Abstract
The profound hypermetabolic response to burn injury is associated with insulin resistance and hyperglycemia, significantly contributing to the incidence of morbidity and mortality in this patient population. These responses are present in all trauma, surgical, or critically ill patients, but the severity, length, and magnitude is unique for burn patients. Although advances in therapeutic strategies to attenuate the post-burn hypermetabolic response have significantly improved the clinical outcome of these patients over the past years, therapeutic approaches to overcome stress-induced hyperglycemia have remained challenging. Intensive insulin therapy has been shown to significantly reduce morbidity and mortality in critically ill patients. High incidence of hypoglycemic events and difficult blood glucose titrations have led to investigation of alternative strategies, including the use of metformin, a biguanide, or fenofibrate, a PPAR-γ agonist. Nevertheless, weaknesses and potential side affects of these drugs reinforces the need for better understanding of the molecular mechanisms underlying insulin resistance post-burn that may lead to novel therapeutic strategies further improving the prognosis of these patients. This review aims to discuss the mechanisms underlying insulin resistance induced hyperglycemia post-burn and outlines current therapeutic strategies that are being used to modulate hyperglycemia following thermal trauma.
Keywords: insulin resistance, insulin, hypermetabolic response, burn injury
INTRODUCTION
More than 500,000 burn injuries occur annually in the United States per year.1 Although most of these burn injuries are minor, approximately 40,000 to 60,000 burn patients require admission to a hospital or major burn center for appropriate treatment.2 The devastating consequences of burns have been recognized by the medical community and significant amounts of resources and research have been dedicated, successfully improving these dismal statistics: Recent reports revealed a 50% decline in burn-related deaths and hospital admissions in the USA over the last 20 years; mainly due to effective prevention strategies, decreasing the number and severity of burns.3, 4 Advances in therapy strategies, based on improved understanding of resuscitation, enhanced wound coverage, more appropriate infection control, improved treatment of inhalation injury and better support of the hypermetabolic response to injury have further improved the clinical outcome of this unique patient population over the past years.5 However, severe burns remain a devastating injury affecting nearly every organ system and leading to significant morbidity and mortality.6 One of the main contributors to adverse outcome of this patient population represents its profound metabolic changes associated with insulin resistance and hyperglycemia.6, 7
Raised glucose levels were first linked to trauma around 150 years ago when Claude Bernard described a state of “diabète traumatique” during hemorrhagic shock.8 Ever since, multiple studies have documented hyperglycemia following burn, trauma, myocardial infarction, stroke or surgery.7, 9–12 Treatment was expectant until recently due to the belief that this phenomenon was a beneficial “fight or flight” response and should not be disturbed, as the risks, such as hypoglycemia, outweighed the benefits.13 Over the last years, however, multiple studies suggested that trauma-induced hyperglycemia may be of serious clinical concern as it has been frequently linked to impaired wound healing14, increased skin graft loss15, increased muscle protein catabolism16, increased incidence of infections17, 18 and mortality.7, 17–21 Thus, various studies have focused on elucidating potential treatment options in order to overcome insulin resistance-induced hyperglycemia in the acute period following trauma.11, 22
In order to provide clinical and pharmacological strategies to overcome insulin resistance post-burn, a general understanding of metabolic and molecular alterations underlying hyperglycemia in severely burned patients is of major importance. This review aims to discuss the mechanisms underlying insulin resistance induced hyperglycemia post-burn and outlines current therapeutic strategies that are being used to modulate hyperglycemia following thermal trauma.
Metabolic changes following severe burn injury
Severe burns covering more than 40% total body surface area (TBSA) are typically followed by a period of stress, inflammation and hypermetabolism, characterized by a hyperdynamic circulatory response with increased body temperature, glycolysis, proteolysis, lipolysis and futile substrate cycling.23–25 These responses are present in all trauma, surgical, or critically ill patients, but the severity, length and magnitude is unique for burn patients.6 Marked and sustained increases in catecholamine, glucocorticoid, glucagon and dopamine secretion are thought to initiate the cascade of events leading to the acute hypermetabolic response with its ensuing catabolic state.23, 26–33 The cause of this complex response is not well understood. However, interleukins 1 and 6, platelet-activating factor, tumor necrosis factor (TNF), endotoxin, neutrophil-adherence complexes, reactive oxygen species, nitric oxide and coagulation as well as complement cascades have also been implicated in regulating this response to burn injury.34 Once these cascades are initiated, their mediators and by-products appear to stimulate the persistent and increased metabolic rate associated with altered glucose metabolism seen after severe burn injury.35
Several studies have indicated that these metabolic phenomena post-burn occur in a timely manner, suggesting two distinct pattern of metabolic regulation following injury.36 The first phase occurs within the first 48 hours of injury and has classically been called the “ebb phase”36, 37, characterized by decreases in cardiac output, oxygen consumption, and metabolic rate as well as impaired glucose tolerance associated with its hyperglycemic state. These metabolic variables gradually increase within the first five days post-injury to a plateau phase (called the “flow” phase), characteristically associated with hyperdynamic circulation and the above mentioned hypermetabolic state. Insulin release during this time period was found to be twice that of controls in response to glucose load38, 39 and plasma glucose levels are markedly elevated, indicating the development of an insulin-resistance.39, 40 Current understanding has been that these metabolic alterations resolve soon after complete wound closure. However, recent studies found that the hypermetabolic response to burn injury may last for more than 12 months after the initial event.23, 26, 33, 41 We found in a recent study that sustained hypermetabolic alterations post-burn, indicated by persistent elevations of total urine cortisol levels, serum cytokines, catecholamines and basal energy requirements, were accompanied by impaired glucose metabolism and insulin sensitivity that persisted for up to three years after the initial burn injury.
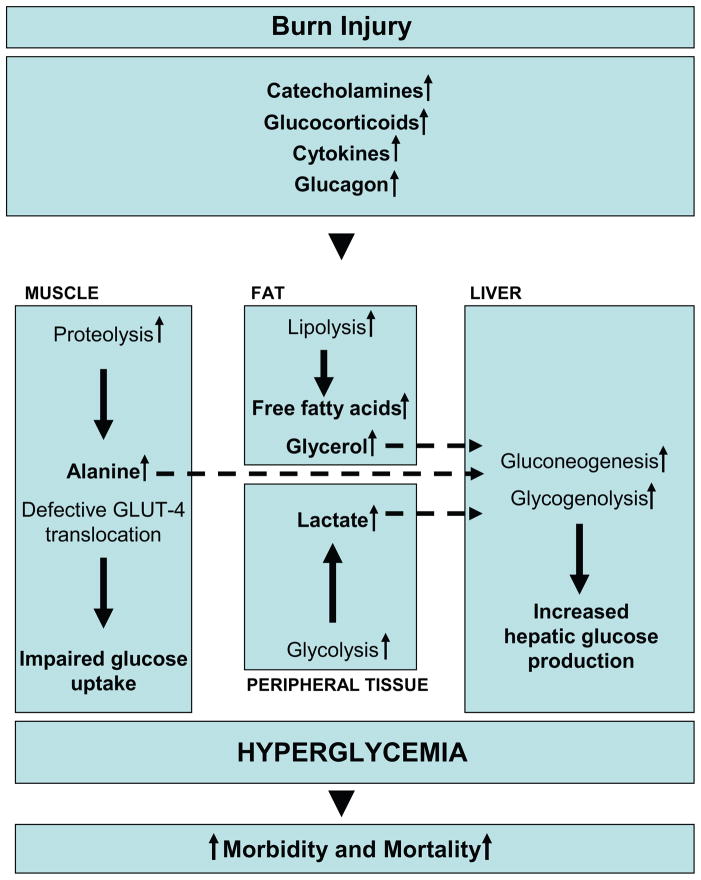
Glucose metabolism in healthy subjects is tightly regulated: under normal circumstances, a postprandial increase in blood glucose concentration stimulates release of insulin from pancreatic β-cells. Insulin mediates peripheral glucose uptake into skeletal muscle and adipose tissue and suppresses hepatic gluconeogenesis, thereby maintaining blood glucose homeostasis.42, 43 In critical illness, however metabolic alterations can cause significant changes in energy substrate metabolism. In order to provide glucose, a major fuel source to vital organs, release of the above mentioned stress mediators oppose the anabolic actions of insulin.44 By enhancing adipose tissue lipolysis45 and skeletal muscle proteolysis46, they increase gluconeogenic substrates, including glycerol, alanine and lactate, thus augmenting hepatic glucose production in burned patients (Figure 1).42, 43, 47 Hyperglycemia fails to suppress hepatic glucose release during this time48 and the suppressive effect of insulin on hepatic glucose release is attenuated, significantly contributing to post-trauma hyperglycemia.49 Catecholamine-mediated enhancement of hepatic glycogenolysis, as well as direct sympathetic stimulation of glycogen breakdown, can further aggravate the hyperglycemia in response to stress.43 Catecholamines have also been shown to impair glucose disposal via alterations of the insulin signaling pathway and GLUT-4 translocation muscle and adipose tissue, resulting in peripheral insulin resistance (Figure 1).42, 50 Cree and colleagues49 showed an impaired activation of Insulin Receptor Substrate-1 at its tyrosine binding site and an inhibition of AKT in muscle biopsies of children at seven days post-burn. Work of Wolfe and colleagues indicates links between impaired liver and muscle mitochondrial oxidative function, altered rates of lipolysis, and impaired insulin signaling post-burn attenuating both the suppressive actions of insulin on hepatic glucose production and on the stimulation of muscle glucose uptake.39, 45, 48, 49 Another counter-regulatory hormone of interest during stress of the critically ill is glucagon. Glucagon, like epinephrine, leads to increased glucose production through both gluconeogenesis and glycogenolysis.51 The action of glucagons alone is not maintained over time; however, its action on gluconeogenesis is sustained in an additive manner with the presence of epinephrine, cortisol, and growth hormone.44, 51 Likewise, epinephrine and glucagon have an additive effect on glycogenolysis.51 Recent studies found that pro-inflammatory cytokines contribute indirectly to post-burn hyperglycemia via enhancing the release of the above mentioned stress hormones.52–54 Other groups showed that inflammatory cytokines, including tumor necrosis factor (TNF), interleukin (IL) -6 and monocyte chemotactic protein (MCP) -1 also act via direct effects on the insulin signal transduction pathway through modification of signaling properties of insulin receptor substrates, contributing to post-burn hyperglycemia via liver and skeletal muscle insulin resistance.55–57 Alterations in metabolic pathways as well as pro-inflammatory cytokines, such as TNF, have also been implicated in significantly contributing to lean muscle protein breakdown, both during the acute and convalescent phases in response to burn injury.58, 59 In contrast to starvation, in which lipolysis and ketosis provide energy and protect muscle reserves, burn injury considerably reduces the ability of the body to utilize fat as an energy source.
Figure 1. Metabolic changes underlying insulin resistance post-burn.
Marked and sustained increases in catecholamine, glucocorticoid, glucagon and cytokine secretion are thought to initiate the cascade of events leading to the acute hypermetabolic response to severe burn injury and oppose the anabolic effects of insulin. By enhancing adipose tissue lipolysis and skeletal muscle proteolysis, they increase gluconeogenic substrates, including glycerol, alanine and lactate, thus augmenting hepatic glucose production in burned patients. Catecholamine-mediated augmentation of hepatic glycogenolysis, as well as direct sympathetic stimulation of glycogen breakdown, further aggravates the hyperglycemia in response to stress. Catecholamines and cytokines, such as IL-1, IL-6, MCP-1 and TNF, have also been shown to impair glucose disposal via alterations of the insulin signaling pathway and GLUT-4 translocation, resulting in peripheral insulin resistance.
Skeletal muscle is thus the major source of fuel in the burned patient, which leads to marked wasting of lean body mass (LBM) within days after injury.6, 60 This muscle breakdown has been demonstrated with whole body and cross leg nitrogen balance studies in which pronounced negative nitrogen balances persisted for 6 and 9 months after injury.61 Since skeletal muscle has been shown to be responsible for 70–80% of whole body insulin-stimulated glucose uptake, decreases in muscle mass may significantly contribute to this persistent insulin resistance post-burn.62 The correlation between hyperglycemia and muscle protein catabolism has been also supported by Flakoll and others63 in which an isotopic tracer of leucine was utilized to index whole-body protein flux in normal volunteers. The group showed a significant increase in proteolysis rates occurring without any alteration in either leucine oxidation or non-oxidative disposal (an estimate of protein synthesis), suggesting an hyperglycemia induced increase in protein breakdown. Flakoll and others63 further demonstrated that elevations of plasma glucose levels resulted in a marked stimulation of whole body proteolysis during hyperinsulinemia. A 10–15% loss in lean body mass has been shown to be associated with significant increases in infection rate and marked delays in wound healing.64 The resultant muscle weakness was further shown to prolong mechanical ventilatory requirements, inhibit sufficient cough reflexes and delay mobilization in protein-malnourished patients, thus markedly contributing to the incidence of mortality in these patients.65 Persistent protein catabolism may also account for delay in growth frequently observed in our pediatric patient population for up to 2 years post-burn.66
In the past years, therapeutic approaches have therefore mainly focused on reversing the hypermetabolic response with its ensuing catabolic state post-burn using a large number of different strategies.
Attenuation of the hypermetabolic response to burn injury
Early excision and closure of the burn wound has been probably the single greatest advancement in the treating patients with severe thermal injuries during the last twenty years; leading to substantially reduced resting energy requirements and subsequent improvement of mortality rates in this particular patient population.67–71 Pharmacological strategies, including growth hormone72, insulin-like growth factor (IGF)-173, oxandrolone74, testosterone75, propranolol76–78 and insulin79, 80 have been successfully utilized in order to attenuate the hypermetabolic response to burn injury. Although effectively improving muscle protein kinetics, maintain muscular growth and decrease donor site healing time81, improving protein metabolism, immune function and attenuating muscle catabolism in catabolic patients82, 83 and improving lean body mass in burned patients84, 85, growth hormone, IGF-1, oxandrolone and testosterone have not been shown to attenuate impaired insulin sensitivity post-burn. In contrast, daily injections of recombinant growth hormone significantly contributed to elevated blood glucose levels post-injury.86 Beta-adrenergic blockade with propranolol represents probably the most efficacious anti-catabolic therapy in the treatment of burns. Long-term use of propranolol during acute care in burn patients, at a dose titrated to reduce heart rate by 15 to 20%, was noted to diminish cardiac work.87 Stable isotope and serial body composition studies showed that administration of propranolol reduces skeletal muscle wasting and increases lean body mass post-burn.76, 88 The underlying mechanism of action of propranolol is still unclear, however, its effect appears to occur due to an increased protein synthesis in the face of a persistent protein breakdown and reduced peripheral lipolysis.78 Recent data suggests that administration of propranolol given at 4 mg/kg BW/q24 also markedly decreased the amount of insulin necessary to decrease elevated glucose level post-burn (unpublished data). Propranolol may thus constitute a promising approach to overcome post-burn insulin resistance. However, future studies are warranted to specifically elucidate its effects and the underlying mechanisms on insulin resistance induced hyperglycemia following thermal injury. The use of ketoconazole, an anti-fungal agent which suppresses the production of cortisol as a side-effect, may constitute another indirect approach to attenuate stress-induced hyperglycemia.
Since trauma-induced hyperglycemia has been suggested in multiple studies to significantly contribute to adverse outcome of critically ill patients, current clinical research has focused on more aggressive maneuvers to normalize plasma glucose in critically injured patients.
Attenuation of hyperglycemia post-burn
Insulin represents probably one of the most extensively studied therapeutic agents and novel therapeutic applications are constantly being found. Besides its ability to decrease blood glucose via mediating peripheral glucose uptake into skeletal muscle and adipose tissue and suppressing hepatic gluconeogenesis, insulin is known to increase DNA replication and protein synthesis via control of amino acid uptake, increase fatty acid synthesis and decreased proteinolysis.13 The latter makes insulin particular attractive for the treatment of hyperglycemia in severely burned patients since insulin given during acute hospitalization has been shown to improve muscle protein synthesis, accelerate donor site healing time, and attenuate lean body mass loss and the acute phase response.80, 89–95 In addition to its anabolic actions, insulin was shown to exert totally unexpected anti-inflammatory effects potentially neutralizing the pro-inflammatory actions of glucose.92, 93 Experiments demonstrating the anti-inflammatory effects of insulin were first performed, in vitro, showing decreased expression of the pro-inflammatory intracellular adhesion molecule (ICAM)-1, chemokine, monocyte chemoattractant protein-1 (MCP-1) and key pro-inflammatory transcription factor, nuclear factor-kappa B (NF-κB) in human aortic endothelial cells after insulin treatment.96, 97 Dandona and colleagues98 then demonstrated that insulin infusions given at a low dose (2 units per hour) to obese subjects suppressed reactive oxygen species (ROS) generation, P47phox expression (an indicator of NADPH oxidase action, the enzyme which generates the superoxide radical), NF-κB binding and increased inhibitor kappa B (IκB)α expression by mononuclear cells. Studies by our group indicated that insulin may restore systemic homeostasis and reduce the drive of the hypermetabolic response in severely burned patients by attenuating the inflammatory response via decreasing pro-inflammatory and increasing the anti-inflammatory cascade.93 A study in pediatric patients using intensive insulin therapy in order to maintain glucose levels between 90 and 120 mg/dl reduced infection rates and improved survival.99 Other studies indicated that insulin given to burn children may reduce increases in C-reactive protein, IL-1β and TNF levels after injury in the absence of normoglycemia.92, 100 These results suggest a dual benefit of insulin administration: reduction of pro-inflammatory effects of glucose by restoration of euglycemia and a proposed additional insulin-mediated anti-inflammatory effect.101 van den Berghe and colleagues11 confirmed the beneficial effects of insulin in large recent milestone study. Insulin administered to maintain glucose at levels below 110 mg/dl decreased mortality, incidence of infections, sepsis and sepsis-associated multi-organ failure in surgically critically ill patients. The same group investigated the effects of insulin in medical ICU patients in an “intent to treat” study.102 Intensive insulin therapy significantly reduced newly acquired kidney injury, accelerated weaning from mechanical ventilation, and accelerated discharge from the ICU and the hospital. The authors further showed that insulin given during the acute phase not only improved acute hospital outcomes but also improved long-term rehabilitation and social reintegration of critically ill patients over a period of 1 year, indicating the advantage of insulin therapy.22, 103 In surgical critically ill patients, the risk of death seemed to be linearly correlated with the degree of hyperglycemia, with no clear cut-off level below which there was no further benefit.104 However, since strict blood glucose control in order to maintain normoglycemia was required to obtain the most clinical benefit, a dialogue has emerged between those who believe that tight glucose control is beneficial for patient outcome and others who fear that high doses of insulin may lead to increased risks for hypoglycemic events and its associated consequences in these patients.11 In fact, a recent multi-center trial in Europe (Efficacy of Volume Substitution and Insulin Therapy in Severe Sepsis [VISEP]) investigated the effects of insulin administration on morbidity and mortality in patients with severe infections and sepsis.105 The authors found that insulin administration did not affect mortality but the rate of severe hypoglycemia was 4-fold higher in patients receiving intensive insulin therapy when compared to the conventional therapy group.105 Another large multi-center study examined the use of a continuous hyperinsulinemic, euglycemic clamp throughout ICU stay and found a dramatic increase in serious hypoglycemic episodes.106 The ideal target glucose range therefore has not been found and several groups are currently undertaking clinical trials in order to define ideal glucose levels for the treatment of ICU and burned patients: A study by Finney and colleagues107 suggests glucose levels of 140 mg/dl and below, while the Surviving Sepsis Campaign recommend to maintain glucose levels below 150 mg/dl.108 However, maintaining a continuous hyperinsulinemic, euglycemic clamp in burn patients is particularly difficult since these patients are being continuously fed large caloric loads via enteral feeding tubes in an attempt to maintain euglycemia. Since burn patients require weekly operations and daily dressing changes, enteral nutrition needs occasionally to be stopped, which may lead to disruption of gastrointestinal motility and increased risk of hypoglycemia.6
Based on the discussion about ideal target glucose ranges clinical research is currently investigating alternative strategies in order to attenuate trauma-related hyperglycemia utilizing other glucose lowering drugs that do not cause hypoglycemia as frequently as insulin.
Metformin (Glucophage), a biguanide, has recently been suggested as an alternative means to correct hyperglycemia in severely injured patients.109 By inhibiting gluconeogenesis and augmenting peripheral insulin sensitivity, metformin directly counters the two main metabolic processes which underlie injury-induced hyperglycemia.110–112 In addition, metformin has been rarely associated with hypoglycemic events, thus possibly eliminating this concern associated with the use of exogenous insulin.113 Experience with metformin in burn patients is limited. In a small randomized study reported by Gore and colleagues metformin reduced plasma glucose concentration, decreased endogenous glucose production and accelerated glucose clearance in severely burned.109 A follow-up study looking at the effects of metformin on muscle protein synthesis, confirmed these observations and demonstrated an increased fractional synthetic rate of muscle protein and improvement in net muscle protein balance in metformin treated patients.112 Metformin may thus, analogous to insulin, have efficacy in critically injured patients as both, an antihyperglycemic and muscle protein anabolic agent. However, the mechanisms by which insulin may improve morbidity and mortality remain unclear. Are these effects due to insulin itself or due to glucose modulation?
Despite the advantages and potential therapeutic uses, treatment with metformin, or other biguanides, has been associated with lactic acidosis.113, 114 Concerns about lactic acidosis associated with biguanide use delayed the introduction of metformin into the U.S. market until May of 1995. This concern prompted prescribing guidelines that identify clinical conditions associated with this complication.115 To avoid metformin-associated lactic acidosis, the use of this medication is contraindicated in certain diseases or illnesses in which there is a potential for impaired lactate elimination (hepatic or renal failure) or tissue hypoxia. However, several reports have questioned the causal relationship between metformin and lactic acidosis.115–117 A study by Brown and colleagues suggested that the rate of lactic acidosis among type two diabetics had not changed since the introduction of metformin to the United States.116 A meta-analysis of trials and cohort studies evaluating the use of metformin did not report any cases of lactic acidosis in 194 trials with 36,893 patient-years of metformin treatment.118 Studies within this analysis that measured blood lactate levels, did not demonstrate significant differences between patients taking metformin and those taking a placebo or a nonbiguanide intervention.118 Nevertheless, as demonstrated in a recent case report by Riesenman and colleagues115, metformin should be used with caution in subacute burn patients. Since experience with the use of metformin in severely burn patients is limited a large prospective study may be warranted to define the safety and appropriate use of this drug in this patient population.
Other ongoing trials in order to decrease post-burn hyperglycemia include the use of Glucagon-Like-Peptide (GLP)-1 and PPAR-γ agonists (e.g., pioglitazone, thioglitazones) or the combination of various anti-diabetic drugs. PPAR-γ agonists, such as fenofibrate, have been shown to improve insulin sensitivity in patients with diabetes. Cree and colleagues found in a recent double-blind, prospective, placebo-controlled randomized trial that fenofibrate treatment significantly decreased plasma glucose significantly decreased plasma glucose concentrations by improving insulin sensitivity and mitochondrial glucose oxidation.49 Fenofibrate also led to significantly increased tyrosine phosphorylation of the insulin receptor (IR) and IRS-1 in muscle tissue after hyperinsulinemic-euglycemic clamp when compared to placebo treated patients, indicating improved insulin receptor signaling.49 Although further studies are warranted to determine the efficiency and applicability of this drug, understanding of the molecular mechanism underlying insulin resistance post-burn may lead to novel therapeutic options.
Molecular mechanisms leading to insulin resistance post-burn
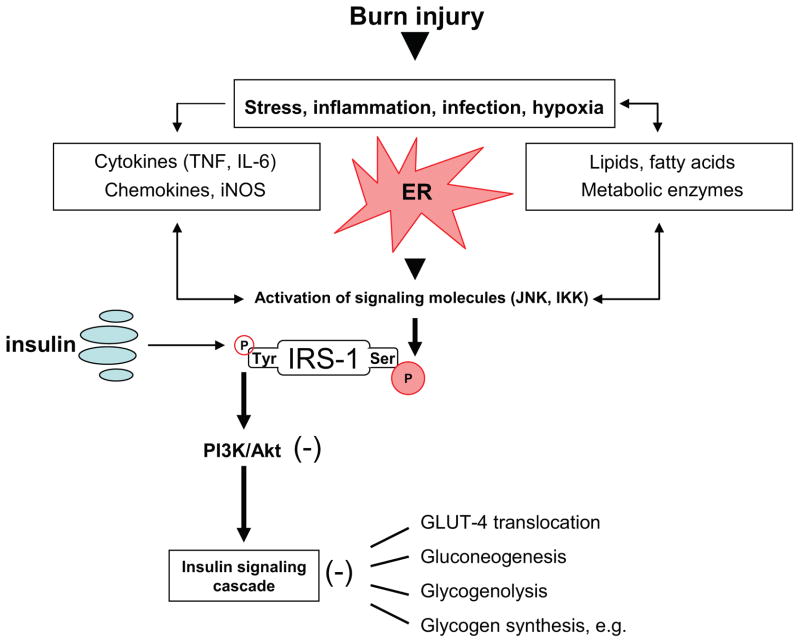
Effects of insulin in order to maintain normoglycemia occur through the insulin signaling cascade.119 Upon binding to the α-subunit on the extracellular portion of its receptor, insulin induces auto-phosphorylation of the β-unit leading to conformational changes and phosphorylation of insulin receptor substrate (IRS)-1 at a critical tyrosine residue, which in turn leads to activation of the phosphatidylinositol-3 kinase (PI3K)/Akt pathway (Figure 2).120, 121 Several studies indicate the major role of PI3 kinase in the regulation of metabolic actions of insulin signaling, including stimulation of glucose transport via phosphorylation of Akt and the resultant plasma membrane localization of the GLUT4 glucose transporter within hepatocytes, skeletal muscle and adipose tissue122–124, as well as stimulation of protein synthesis through activation of the protein kinase mTOR (mammalian target of rapamycin).125 Activation of AKT has also been shown to be of major importance for the regulation of hepatic glucose homeostasis via insulin signaling cascades, including inhibition of gluconeogenesis and glycogenolysis pathways via activation or inactivation of several key enzymes.126 Other effects include activation of glycogen synthesis, lipid synthesis, inhibition of lipolysis and lipocyte apoptosis.125, 127–129 Recent work now suggests that stress-induced insulin resistance may in part be due to phosphorylation-based negative-feedback, which may uncouple the insulin receptor or insulin receptor-associated proteins from its downstream signaling pathways, altering insulin action.121 Epinephrine for example seems to exerts its effects on peripheral insulin resistance via this mechanism.50 Specifically, phosphorylation of IRS-1 at serine residues by various kinases may preclude its tyrosine phosphorylation by the insulin receptor tyrosine kinase, thus inhibiting insulin receptor trafficking (Figure 2).130, 131 Among these IRS-modifying enzymes, mounting evidence indicates that activation of c-Jun N-terminal kinase (JNK) and inhibitor of NF-κB kinase-β, (IKK) may be central in mediating insulin resistance in response to various stresses that occur in obesity and other conditions of insulin resistance (Figure 2).124 Both have been found to inhibit insulin action by serine phosphorylation of IRS-1131, 132; even though the activity of IKK in this regard has not yet been well established under physiological conditions. Several studies found JNK to be activated upon specific stimuli, including presence of various cytokines, such as IL-6, IL-8, MCP-1 and TNF-α, and internal cues, including endoplasmic reticulum stress, all of which are present under conditions leading to hyperglycemia, such as obesity, diabetes mellitus and stress.131, 133–135 It has been well established that a variety of cellular stress signaling and inflammatory pathways are activated as a consequence of burn. A key player in the cellular stress response is the endoplasmic reticulum (ER), a membranous organelle that functions in the synthesis and processing of secretory and membrane proteins.136 Certain pathological stress conditions disrupt ER homeostasis and lead to accumulation of unfolded or misfolded proteins in the ER lumen.136–138 The ER stress response limits unfolded protein burden in the ER lumen by inhibiting translation and inducing the nuclear transcription of additional chaperone proteins. If the unfolding protein burden can not be reversed, apoptotic cell death ensues. To cope with this stress, cells activate a signal transduction system linking the ER lumen with the cytoplasm and nucleus, called the unfolded protein response (UPR).137, 138 ER stress is detected by transmembrane proteins which monitor the load of unfolded proteins in the ER lumen, and transmit this signal to the cytosol.136 Two of these proteins, inositol requiring enzyme-1 and PKR-like ER kinase, undergo oligomerization and phosphorylation in response to increased ER stress.136 Work in our laboratory has recently demonstrated increased phosphorylation of IRE-1 and PERK in rat livers isolated 24 and 72 hours after burn injury, indicating activation of ER-stress signaling pathways post-burn. We also found IRE-1 to be activated for up to 60 days after the initial burn injury in muscle samples of pediatric patients (Jeschke and colleagues, unpublished data).
Figure 2. Molecular mechanisms underlying insulin resistance following thermal injury.
Activation of JNK or IKK by cytokine signaling or lipid products during ER stress may lead to phosphorylation of IRS-1 at serine residues which may preclude its tyrosine phosphorylation by the insulin receptor tyrosine kinase, thus resulting in impaired PI3K/Akt signaling and insulin resistance with its associated consequences.
Notably, activity of JNK has been linked to IRE-1 and PERK activity during ER stress.139 Consistent with this phenomenon, we found total JNK activity, indicated by c-Jun phosphorylation, to be markedly elevated upon burn injury, associated with increased glucose and insulin levels. Inhibition of JNK activity in obese mice with the synthetic inhibitor SP600125 was recently found to reverse ER stress-induced serine phosphorylation of IRS-1.135, 140 Activation status of c-Jun N-terminal kinase may thus represent a central and integrating mechanism linking ER-stress and intracellular glucose homeostasis, since serine phosphorylation of IRS-1 has been shown to impair insulin receptor signaling.141
Indeed, interventions in order to block JNK activity in established models of obesity and diabetes improved systemic glucose homeostasis and insulin sensitivity, as well as atherosclerosis, suggesting that JNK inhibition might be one promising therapeutic avenue for diabetes.142–144 We are currently utilizing orally active small-molecule chemical chaperones in order to attenuate ER stress by increasing cellular folding capacity. These chaperones have been previously shown to markedly alleviate obesity-induced ER stress and JNK activation, as well as treating insulin resistance and type 2 diabetes in mice.126, 145 They may represent another promising potential therapeutic approach, if this concept is applicable to humans. Cytokines and their receptors represent other obvious potential targets in order to attenuate insulin resistance post-trauma. However, even though there have been encouraging results using anti-TNF or anti-CCR2 (chemokine (C–C motif) receptor 2), the benefit of targeting cytokine or signaling receptor is likely to be limited.134, 146 Thus, tackling a more central locus rather than targeting single molecules may proof valuable for the development of novel and effective therapeutics, in order to overcome insulin-resistance post-burn.
However, it remains to be determined whether serine/threonine phosphorylation of IRS-1 or IRS-2 can largely account for the insulin-desensitizing effects of JNK, IKK or ER stress on insulin resistance in vivo. Rather, a role of serine/threonine phosphorylation of IRS-1 in insulin resistance could be more complex.
Inducible nitric oxide synthase (iNOS), a mediator of inflammation and a variety of pathophysiological processes, has emerged as an important player in insulin resistance.147 iNOS was originally identified in activated macrophages148, but is also expressed in various tissues, including skeletal muscle, liver, and adipose tissue, even under a normal, unstressed condition.149 The IKK-NF-κB pathway 150 and JNK 151 are major upregulators of iNOS expression. Increased nitrosative stress, particularly protein S-nitrosylation, has been proposed to be involved in the pathogenesis of iNOS-mediated insulin resistance by impairing intracellular insulin signaling.149 However, molecular mechanisms by which iNOS mediates insulin resistance remain largely unknown, although iNOS has been shown to impair insulin signaling at multiple levels.149 Work of Perreault et al.152, for example, indicated that iNOS disruption reversed high-fat diet–induced impaired insulin-stimulated tyrosine phosphorylation of IR and IRS-1, IRS-1–associated PI3K activity, and phosphorylation of Akt/PKB in skeletal muscle. Mounting evidence suggests that iNOS may function as both a downstream effector and an upstream amplifier of sustained activation of inflammatory/stress-signaling pathways, forming a vicious cycle, which causes and/or exacerbates insulin resistance.149 Interestingly, insulin sensitizers, such as thiazolidinediones and metformin have been recently found to suppress iNOS expression in cultured cells as well as in diabetic rodents via activating AMP-activated protein kinase (AMPK), thus leading to improved insulin sensitivity.153, 154 These results may place the use of drugs like metformin or thiazolidinediones in a different light and may help to further elucidate the molecular mechanisms underlying insulin resistance post-burn.
Conclusion
The profound metabolic alterations post-burn associated with persistent changes in glucose metabolism and impaired insulin sensitivity significantly contribute to adverse outcome of this patient population. Even though advances in therapy strategies in order to attenuate the hypermetabolic response to burn have significantly improved the clinical outcome of these patients over the past years, therapeutic approaches to overcome stress-induced hyperglycemia have remained challenging. Maintaining blood glucose at levels below 110 mg/dl using intensive insulin therapy has been shown to reduce mortality and morbidity in critically ill patients, however, associated hypoglycemic events have led to the investigation of alternative strategies, including the use of metformin and the PPAR-γ agonist fenofibrate. Nevertheless, further studies are warranted to determine ideal glucose ranges and the safety and appropriate use of these drugs in severely burned patients. Aside from the discussion about the ideal target glucose range, we currently lack understanding by which mechanisms insulin administration may improve morbidity and mortality in severely burned patients. Are these effects due to insulin itself or due to glucose modulation? Better understanding of the molecular mechanisms underlying insulin resistance post-burn may help solving this question and may lead to the development of novel therapeutic options in order to treat stress-induced hyperglycemia thus further improving the prognosis of this unique patient population.
Acknowledgments
This study was supported by grants from Shriners Hospitals for Children (8660, 8760, 9145 and 8640), National Institutes of Health (R01-GM56687, T32 GM008256, and P50 GM60338), NIDRR (H133A020102), and American Surgical Association Foundation.
References
- 1.Guidelines for the operation of burn centers. J Burn Care Res. 2007;28:134–41. doi: 10.1097/BCR.0b013e31802c8861. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen TT, Gilpin DA, Meyer NA, et al. Current treatment of severely burned patients. Ann Surg. 1996;223:14–25. doi: 10.1097/00000658-199601000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brigham PA, McLoughlin E. Burn incidence and medical care use in the United States: estimates, trends, and data sources. J Burn Care Rehabil. 1996;17:95–107. doi: 10.1097/00004630-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Wolf SE. Critical care in the severely burned: organ support and management of complications. In: Herndon DN, editor. Total Burn Care. 3. London: Saunders Elsevier; 2007. pp. 454–76. [Google Scholar]
- 5.Herndon DN. Total Burn Care. 3. Philadelphia: Saunders Elsevier; 2007. [Google Scholar]
- 6.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363:1895–902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 7.McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001;17:107–24. doi: 10.1016/s0749-0704(05)70154-8. [DOI] [PubMed] [Google Scholar]
- 8.Bernard C. Leçons sur les phénomènes de la vie communs aux animaux et aux végétaux. Vol. 1. Paris, France: J.B. Baillière et Fils; 1878. [Google Scholar]
- 9.Kagansky N, Levy S, Knobler H. The role of hyperglycemia in acute stroke. Arch Neurol. 2001;58:1209–12. doi: 10.1001/archneur.58.8.1209. [DOI] [PubMed] [Google Scholar]
- 10.Mizock BA. Alterations in fuel metabolism in critical illness: hyperglycaemia. Best Pract Res Clin Endocrinol Metab. 2001;15:533–51. doi: 10.1053/beem.2001.0168. [DOI] [PubMed] [Google Scholar]
- 11.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 12.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–8. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 13.Pidcoke HF, Wade CE, Wolf SE. Insulin and the burned patient. Crit Care Med. 2007;35:S524–30. doi: 10.1097/01.CCM.0000278065.72486.31. [DOI] [PubMed] [Google Scholar]
- 14.McMurry JF., Jr Wound healing with diabetes mellitus. Better glucose control for better wound healing in diabetes. Surg Clin North Am. 1984;64:769–78. doi: 10.1016/s0039-6109(16)43393-1. [DOI] [PubMed] [Google Scholar]
- 15.Mowlavi A, Andrews K, Milner S, et al. The effects of hyperglycemia on skin graft survival in the burn patient. Ann Plast Surg. 2000;45:629–32. doi: 10.1097/00000637-200045060-00010. [DOI] [PubMed] [Google Scholar]
- 16.Gore DC, Chinkes DL, Hart DW, et al. Hyperglycemia exacerbates muscle protein catabolism in burn-injured patients. Crit Care Med. 2002;30:2438–42. doi: 10.1097/00003246-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Guvener M, Pasaoglu I, Demircin M, et al. Perioperative hyperglycemia is a strong correlate of postoperative infection in type II diabetic patients after coronary artery bypass grafting. Endocr J. 2002;49:531–7. doi: 10.1507/endocrj.49.531. [DOI] [PubMed] [Google Scholar]
- 18.Gore DC, Chinkes D, Heggers J, et al. Association of hyperglycemia with increased mortality after severe burn injury. J Trauma. 2001;51:540–4. doi: 10.1097/00005373-200109000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Thorell A, Efendic S, Gutniak M, et al. Development of postoperative insulin resistance is associated with the magnitude of operation. Eur J Surg. 1993;159:593–9. [PubMed] [Google Scholar]
- 20.Garcia-Avello A, Lorente JA, Cesar-Perez J, et al. Degree of hypercoagulability and hyperfibrinolysis is related to organ failure and prognosis after burn trauma. Thromb Res. 1998;89:59–64. doi: 10.1016/s0049-3848(97)00291-0. [DOI] [PubMed] [Google Scholar]
- 21.Christiansen C, Toft P, Jorgensen HS, et al. Hyperglycaemia and mortality in critically ill patients. A prospective study. Intensive Care Med. 2004;30:1685–8. doi: 10.1007/s00134-004-2325-2. [DOI] [PubMed] [Google Scholar]
- 22.Ingels C, Debaveye Y, Milants I, et al. Strict blood glucose control with insulin during intensive care after cardiac surgery: impact on 4-years survival, dependency on medical care, and quality-of-life. Eur Heart J. 2006;27:2716–24. doi: 10.1093/eurheartj/ehi855. [DOI] [PubMed] [Google Scholar]
- 23.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312–9. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 24.Reiss E, Pearson E, Artz CP. The metabolic response to burns. J Clin Invest. 1956;35:62–77. doi: 10.1172/JCI103253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu YM, Tompkins RG, Ryan CM, et al. The metabolic basis of the increase of the increase in energy expenditure in severely burned patients. JPEN J Parenter Enteral Nutr. 1999;23:160–8. doi: 10.1177/0148607199023003160. [DOI] [PubMed] [Google Scholar]
- 26.Mlcak RP, Jeschke MG, Barrow RE, et al. The influence of age and gender on resting energy expenditure in severely burned children. Ann Surg. 2006;244:121–30. doi: 10.1097/01.sla.0000217678.78472.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Przkora R, Barrow RE, Jeschke MG, et al. Body composition changes with time in pediatric burn patients. J Trauma. 2006;60:968–71. doi: 10.1097/01.ta.0000214580.27501.19. [DOI] [PubMed] [Google Scholar]
- 28.Dolecek R. Endocrine changes after burn trauma--a review. Keio J Med. 1989;38:262–76. doi: 10.2302/kjm.38.262. [DOI] [PubMed] [Google Scholar]
- 29.Jeffries MK, Vance ML. Growth hormone and cortisol secretion in patients with burn injury. J Burn Care Rehabil. 1992;13:391–5. doi: 10.1097/00004630-199207000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Klein GL, Bi LX, Sherrard DJ, et al. Evidence supporting a role of glucocorticoids in short-term bone loss in burned children. Osteoporos Int. 2004;15:468–74. doi: 10.1007/s00198-003-1572-3. [DOI] [PubMed] [Google Scholar]
- 31.Goodall M, Stone C, Haynes BW., Jr Urinary output of adrenaline and noradrenaline in severe thermal burns. Ann Surg. 1957;145:479–87. doi: 10.1097/00000658-195704000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coombes EJ, Batstone GF. Urine cortisol levels after burn injury. Burns Incl Therm Inj. 1982;8:333–7. doi: 10.1016/0305-4179(82)90033-x. [DOI] [PubMed] [Google Scholar]
- 33.Norbury WB, Herndon DN. Modulation of the hypermetabolic response after burn injury. In: Herndon DN, editor. Total Burn Care. 3. New York: Saunders Elsevier; 2007. pp. 420–33. [Google Scholar]
- 34.Sheridan RL. A great constitutional disturbance. N Engl J Med. 2001;345:1271–2. doi: 10.1056/NEJM200110253451710. [DOI] [PubMed] [Google Scholar]
- 35.Pereira C, Murphy K, Jeschke M, et al. Post burn muscle wasting and the effects of treatments. Int J Biochem Cell Biol. 2005;37:1948–61. doi: 10.1016/j.biocel.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Wolfe RR. Review: acute versus chronic response to burn injury. Circ Shock. 1981;8:105–15. [PubMed] [Google Scholar]
- 37.Cuthbertson DP, Angeles Valero Zanuy MA, Leon Sanz ML. Post-shock metabolic response 1942. Nutr Hosp. 2001;16:175–82. [PubMed] [Google Scholar]
- 38.Galster AD, Bier DM, Cryer PE, et al. Plasma palmitate turnover in subjects with thermal injury. J Trauma. 1984;24:938–45. doi: 10.1097/00005373-198411000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Cree MG, Aarsland A, Herndon DN, et al. Role of fat metabolism in burn trauma-induced skeletal muscle insulin resistance. Crit Care Med. 2007;35:S476–83. doi: 10.1097/01.CCM.0000278066.05354.53. [DOI] [PubMed] [Google Scholar]
- 40.Childs C, Heath DF, Little RA, et al. Glucose metabolism in children during the first day after burn injury. Arch Emerg Med. 1990;7:135–47. doi: 10.1136/emj.7.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeschke MG, Mlcak RP, Finnerty CC, et al. Burn size determines the inflammatory and hypermetabolic response. Crit Care. 2007;11:R90. doi: 10.1186/cc6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gearhart MM, Parbhoo SK. Hyperglycemia in the critically ill patient. AACN Clin Issues. 2006;17:50–5. doi: 10.1097/00044067-200601000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Robinson LE, van Soeren MH. Insulin resistance and hyperglycemia in critical illness: role of insulin in glycemic control. AACN Clin Issues. 2004;15:45–62. doi: 10.1097/00044067-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Khani S, Tayek JA. Cortisol increases gluconeogenesis in humans: its role in the metabolic syndrome. Clin Sci (Lond) 2001;101:739–47. doi: 10.1042/cs1010739. [DOI] [PubMed] [Google Scholar]
- 45.Wolfe RR, Herndon DN, Jahoor F, et al. Effect of severe burn injury on substrate cycling by glucose and fatty acids. N Engl J Med. 1987;317:403–8. doi: 10.1056/NEJM198708133170702. [DOI] [PubMed] [Google Scholar]
- 46.Gore DC, Jahoor F, Wolfe RR, et al. Acute response of human muscle protein to catabolic hormones. Ann Surg. 1993;218:679–84. doi: 10.1097/00000658-199321850-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carlson GL. Insulin resistance and glucose-induced thermogenesis in critical illness. Proc Nutr Soc. 2001;60:381–8. doi: 10.1079/pns200193. [DOI] [PubMed] [Google Scholar]
- 48.Wolfe RR, Durkot MJ, Allsop JR, et al. Glucose metabolism in severely burned patients. Metabolism. 1979;28:1031–9. doi: 10.1016/0026-0495(79)90007-6. [DOI] [PubMed] [Google Scholar]
- 49.Cree MG, Zwetsloot JJ, Herndon DN, et al. Insulin sensitivity and mitochondrial function are improved in children with burn injury during a randomized controlled trial of fenofibrate. Ann Surg. 2007;245:214–21. doi: 10.1097/01.sla.0000250409.51289.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hunt DG, Ivy JL. Epinephrine inhibits insulin-stimulated muscle glucose transport. J Appl Physiol. 2002;93:1638–43. doi: 10.1152/japplphysiol.00445.2002. [DOI] [PubMed] [Google Scholar]
- 51.Gustavson SM, Chu CA, Nishizawa M, et al. Interaction of glucagon and epinephrine in the control of hepatic glucose production in the conscious dog. Am J Physiol Endocrinol Metab. 2003;284:E695–707. doi: 10.1152/ajpendo.00308.2002. [DOI] [PubMed] [Google Scholar]
- 52.Mastorakos G, Chrousos GP, Weber JS. Recombinant interleukin-6 activates the hypothalamic-pituitary-adrenal axis in humans. J Clin Endocrinol Metab. 1993;77:1690–4. doi: 10.1210/jcem.77.6.8263159. [DOI] [PubMed] [Google Scholar]
- 53.Lang CH, Dobrescu C, Bagby GJ. Tumor necrosis factor impairs insulin action on peripheral glucose disposal and hepatic glucose output. Endocrinology. 1992;130:43–52. doi: 10.1210/endo.130.1.1727716. [DOI] [PubMed] [Google Scholar]
- 54.Akita S, Akino K, Ren SG, et al. Elevated circulating leukemia inhibitory factor in patients with extensive burns. J Burn Care Res. 2006;27:221–5. doi: 10.1097/01.BCR.0000197679.08671.A5. [DOI] [PubMed] [Google Scholar]
- 55.Fan J, Li YH, Wojnar MM, et al. Endotoxin-induced alterations in insulin-stimulated phosphorylation of insulin receptor, IRS-1, and MAP kinase in skeletal muscle. Shock. 1996;6:164–70. [PubMed] [Google Scholar]
- 56.del Aguila LF, Claffey KP, Kirwan JP. TNF-alpha impairs insulin signaling and insulin stimulation of glucose uptake in C2C12 muscle cells. Am J Physiol. 1999;276:E849–55. doi: 10.1152/ajpendo.1999.276.5.E849. [DOI] [PubMed] [Google Scholar]
- 57.Sell H, Dietze-Schroeder D, Kaiser U, et al. Monocyte chemotactic protein-1 is a potential player in the negative cross-talk between adipose tissue and skeletal muscle. Endocrinology. 2006;147:2458–67. doi: 10.1210/en.2005-0969. [DOI] [PubMed] [Google Scholar]
- 58.Baracos V, Rodemann HP, Dinarello CA, et al. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med. 1983;308:553–8. doi: 10.1056/NEJM198303103081002. [DOI] [PubMed] [Google Scholar]
- 59.Jahoor F, Desai M, Herndon DN, et al. Dynamics of the protein metabolic response to burn injury. Metabolism. 1988;37:330–7. doi: 10.1016/0026-0495(88)90132-1. [DOI] [PubMed] [Google Scholar]
- 60.Saffle JR, Graves C. Nutritional support of the burned patient. In: Herndon DN, editor. Total Burn Care. 3. London: Saunders Elsevier; 2007. pp. 398–419. [Google Scholar]
- 61.Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232:455–65. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DeFronzo RA, Jacot E, Jequier E, et al. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–7. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 63.Flakoll PJ, Hill JO, Abumrad NN. Acute hyperglycemia enhances proteolysis in normal man. Am J Physiol. 1993;265:E715–21. doi: 10.1152/ajpendo.1993.265.5.E715. [DOI] [PubMed] [Google Scholar]
- 64.McClave SA, Snider HL. Use of indirect calorimetry in clinical nutrition. Nutr Clin Pract. 1992;7:207–21. doi: 10.1177/0115426592007005207. [DOI] [PubMed] [Google Scholar]
- 65.Arora NS, Rochester DF. Respiratory muscle strength and maximal voluntary ventilation in undernourished patients. Am Rev Respir Dis. 1982;126:5–8. doi: 10.1164/arrd.1982.126.1.5. [DOI] [PubMed] [Google Scholar]
- 66.Rutan RL, Herndon DN. Growth delay in postburn pediatric patients. Arch Surg. 1990;125:392–5. doi: 10.1001/archsurg.1990.01410150114021. [DOI] [PubMed] [Google Scholar]
- 67.Atiyeh BS, Dham R, Kadry M, et al. Benefit-cost analysis of moist exposed burn ointment. Burns. 2002;28:659–63. doi: 10.1016/s0305-4179(02)00075-x. [DOI] [PubMed] [Google Scholar]
- 68.Lofts JA. Cost analysis of a major burn. N Z Med J. 1991;104:488–90. [PubMed] [Google Scholar]
- 69.Munster AM, Smith-Meek M, Sharkey P. The effect of early surgical intervention on mortality and cost-effectiveness in burn care, 1978–91. Burns. 1994;20:61–4. doi: 10.1016/0305-4179(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 70.Ramzy PI, Barret JP, Herndon DN. Thermal injury. Crit Care Clin. 1999;15:333–52. ix. doi: 10.1016/s0749-0704(05)70058-0. [DOI] [PubMed] [Google Scholar]
- 71.Chan BP, Kochevar IE, Redmond RW. Enhancement of porcine skin graft adherence using a light-activated process. J Surg Res. 2002;108:77–84. doi: 10.1006/jsre.2002.6516. [DOI] [PubMed] [Google Scholar]
- 72.Gore DC, Honeycutt D, Jahoor F, et al. Effect of exogenous growth hormone on whole-body and isolated-limb protein kinetics in burned patients. Arch Surg. 1991;126:38–43. doi: 10.1001/archsurg.1991.01410250042006. [DOI] [PubMed] [Google Scholar]
- 73.Debroy MA, Wolf SE, Zhang XJ, et al. Anabolic effects of insulin-like growth factor in combination with insulin-like growth factor binding protein-3 in severely burned adults. J Trauma. 1999;47:904–11. doi: 10.1097/00005373-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 74.Hart DW, Wolf SE, Ramzy PI, et al. Anabolic effects of oxandrolone after severe burn. Ann Surg. 2001;233:556–64. doi: 10.1097/00000658-200104000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferrando AA, Sheffield-Moore M, Wolf SE, et al. Testosterone administration in severe burns ameliorates muscle catabolism. Crit Care Med. 2001;29:1936–42. doi: 10.1097/00003246-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 76.Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–9. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 77.Pereira CT, Murphy KD, Herndon DN. Altering metabolism. J Burn Care Rehabil. 2005;26:194–9. [PubMed] [Google Scholar]
- 78.Pereira CT, Jeschke MG, Herndon DN. Beta-blockade in burns. Novartis Found Symp. 2007;280:238–51. doi: 10.1002/9780470059593.ch16. [DOI] [PubMed] [Google Scholar]
- 79.Sakurai Y, Aarsland A, Herndon DN, et al. Stimulation of muscle protein synthesis by long-term insulin infusion in severely burned patients. Ann Surg. 1995;222:283–94. 94–7. doi: 10.1097/00000658-199509000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ferrando AA, Chinkes DL, Wolf SE, et al. A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg. 1999;229:11–8. doi: 10.1097/00000658-199901000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Herndon DN, Barrow RE, Kunkel KR, et al. Effects of recombinant human growth hormone on donor-site healing in severely burned children. Ann Surg. 1990;212:424–31. doi: 10.1097/00000658-199010000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moller S, Jensen M, Svensson P, et al. Insulin-like growth factor 1 (IGF-1) in burn patients. Burns. 1991;17:279–81. doi: 10.1016/0305-4179(91)90039-j. [DOI] [PubMed] [Google Scholar]
- 83.Herndon DN, Ramzy PI, DebRoy MA, et al. Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg. 1999;229:713–22. doi: 10.1097/00000658-199905000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Demling RH, DeSanti L. Oxandrolone, an anabolic steroid, significantly increases the rate of weight gain in the recovery phase after major burns. J Trauma. 1997;43:47–51. doi: 10.1097/00005373-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 85.Wolf SE, Thomas SJ, Dasu MR, et al. Improved net protein balance, lean mass, and gene expression changes with oxandrolone treatment in the severely burned. Ann Surg. 2003;237:801–11. doi: 10.1097/01.SLA.0000071562.12637.3E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh KP, Prasad R, Chari PS, et al. Effect of growth hormone therapy in burn patients on conservative treatment. Burns. 1998;24:733–8. doi: 10.1016/s0305-4179(98)00113-2. [DOI] [PubMed] [Google Scholar]
- 87.Baron PW, Barrow RE, Pierre EJ, et al. Prolonged use of propranolol safely decreases cardiac work in burned children. J Burn Care Rehabil. 1997;18:223–7. doi: 10.1097/00004630-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 88.Gore DC, Honeycutt D, Jahoor F, et al. Propranolol diminishes extremity blood flow in burned patients. Ann Surg. 1991;213:568–74. doi: 10.1097/00000658-199106000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pierre EJ, Barrow RE, Hawkins HK, et al. Effects of insulin on wound healing. J Trauma. 1998;44:342–5. doi: 10.1097/00005373-199802000-00019. [DOI] [PubMed] [Google Scholar]
- 90.Thomas SJ, Morimoto K, Herndon DN, et al. The effect of prolonged euglycemic hyperinsulinemia on lean body mass after severe burn. Surgery. 2002;132:341–7. doi: 10.1067/msy.2002.126871. [DOI] [PubMed] [Google Scholar]
- 91.Zhang XJ, Chinkes DL, Wolf SE, et al. Insulin but not growth hormone stimulates protein anabolism in skin would and muscle. Am J Physiol. 1999;276:E712–E20. doi: 10.1152/ajpendo.1999.276.4.E712. [DOI] [PubMed] [Google Scholar]
- 92.Jeschke MG, Klein D, Bolder U, et al. Insulin attenuates the systemic inflammatory response in endotoxemic rats. Endocrinology. 2004;145:4084–93. doi: 10.1210/en.2004-0592. [DOI] [PubMed] [Google Scholar]
- 93.Jeschke MG, Klein D, Herndon DN. Insulin treatment improves the systemic inflammatory reaction to severe trauma. Ann Surg. 2004;239:553–60. doi: 10.1097/01.sla.0000118569.10289.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jeschke MG, Rensing H, Klein D, et al. Insulin prevents liver damage and preserves liver function in lipopolysaccharide-induced endotoxemic rats. J Hepatol. 2005;42:870–9. doi: 10.1016/j.jhep.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 95.Klein D, Schubert T, Horch RE, et al. Insulin treatment improves hepatic morphology and function through modulation of hepatic signals after severe trauma. Ann Surg. 2004;240:340–9. doi: 10.1097/01.sla.0000133353.57674.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aljada A, Saadeh R, Assian E, et al. Insulin inhibits the expression of intercellular adhesion molecule-1 by human aortic endothelial cells through stimulation of nitric oxide. J Clin Endocrinol Metab. 2000;85:2572–5. doi: 10.1210/jcem.85.7.6677. [DOI] [PubMed] [Google Scholar]
- 97.Aljada A, Dandona P. Effect of insulin on human aortic endothelial nitric oxide synthase. Metabolism. 2000;49:147–50. doi: 10.1016/s0026-0495(00)91039-4. [DOI] [PubMed] [Google Scholar]
- 98.Dandona P, Aljada A, Mohanty P, et al. Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab. 2001;86:3257–65. doi: 10.1210/jcem.86.7.7623. [DOI] [PubMed] [Google Scholar]
- 99.Pham TN, Warren AJ, Phan HH, et al. Impact of tight glycemic control in severely burned children. J Trauma. 2005;59:1148–54. doi: 10.1097/01.ta.0000188933.16637.68. [DOI] [PubMed] [Google Scholar]
- 100.Hansen TK, Thiel S, Wouters PJ, et al. Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J Clin Endocrinol Metab. 2003;88:1082–8. doi: 10.1210/jc.2002-021478. [DOI] [PubMed] [Google Scholar]
- 101.Dandona P, Chaudhuri A, Mohanty P, et al. Anti-inflammatory effects of insulin. Curr Opin Clin Nutr Metab Care. 2007;10:511–7. doi: 10.1097/MCO.0b013e3281e38774. [DOI] [PubMed] [Google Scholar]
- 102.Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–61. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 103.Ellger B, Debaveye Y, Vanhorebeek I, et al. Survival benefits of intensive insulin therapy in critical illness: impact of maintaining normoglycemia versus glycemia-independent actions of insulin. Diabetes. 2006;55:1096–105. doi: 10.2337/diabetes.55.04.06.db05-1434. [DOI] [PubMed] [Google Scholar]
- 104.Van den Berghe G, Wouters PJ, Bouillon R, et al. Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control. Crit Care Med. 2003;31:359–66. doi: 10.1097/01.CCM.0000045568.12881.10. [DOI] [PubMed] [Google Scholar]
- 105.Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–39. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 106.Langouche L, Vanhorebeek I, Van den Berghe G. Therapy insight: the effect of tight glycemic control in acute illness. Nat Clin Pract Endocrinol Metab. 2007;3:270–8. doi: 10.1038/ncpendmet0426. [DOI] [PubMed] [Google Scholar]
- 107.Finney SJ, Zekveld C, Elia A, et al. Glucose control and mortality in critically ill patients. JAMA. 2003;290:2041–7. doi: 10.1001/jama.290.15.2041. [DOI] [PubMed] [Google Scholar]
- 108.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 109.Gore DC, Wolf SE, Herndon DN, et al. Metformin blunts stress-induced hyperglycemia after thermal injury. J Trauma. 2003;54:555–61. doi: 10.1097/01.TA.0000026990.32856.58. [DOI] [PubMed] [Google Scholar]
- 110.DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med. 1995;333:541–9. doi: 10.1056/NEJM199508313330902. [DOI] [PubMed] [Google Scholar]
- 111.Stumvoll M, Nurjhan N, Perriello G, et al. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:550–4. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- 112.Gore DC, Herndon DN, Wolfe RR. Comparison of peripheral metabolic effects of insulin and metformin following severe burn injury. J Trauma. 2005;59:316–23. doi: 10.1097/01.ta.0000180387.34057.5a. [DOI] [PubMed] [Google Scholar]
- 113.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–9. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 114.Luft D, Schmulling RM, Eggstein M. Lactic acidosis in biguanide-treated diabetics: a review of 330 cases. Diabetologia. 1978;14:75–87. doi: 10.1007/BF01263444. [DOI] [PubMed] [Google Scholar]
- 115.Riesenman PJ, Braithwaite SS, Cairns BA. Metformin-associated lactic acidosis in a burn patient. J Burn Care Res. 2007;28:342–7. doi: 10.1097/BCR.0B013E318031A1FE. [DOI] [PubMed] [Google Scholar]
- 116.Brown JB, Pedula K, Barzilay J, et al. Lactic acidosis rates in type 2 diabetes. Diabetes Care. 1998;21:1659–63. doi: 10.2337/diacare.21.10.1659. [DOI] [PubMed] [Google Scholar]
- 117.Lalau JD, Lacroix C, Compagnon P, et al. Role of metformin accumulation in metformin-associated lactic acidosis. Diabetes Care. 1995;18:779–84. doi: 10.2337/diacare.18.6.779. [DOI] [PubMed] [Google Scholar]
- 118.Salpeter SR, Greyber E, Pasternak GA, et al. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2003;163:2594–602. doi: 10.1001/archinte.163.21.2594. [DOI] [PubMed] [Google Scholar]
- 119.White MF. The insulin signalling system and the IRS proteins. Diabetologia. 1997;40 (Suppl 2):S2–17. doi: 10.1007/s001250051387. [DOI] [PubMed] [Google Scholar]
- 120.Kahn CR, White MF, Shoelson SE, et al. The insulin receptor and its substrate: molecular determinants of early events in insulin action. Recent Prog Horm Res. 1993;48:291–339. doi: 10.1016/b978-0-12-571148-7.50015-4. [DOI] [PubMed] [Google Scholar]
- 121.Le Roith D, Zick Y. Recent advances in our understanding of insulin action and insulin resistance. Diabetes Care. 2001;24:588–97. doi: 10.2337/diacare.24.3.588. [DOI] [PubMed] [Google Scholar]
- 122.Ruderman NB, Kapeller R, White MF, et al. Activation of phosphatidylinositol 3–kinase by insulin. Proc Natl Acad Sci U S A. 1990;87:1411–5. doi: 10.1073/pnas.87.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Folli F, Saad MJ, Backer JM, et al. Insulin stimulation of phosphatidylinositol 3-kinase activity and association with insulin receptor substrate 1 in liver and muscle of the intact rat. J Biol Chem. 1992;267:22171–7. [PubMed] [Google Scholar]
- 124.Hotamisligil GS, Budavari A, Murray D, et al. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J Clin Invest. 1994;94:1543–9. doi: 10.1172/JCI117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Saltiel AR, Pessin JE. Insulin signaling in microdomains of the plasma membrane. Traffic. 2003;4:711–6. doi: 10.1034/j.1600-0854.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 126.Yu XX, Pandey SK, Booten SL, et al. Reduced adiposity and improved insulin sensitivity in obese mice with antisense suppression of 4E-BP2 expression. Am J Physiol Endocrinol Metab. 2008;294:E530–9. doi: 10.1152/ajpendo.00350.2007. [DOI] [PubMed] [Google Scholar]
- 127.Ikezu T, Okamoto T, Yonezawa K, et al. Analysis of thermal injury-induced insulin resistance in rodents. Implication of postreceptor mechanisms. J Biol Chem. 1997;272:25289–95. doi: 10.1074/jbc.272.40.25289. [DOI] [PubMed] [Google Scholar]
- 128.Chang L, Chiang SH, Saltiel AR. Insulin signaling and the regulation of glucose transport. Mol Med. 2004;10:65–71. doi: 10.2119/2005-00029.Saltiel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thurmond DC, Pessin JE. Molecular machinery involved in the insulin-regulated fusion of GLUT4-containing vesicles with the plasma membrane (review) Mol Membr Biol. 2001;18:237–45. doi: 10.1080/09687680110082400. [DOI] [PubMed] [Google Scholar]
- 130.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 131.Aguirre V, Uchida T, Yenush L, et al. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–54. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 132.Gao Z, Hwang D, Bataille F, et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277:48115–21. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 133.Gauglitz GG, Song J, Herndon DN, et al. Characterization of the inflammatory response during acute and postacute phases after severe burn. Shock. 2008 Apr 3; doi: 10.1097/SHK.0b013e31816e3373. epub ahead of print. [DOI] [PMC free article] [PubMed]
- 134.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 136.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 137.Hampton RY. ER stress response: getting the UPR hand on misfolded proteins. Curr Biol. 2000;10:R518–21. doi: 10.1016/s0960-9822(00)00583-2. [DOI] [PubMed] [Google Scholar]
- 138.Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–4. doi: 10.1016/s0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- 139.Urano F, Wang X, Bertolotti A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–6. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 140.Bennett BL, Sasaki DT, Murray BW, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–6. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 142.Ricci R, Sumara G, Sumara I, et al. Requirement of JNK2 for scavenger receptor A-mediated foam cell formation in atherogenesis. Science. 2004;306:1558–61. doi: 10.1126/science.1101909. [DOI] [PubMed] [Google Scholar]
- 143.Kaneto H, Nakatani Y, Miyatsuka T, et al. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med. 2004;10:1128–32. doi: 10.1038/nm1111. [DOI] [PubMed] [Google Scholar]
- 144.Liu G, Rondinone CM. JNK: bridging the insulin signaling and inflammatory pathway. Curr Opin Investig Drugs. 2005;6:979–87. [PubMed] [Google Scholar]
- 145.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sugita H, Fujimoto M, Yasukawa T, et al. Inducible nitric-oxide synthase and NO donor induce insulin receptor substrate-1 degradation in skeletal muscle cells. J Biol Chem. 2005;280:14203–11. doi: 10.1074/jbc.M411226200. [DOI] [PubMed] [Google Scholar]
- 148.Xie QW, Cho HJ, Calaycay J, et al. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–8. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- 149.Kaneki M, Shimizu N, Yamada D, et al. Nitrosative stress and pathogenesis of insulin resistance. Antioxid Redox Signal. 2007;9:319–29. doi: 10.1089/ars.2006.1464. [DOI] [PubMed] [Google Scholar]
- 150.Krishnegowda G, Hajjar AM, Zhu J, et al. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem. 2005;280:8606–16. doi: 10.1074/jbc.M413541200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pawate S, Bhat NR. C-Jun N-terminal kinase (JNK) regulation of iNOS expression in glial cells: predominant role of JNK1 isoform. Antioxid Redox Signal. 2006;8:903–9. doi: 10.1089/ars.2006.8.903. [DOI] [PubMed] [Google Scholar]
- 152.Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat Med. 2001;7:1138–43. doi: 10.1038/nm1001-1138. [DOI] [PubMed] [Google Scholar]
- 153.Carvalho-Filho MA, Ueno M, Hirabara SM, et al. S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: a novel mechanism of insulin resistance. Diabetes. 2005;54:959–67. doi: 10.2337/diabetes.54.4.959. [DOI] [PubMed] [Google Scholar]
- 154.Pilon G, Dallaire P, Marette A. Inhibition of inducible nitric-oxide synthase by activators of AMP-activated protein kinase: a new mechanism of action of insulin-sensitizing drugs. J Biol Chem. 2004;279:20767–74. doi: 10.1074/jbc.M401390200. [DOI] [PubMed] [Google Scholar]