mV) assessed by an envelope of tails test (see inset for clamp protocol) before (left) and after exposure to ChT for 1 min (300 µM) (right). B, pooled data (n = 7) for the activation time courses, which were plots of the tail currents recorded at −100 mV against the activation pulse (+30 mV) duration. C, Activation time course of hERG current (at 0 mV) assessed by an envelope of tails test (see inset for clamp protocol) before (left) and after exposure to ChT for 1 min (300 µM) (right). D, Pooled data (n = 6) for the activation time courses, which were plots of the tail currents recorded at −100 mV against the activation pulse (0 mV) duration. The time course was fit with a single exponential function.
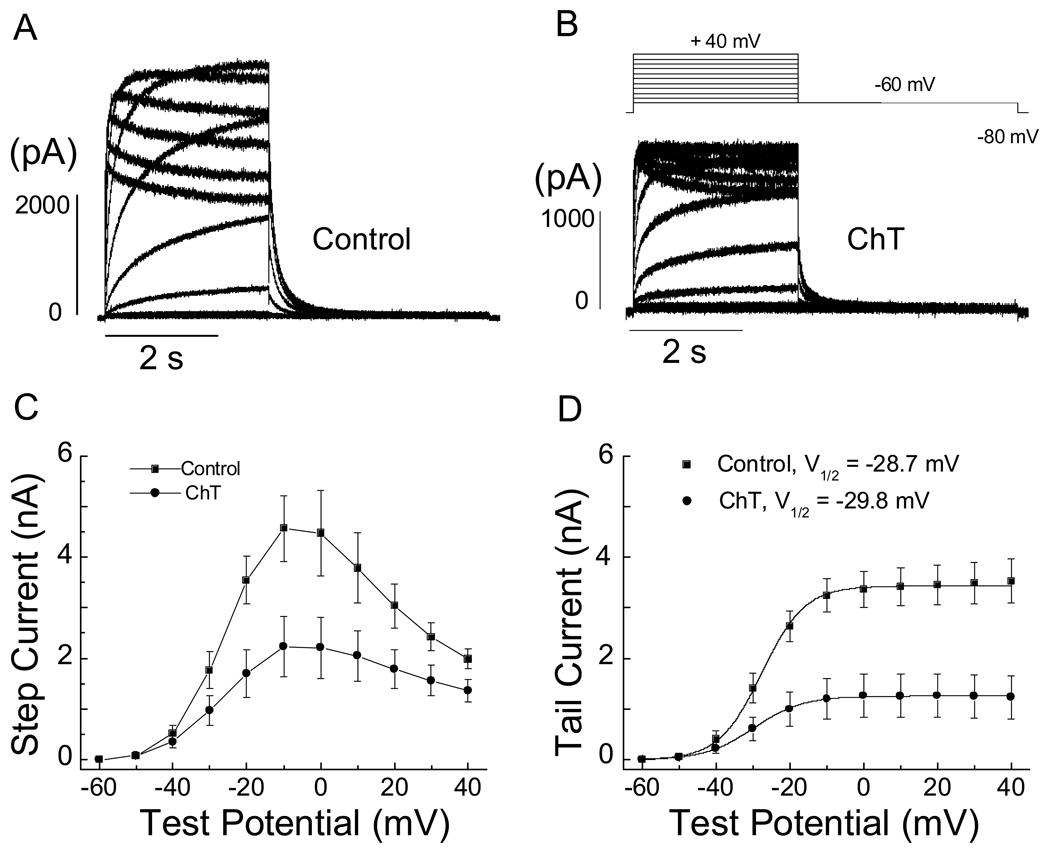
To examine the effect of Met oxidation on the voltage dependence of hERG current activation, we determined the mid-point activation voltage (V1/2) and slope factor by fitting the current-voltage results obtained from tail currents at −60 mV with a Boltzmann function (Fig. 5). In this set of experiment designed to obtain the steady-state activation curve, the repolarization pulse (−60 mV) was proceded by a 3-sec depolarizing step (−60 to +40 mV, in 10-mV increments, Fig. 5B). Representative recordings obtained before (control) and after treatment with ChT (short perfusion procedure) are shown in Fig. 5A and B, respectively. Both activation (from −30 to +40 mV) and tail currents are dramatically decreased by ChT (Fig. 5C, D). The slope factor was increased by ChT (5.5 ± 0.8 vs 6.2 ± 0.3 mV, n = 9, P = 0.005) but the V1/2 (−28.7 ± 1.0 vs −29.8 ± 0.8 mV, n = 9) was not altered (P > 0.05) (Fig. 5D).
Fig. 5.
Current-voltage relationships of hERG K+ current in HEK 293 cells before and after ChT application. hERG current was activated by applying voltage pulses for 3 s from −60 to +40 mV (10-mV increments). Outward tail current was recorded upon repolarization to −60 mV for 4 sec. Holding potential was −80 mV. Representative current traces before (A) and after application of 300 µM ChT for 1 min (B). C, pooled current-voltage plot for activating current measured at the end of the depolarizing voltage steps (n = 9). Straight lines connect the data points. D, Pooled current-voltage plot for peak outward tail current (activation curve). The activation curve was fit with a Boltzmann function. The slope factor was increased by ChT (5.5 ± 0.8 vs 6.2 ± 0.3 mV, n = 9, P = 0.005) but the V1/2 (−28.7 ± 1.0 vs −29.8 ± 0.8 mV, n = 9) was not altered by ChT (P > 0.05).
3.3. Effect of ChT on voltage dependence and kinetics of hERG channel inactivation
A three-pulse protocol (Fig. 6A) was used to assess the time course of the inactivation of hERG channel [30,31]. Following a 500-ms pulse to +60 mV to allow the channels to fully activate and inactivate, a 2-ms pulse was applied to −100 mV to remove inactivation. Test pulses to potentials ranging from −20 to +60 mV were then applied to induce channel inactivation. The inactivating currents were fitted by a single exponential function, and such analyses showed that the inactivation time constants were not significantly changed by ChT over the range of −20 to +60 mV (Fig. 6B; n = 5, P > 0.05).
Fig. 6.
Effect of ChT on hERG channel inactivation in HEK 293 cells. A, Representative traces showing the time course of hERG channel inactivation before (left) and after 1-min application of ChT (300 µM) (right). A three-pulse protocol was used to assess inactivation kinetics (see inset). B, Inactivation time constant plotted as a function of test voltage. The time constants were determined by fitting a single exponential function to the inactivating current (in A). At all voltages, the time constants were not significantly influenced by ChT (n = 7). Straight lines connect the data points. C, Representative data showing steady-state inactivation of hERG channels before (left) and after 1-min application of ChT (300 µM) (right). Currents were elicited by the protocol shown in inset in which inactivation was allowed to relax to steady-state during 20-ms test pulses to potentials ranging from −120 to +10 mV. D, Steady-state inactivation curves. Peak outward currents elicited by the second step to +20 mV were corrected for channel closing and plotted as a function of the preceding test pulse potential. Inactivation curve was fit with a Boltzmann function. The estimated peak amplitudes were 15.0 ± 2.5 and 7.4 ± 1.4 nA (n = 6).
ChT also failed to alter the voltage dependence of steady-state inactivation of hERG channels (Fig. 6C, D). After a 1-sec pulse to +20 mV, the membrane potential was clamped to various test voltages for 20 ms to allow inactivation to relax to a steady state. The 20-ms test pulse was followed by a return step to +20 mV. At negative test potentials, the current declined because channel closing occurred through deactivation. Thus, peak current amplitudes at the return step (+20 mV) were corrected for channel closing by applying a correction factor, which was the ratio of the peak tail amplitude of the test pulse (indicated by “a” in the control of panel C) over the current amplitude at the end of the test pulse (indicated by “b” in the control of panel C). The correction factor was calculated for each test pulse at negative potentials where the current declined because of channel closure. The corrected current at the return step (+20 mV) was plotted as a function of voltage of the 20-ms test pulses to obtain the steady-state inactivation curve [27,31,32]. Peak current amplitudes at the return step to +20 mV were prominently decreased by ChT (300 µM), consistent with the results presented thus far. The voltage dependence of inactivation was quantified by using a Boltzmann function (Fig. 6D). The V1/2 values before and after ChT treatment were −78.1 ± 5.1 and −84.1 ± 5.3 mV, respectively, and the difference was not significant (n = 6, P = 0.345). The slope factor value did not change either (29 ± 2.0 vs 30.0 ± 2.2 mV, n = 6, P = 0.916).
3.4. Effect of ChT on deactivation kinetics of hERG channels
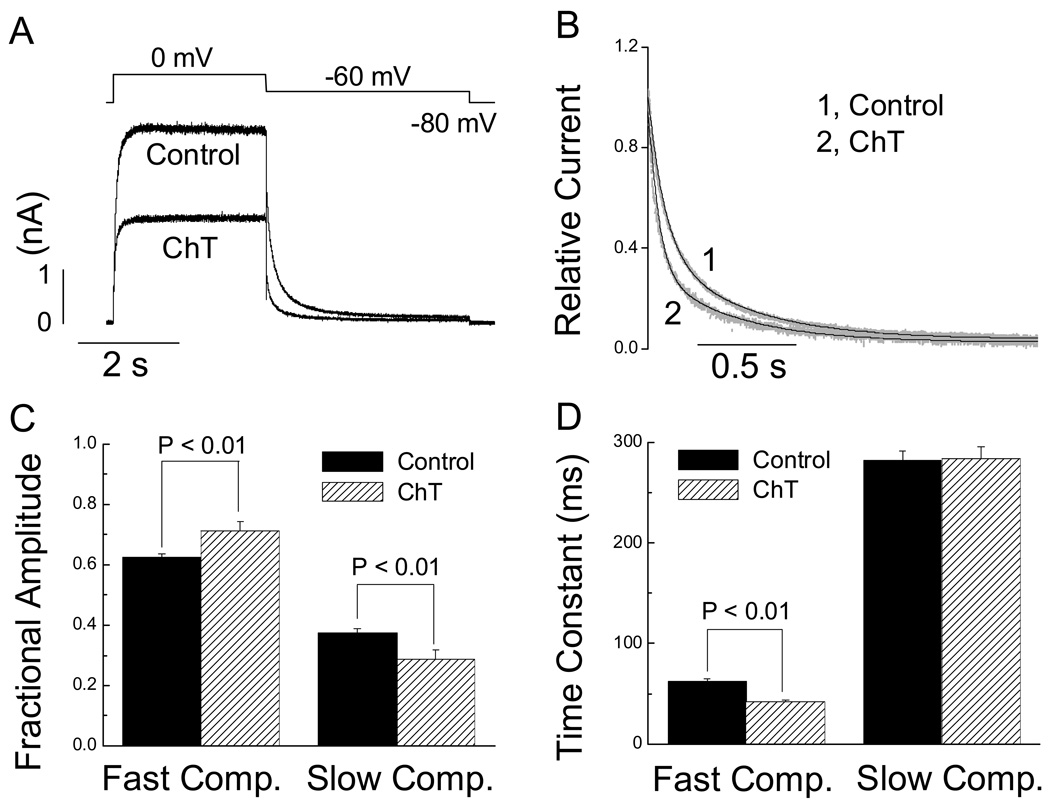
Treatment with ChT (short perfusion procedure) accelerated the overall time course of the tail current recorded at −60 mV (Fig. 7A, B). Double exponential fits of the tail currents revealed that the acceleration was associated with an increase in the fractional amplitude (Fig. 7C) and a decrease in the time constant (Fig. 7D) of the fast component. The time constant of the slow component remained unaltered by ChT (Fig. 7D) although the fractional amplitude of the slow component was decreased (Fig. 7C). Time course of the tail currents at −100 mV was also accelerated, with both slow and fast time constant decreased significantly (slow τ: 20.8 ± 2.5 vs 29.9 ± 1.8 ms, P < 0.01; fast τ: 4.1 ± 0.3 vs 7.1 ± 0.4 ms, P < 0.01)
Fig. 7.
ChT alters hERG tail current kinetics in HEK 293 cells. A, tail currents recorded at −60 mV following a depolarizing step to 0 mV before (control) and after exposure to ChT (300 µM for 1 min). B, Comparison of scaled representative tail currents recorded at −60 mV before and after treatment with ChT. The black lines are double exponential fit of the scaled tail currents plotted in grey. C and D, Pooled data (n = 7) showing changes in the double exponential parameters caused by ChT. Fractional amplitude for the fast or slow component was calculated respectively using the formula: A1/(A1 +A2) or A2/(A1 +A2), where A1 is the amplitude of the fast component of the tail and A2 the amplitude of the slow component.
3.5. Effect of ChT on hERG channel ion selectivity
To examine if ChT affected ion selectivity of the hERG channel, we measured the reversal potential of hERG current before and after exposure to ChT (300 µM) for 1 min in our standard recording solutions (containing primarily Na+ and K+). Both the hERG activation and tail currents were suppressed by ChT (Fig. 8) but the reversal potential was not significantly changed. The average reversal potentials measured before and after exposure to ChT were −81.4 ± 1.1 mV and −80.9 ± 0.4 mV (P > 0.05, n = 6), respectively. Treatment with ChT thus preferentially altered selected gating properties of hERG channels without affecting the selectivity between K+ and Na+.
4. Discussion
This study demonstrates that application of the oxidant ChT, which preferentially oxidizes Met in many systems, leads to dramatic functional alterations in hERG channels expressed in mammalian cells at a physiological temperature. The eventual and most prominent effect caused by extended treatment of hERG channels with ChT is a near total inhibition of the current. In addition, before the channels are completely inhibited by ChT, selected gating properties also undergo noticeable changes such as overall acceleration of tail currents. The ChT-mediated inhibition of hERG current was significantly attenuated by MSRA, an enzyme that specifically repairs oxidized Met residues [20,24]. The incomplete protection by MSRA (Fig. 3) against ChT-mediated inhibition of hERG current is observed probably because MetO occurs in two epimeric configurations (Met-S-O and Met-R-O) and MSRA only reduces Met-S-O to Met [24]. These results indicate that ChT alters hERG channel by oxidizing the Met residues and not by acting as a simple pore blocker. Cytoplasmic Met residues are likely important in mediating the inhibitory effect of ChT because the presence of MSRA in the cytoplasmic side antagonizes the inhibitory effect. Multiple Met residues are probably oxidized by ChT to induce the two distinct components of the functional changes observed: the gating changes and the removal from the active pool available to open. ChT is an oxidizing agent that preferentially oxidizes methionine to methionine sulfoxide (MetO) but it can also oxidize cysteine. Selective oxidation of methionine to MetO by ChT (< 10 mM) has been demonstrated in different proteins [33–40]. Particularly in other ion channels, ChT does indeed act as a Met-preferring oxidizing agent as demonstrated by using electrophysiology and site-directed mutagenesis[20,23]. Substitution of critical Met residues with less readily oxidized residues, such as Leu, eliminates the ChT sensitivity [20, 21, 23]. Our observation that DTT does not diminish the ChT effect on hERG current (Fig. 3) also indicates that cysteine oxidation may not be involved in ChT-induced inhibition of hERG channel function and further supports the selective nature of ChT as a methionine-oxidizing agent.
Previous studies have reported that functional properties of hERG channels are altered by RS [14–17,41]. However, different effects on hERG channel functions were shown for different RS. For example, experimental generation of RS using the iron/ascorbate reaction increases outward currents through hERG channels expressed in Xenopus oocytes [14]. The current enhancing effect requires two histidine residues at positions 578 and 587 in the S5-S6 linker region of the channel [17]. Direct application of hydrogen peroxide also alters hERG current by accelerating the activation and deactivation kinetics [15]. In these studies, involvement of cysteine oxidation is probable but it was not directly addressed. In contrast, the hERG channel function was impaired by RS that were generated by exposure to tumor necrosis factor-α and hyperglycemia [16,41]. There are several explanations for the observed different effects. The first, and also the most important reason is that different reactive species are used or generated under various experimental settings. Second, such reactive species may oxidize different amino acid residues and a single reactive species may oxidize more than one type of amino acid. Therefore, it is very likely that more than one type of amino acid in hERG channels are oxidized during exposure to RS. Third, variations in the hERG channel-expressing systems, such as oocytes and mammalian cell lines, could be another reason contributing to the different effects. For example, hydrogen peroxide has been shown to alter hERG current expressed in HEK-293 cells by accelerating the activation and deactivation kinetics [15]. However, other studies using Xenopus oocytes reported no effect of hydrogen peroxide on hERG channels [42,43]. Undoubtedly, a more systematic effort is needed to better understand RS-mediated modulation of hERG channel. Nevertheless, the results presented in this study indicate that Met residue in hERG channel is one of the amino acids that confer sensitivity to oxidative modification imposed by RS, although it is not known which Met residue(s) is (are) responsible for the functional change of hERG channels exposed to ChT. A systematic mutagenesis of hERG with 24 Met residues may be required to further address this issue. It should also be mentioned that our results do not readily exclude the possibility that the effect of ChT on hERG could be modulated by the ancillary subunits taking part in forming functional Ikr channels.
Normal cardiac electrical rhythm requires appropriate termination of the cardiac action potential, in which hERG K+ channels play a critical role [3,4]. Therefore, the susceptibility of hERG channels to RS-mediated oxidative modification is likely to be physiologically and pathophysiologically important. For instance, cardiac reperfusion injury, wherein a burst of RS occurs, is often complicated by cardiac arrhythmias [9–11]. Our results suggest that one important contributing factor for the abnormal rhythm may be insufficient protection of hERG K+ channels against Met oxidation. Additionally, functional levels of MSRA in selected tissues are reported to decrease with age and are lower in patients with Alzheimer’s disease [44,45]. Both of these conditions are associated with an increased risk of cardiac arrhythmia [46,47]. hERG channels are also expressed in neuronal cells where they are postulated to be involved in regulation of neuronal excitability [8]. Excess liberation of RS in the central nervous system could therefore affect the neuronal function via the modification of hERG channels. In fact, indications that hERG oxidation may play a role in neuronal diseases such as epilepsy do exist [48]. In conclusion, the function of hERG channels to conduct K+ current is modulated by the redox status of the Met residues. Dysfunction of hERG channels caused by Met oxidation is likely to occur during oxidative stress mediated by an imbalance of oxidant generation and elimination and could be an important mechanism for abnormal electrical activities.
ACKNOWLEDGEMENTS
This study was supported in part by grants from National Institutes of Health (T.H.) and the Deutsche Forschungsgemeinschaft (HE2993/6 to S.H.H.). We are indebted to Kathryn Houseman for maintaining the HEK 293 cell line.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 2.Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- 3.Viskin S. Long QT syndromes and torsade de pointes. Lancet. 1999;354:1625–1633. doi: 10.1016/S0140-6736(99)02107-8. [DOI] [PubMed] [Google Scholar]
- 4.Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104:569–580. doi: 10.1016/s0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 5.Keating MT. Genetic approaches to cardiovascular disease. Supravalvular aortic stenosis, Williams syndrome, and long-QT syndrome. Circulation. 1995;92:142–147. doi: 10.1161/01.cir.92.1.142. [DOI] [PubMed] [Google Scholar]
- 6.Cavero I, Mestre M, Guillon JM, Crumb W. Drugs that prolong QT interval as an unwanted effect: assessing their likelihood of inducing hazardous cardiac dysrhythmias. Expert Opin Pharmacother. 2000;1:947–973. doi: 10.1517/14656566.1.5.947. [DOI] [PubMed] [Google Scholar]
- 7.De Ponti F, Poluzzi E, Cavalli A, Recanatini M, Montanaro N. Safety of non-antiarrhythmic drugs that prolong the QT interval or induce torsade de pointes: an overview. Drug Safety. 2002;25:263–286. doi: 10.2165/00002018-200225040-00004. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz JR, Bauer CK. Functions of erg K+ channels in excitable cells. J Cell Mol Med. 2004;8:22–30. doi: 10.1111/j.1582-4934.2004.tb00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birnbaum Y, Leor J, Kloner RA. Pathobiology and clinical impact of reperfusion injury. J Thromb Thrombolysis. 1997;4:185–195. doi: 10.1023/a:1008866111951. [DOI] [PubMed] [Google Scholar]
- 10.Gazmuri RJ, Ayoub IM, Kolarova J. Myocardial protection during resuscitation from cardiac arrest. Curr Opin Crit Care. 2003;9:199–204. doi: 10.1097/00075198-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Kevin LG, Novalija E, Stowe DF. Reactive oxygen species as mediators of cardiac injury and protection: the relevance to anesthesia practice. Anesth Analg. 2005;101:1275–1287. doi: 10.1213/01.ANE.0000180999.81013.D0. [DOI] [PubMed] [Google Scholar]
- 12.Ruppersberg JP, Stocker M, Pongs O, Heinemann SH, Frank R, Koenen M. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature. 1991;352:771–774. doi: 10.1038/352711a0. [DOI] [PubMed] [Google Scholar]
- 13.Rettig J, Heinemann SH, Wunder F, Lorra C, Parcej DN, Dolly JO, Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of b-subunit. Nature. 1994;369:289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- 14.Taglialatela M, Castaldo P, Iossa S, Pannaccione A, Fresi A, Ficker E, Annunziato L. Regulation of the human ether-a-gogo related gene (HERG) K+ channels by reactive oxygen species. Proc Natl Acad Sci USA. 1997;94:11698–11703. doi: 10.1073/pnas.94.21.11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berube J, Caouette D, Daleau P. Hydrogen peroxide modifies the kinetics of HERG channel expressed in a mammalian cell line. J Pharmacol Exp Ther. 2001;297:96–102. [PubMed] [Google Scholar]
- 16.Wang J, Wang H, Zhang Y, Gao H, Nattel S, Wang Z. Impairment of HERG K+ channel function by tumor necrosis factor-alpha: role of reactive oxygen species as a mediator. J Biol Chem. 2004;279:13289–13292. doi: 10.1074/jbc.C400025200. [DOI] [PubMed] [Google Scholar]
- 17.Pannaccione A, Castaldo P, Ficker E, Annunziato L, Taglialatela M. Histidines 578 and 587 in the S5-S6 linker of the human Ether-a-go-go related gene-1 K+ channels confer sensitivity to reactive oxygen species. J Biol Chem. 2002;277:8912–8919. doi: 10.1074/jbc.M111353200. [DOI] [PubMed] [Google Scholar]
- 18.Black SD. Development of hydrophobicity parameters for prenylated proteins. Biochem Biophys Res Commun. 1992;186:1437–1442. doi: 10.1016/s0006-291x(05)81567-0. [DOI] [PubMed] [Google Scholar]
- 19.Hoshi T, Heinemann SH. Regulation of cell function by methionine oxidation and reduction. J Physiol (Lond) 2001;531:1–11. doi: 10.1111/j.1469-7793.2001.0001j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciorba MA, Heinemann SH, Weissbach H, Brot N, Hoshi T. Modulation of potassium channel function by methionine oxidation and reduction. Proc Natl Acad Sci USA. 1997;94:9932–9937. doi: 10.1073/pnas.94.18.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Avdonin V, Ciorba MA, Heinemann SH, Hoshi T. Acceleration of P/C-type inactivation in voltage-gated K+ channels by methionine oxidation. Biophys J. 2000;78:174–187. doi: 10.1016/S0006-3495(00)76583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang XD, Daggett H, Hanner M, Garcia ML, McManus OB, Brot N, Weissbach H, Heinemann SH, Hoshi T. Oxidative regulation of large conductance calcium-activated potassium channels. J Gen Physiol. 2001;117:253–274. doi: 10.1085/jgp.117.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santarelli LC, Wassef R, Heinemann SH, Hoshi T. Three methionine residues located within the regulator of conductance for K+ (RCK) domains confer oxidative sensitivity to large-conductance Ca2+-activated K+ channels. J Physiol (Lond) 2006;571:329–348. doi: 10.1113/jphysiol.2005.101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weissbach H, Resnick L, Brot N. Methionine sulfoxide reductases: history and cellular role in protecting against oxidative damage. Biochim Biophys Acta. 2005;1703:203–212. doi: 10.1016/j.bbapap.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Moskovitz J, Jenkins NA, Gilbert DJ, Copeland NG, Jursky F, Weissbach H, Brot N. Chromosomal localization of the mammalian peptide-methionine sulfoxide reductase gene and its differential expression in various tissues. Proc Natl Acad Sci USA. 1996;93:3205–3208. doi: 10.1073/pnas.93.8.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci USA. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith PL, Baukrowitz T, Yellen G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature. 1996;379:833–836. doi: 10.1038/379833a0. [DOI] [PubMed] [Google Scholar]
- 28.Schönherr R, Heinemann SH. Molecular determinants for activation and inactivation of HERG, a human inward rectifier potassium channel. J Physiol (Lond) 1996;493:635–642. doi: 10.1113/jphysiol.1996.sp021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warmke JW, Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci USA. 1994;91:3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Z, Gong Q, Ye B, Fan Z, Makielski JC, Robertson GA, January CT. Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys J. 1998;74:230–241. doi: 10.1016/S0006-3495(98)77782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su Z, Chen J, Martin R, McDermott JS, Cox BF, Gopalakrishnan M, Gintant G. Block of hERG channel by ziprasidone: Biophysical properties and molecular determinants. Biochem Pharmacol. 2006;71:278–286. doi: 10.1016/j.bcp.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 32.Caballero R, Moreno I, Gonzalez T, Arias C, Valenzuela C, Delpon E, Tamargo J. Spironolactone and its main metabolite, canrenioc acid, block human ether-á-go-go-related gene channels. Circulation. 2003;107:889–895. doi: 10.1161/01.cir.0000048189.58449.f7. [DOI] [PubMed] [Google Scholar]
- 33.Shechter Y, Burstein Y, Patchornik A. Selective oxidation of methionine residues in proteins. Biochemistry. 1975;14:4497–4503. doi: 10.1021/bi00691a025. [DOI] [PubMed] [Google Scholar]
- 34.Wang GK, Brodwick MS, Eaton DC. Removal of sodium channel inactivation in squid axon by the oxidant chloramine-T. J Gen Physiol. 1985;86:289–302. doi: 10.1085/jgp.86.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beck-Speier I, Leuschel L, Luippold G, Maier KL. Proteins released from stimulated neutrophils contain very high levels of oxidized methionine. FEBS Lett. 1988;227:1–4. doi: 10.1016/0014-5793(88)81401-7. [DOI] [PubMed] [Google Scholar]
- 36.Miles AM, Smith RL. Functional methionines in the collagen/gelatin binding domain of plasma fibronectin: effects of chemical modification by chloramine-T. Biochemistry. 1993;32:8168–8178. doi: 10.1021/bi00083a017. [DOI] [PubMed] [Google Scholar]
- 37.Schlief T, Schonherr R, Heinemann SH. Modification of C-type inactivating Shaker potassium channels by chloramine-T. Pflugers Arch. 1996;431:483–493. doi: 10.1007/BF02191894. [DOI] [PubMed] [Google Scholar]
- 38.Nedkov P, Spassov V, Tzokov S. Relationship between accessibility and reactivity of Lys, Met and Tyr in subtilisins DY and Carlsberg. Biol Chem. 1996;377:653–659. doi: 10.1515/bchm3.1996.377.10.653. [DOI] [PubMed] [Google Scholar]
- 39.Fu Q, Mcphie P, Gowda DC. Methionine modification impairs the C5-cleavage function of cobra venom factor-dependent C3/C5 convertase. Biochem Mol Biol Int. 1998;45:133–144. doi: 10.1080/15216549800202502. [DOI] [PubMed] [Google Scholar]
- 40.Stief TW, Kurz J, Doss MO, Fareed J. Singlet oxygen inactivates fibrinogen, factor V, factor VII, factor X, and platelet aggregation of human blood. Thromb Res. 2000;97:473–480. doi: 10.1016/s0049-3848(99)00211-x. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Han H, Wang J, Wang H, Yang B, Wang Z. Impairment of human ether-a-go-go-related gene (hERG) K+ channels function by hypoglycemia and hyperglycemia. J Biol Chem. 2003;278:10417–10426. doi: 10.1074/jbc.M211044200. [DOI] [PubMed] [Google Scholar]
- 42.Fan JS, Jiang M, Dun W, McDonald TV, Tseng GN. Effects of outer mouth mutations on hERG channel function: a comparison with similar mutations in the Shaker channel. Biophys J. 1999;76:3128–3140. doi: 10.1016/S0006-3495(99)77464-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dun W, Jiang M, Tseng GN. Allosteric effects of mutations in the extracellular S5-P loop on the gating and ion permeation properties of the hERG potassium channel. Pflugers Arch. 1999;439:141–149. doi: 10.1007/s004249900101. [DOI] [PubMed] [Google Scholar]
- 44.Petropoulos I, Mary J, Perichon M, Friguet B. Rat peptide methionine sulphoxide reductase: cloning of the cDNA, and down-regulation of gene expression and enzyme activity during aging. Biochem J. 2001;355:819–825. doi: 10.1042/bj3550819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gabbita SP, Aksenov MY, Lovell MA, Markesbery WR. Decrease in peptide methionine sulfoxide reductase in Alzheimer's disease brain. J Neurochem. 1999;73:1660–1666. doi: 10.1046/j.1471-4159.1999.0731660.x. [DOI] [PubMed] [Google Scholar]
- 46.Go AS. The epidemiology of atrial fibrillation in elderly persons: the tip of the iceberg. Am J Geriatr Cardiol. 2005;14:56–61. doi: 10.1111/j.1076-7460.2005.02278.x. [DOI] [PubMed] [Google Scholar]
- 47.Roman GC. Vascular dementia prevention: a risk factor analysis. Cerebrovasc Dis. 2005;20(Suppl 2):91–100. doi: 10.1159/000089361. [DOI] [PubMed] [Google Scholar]
- 48.Azarbayjani F, Danielsson BR. Embryonic arrhythmia by inhibition of HERG channels: a common hypoxia-related teratogenic mechanism for antiepileptic drugs? Epilepsia. 2002;43:457–468. doi: 10.1046/j.1528-1157.2002.28999.x. [DOI] [PubMed] [Google Scholar]