Abstract
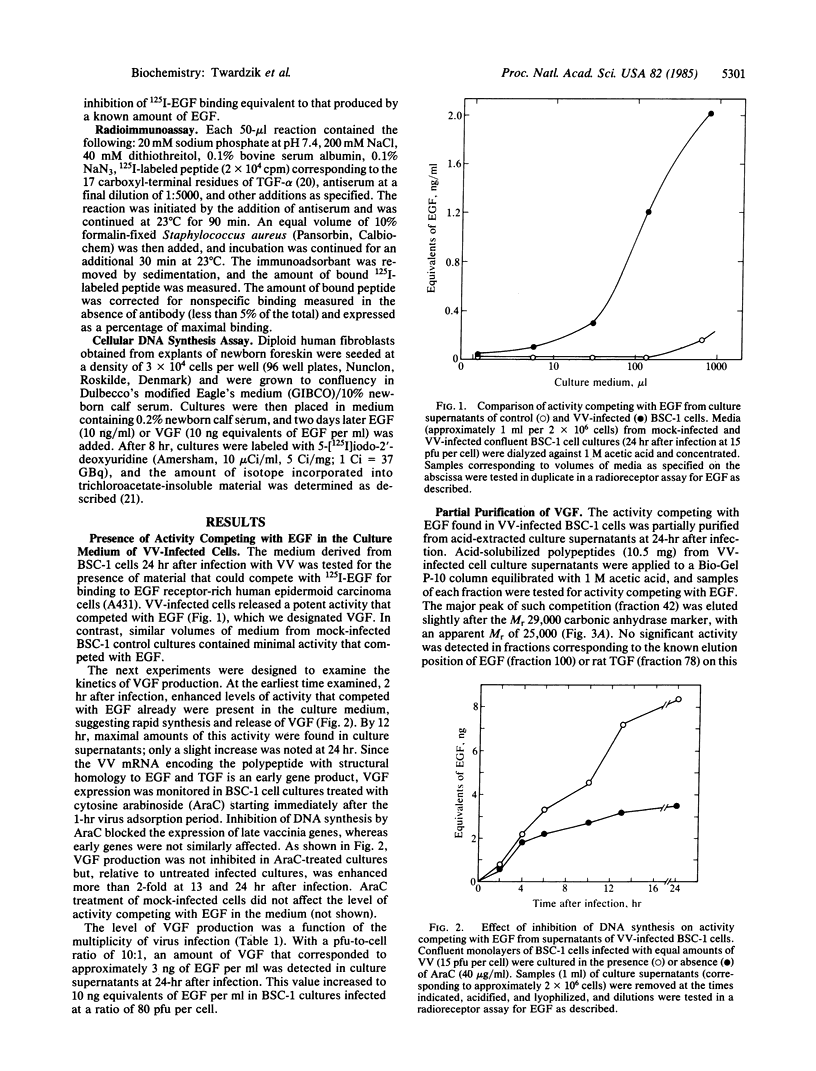
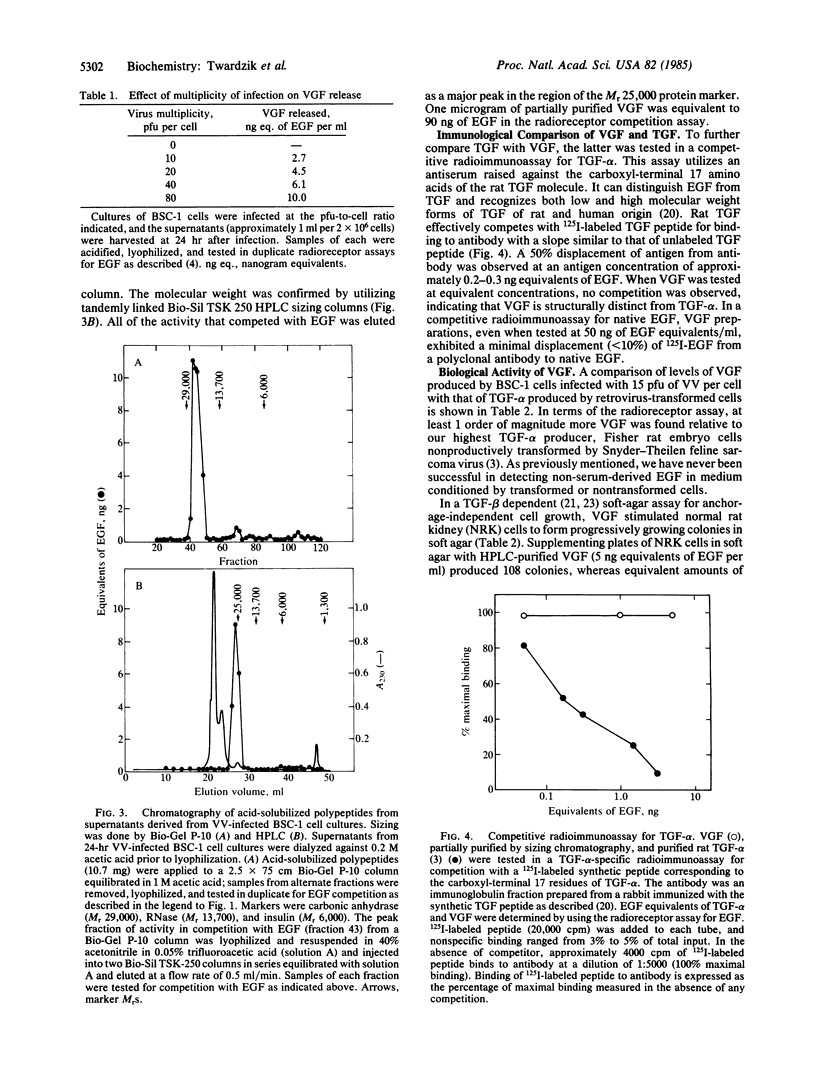
The recent discovery, that a vaccinia virus (VV) gene encodes a polypeptide with structural homology to transforming growth factor (TGF-alpha) and epidermal growth factor (EGF), led us to look for a virus-induced protein with the predicted biological activity. The supernatants of VV-infected cell cultures were found to contain an acid stable Mr 25,000 polypeptide that competes with EGF for binding to EGF membrane receptors. This VV-induced growth factor (VGF) like EGF and TGF-alpha is mitogenic and stimulates anchorage-independent cell growth in the presence of TGF-beta. However, VGF did not cross-react in a radioimmunoassay specific for small and large forms of TGF-alpha and exhibited minimal cross-reactivity with antisera to EGF. VGF was detectable in the culture medium within 2 hr, and maximal amounts were present 12 hr after infection. The level of VGF was proportional to the multiplicity of VV used. Inhibition of viral DNA synthesis enhanced VGF production, consistent with the hypothesis that VGF is an early gene product encoded by VV. The demonstration of a novel growth factor, released from cells infected with VV, may have important implications regarding the nature of virus-host interactions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anzano M. A., Roberts A. B., Meyers C. A., Komoriya A., Lamb L. C., Smith J. M., Sporn M. B. Synergistic interaction of two classes of transforming growth factors from murine sarcoma cells. Cancer Res. 1982 Nov;42(11):4776–4778. [PubMed] [Google Scholar]
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Blomquist M. C., Hunt L. T., Barker W. C. Vaccinia virus 19-kilodalton protein: relationship to several mammalian proteins, including two growth factors. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7363–7367. doi: 10.1073/pnas.81.23.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. P., Twardzik D. R., Marquardt H., Todaro G. J. Vaccinia virus encodes a polypeptide homologous to epidermal growth factor and transforming growth factor. Nature. 1985 Feb 7;313(6002):491–492. doi: 10.1038/313491a0. [DOI] [PubMed] [Google Scholar]
- COHEN S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962 May;237:1555–1562. [PubMed] [Google Scholar]
- Cohen S., Carpenter G. Human epidermal growth factor: isolation and chemical and biological properties. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1317–1321. doi: 10.1073/pnas.72.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Carpenter G., King L., Jr Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980 May 25;255(10):4834–4842. [PubMed] [Google Scholar]
- Linsley P. S., Hargreaves W. R., Twardzik D. R., Todaro G. J. Detection of larger polypeptides structurally and functionally related to type I transforming growth factor. Proc Natl Acad Sci U S A. 1985 Jan;82(2):356–360. doi: 10.1073/pnas.82.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt H., Hunkapiller M. W., Hood L. E., Todaro G. J. Rat transforming growth factor type 1: structure and relation to epidermal growth factor. Science. 1984 Mar 9;223(4640):1079–1082. doi: 10.1126/science.6320373. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Hunkapiller M. W., Hood L. E., Twardzik D. R., De Larco J. E., Stephenson J. R., Todaro G. J. Transforming growth factors produced by retrovirus-transformed rodent fibroblasts and human melanoma cells: amino acid sequence homology with epidermal growth factor. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4684–4688. doi: 10.1073/pnas.80.15.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt H., Todaro G. J. Human transforming growth factor. Production by a melanoma cell line, purification, and initial characterization. J Biol Chem. 1982 May 10;257(9):5220–5225. [PubMed] [Google Scholar]
- Moses H. L., Branum E. L., Proper J. A., Robinson R. A. Transforming growth factor production by chemically transformed cells. Cancer Res. 1981 Jul;41(7):2842–2848. [PubMed] [Google Scholar]
- Ozanne B., Fulton R. J., Kaplan P. L. Kirsten murine sarcoma virus transformed cell lines and a spontaneously transformed rat cell-line produce transforming factors. J Cell Physiol. 1980 Oct;105(1):163–180. doi: 10.1002/jcp.1041050118. [DOI] [PubMed] [Google Scholar]
- Postlethwaite R. Molluscum contagiosum. Arch Environ Health. 1970 Sep;21(3):432–452. doi: 10.1080/00039896.1970.10667262. [DOI] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Todaro G. J., Fryling C., Stephenson J. R. Human transforming growth factors induce tyrosine phosphorylation of EGF receptors. Nature. 1981 Jul 16;292(5820):259–262. doi: 10.1038/292259a0. [DOI] [PubMed] [Google Scholar]
- Rouhandeh H., Vafai A. A novel cell transformation with DNA-containing cytoplasmic Yaba tumor poxvirus. Virology. 1982 Jul 15;120(1):77–92. doi: 10.1016/0042-6822(82)90008-3. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Inagami T., Cohen S. The primary structure of epidermal growth factor. J Biol Chem. 1972 Dec 10;247(23):7612–7621. [PubMed] [Google Scholar]
- Twardzik D. R., Ranchalis J. E., Todaro G. J. Mouse embryonic transforming growth factors related to those isolated from tumor cells. Cancer Res. 1982 Feb;42(2):590–593. [PubMed] [Google Scholar]
- Twardzik D. R., Todaro G. J., Marquardt H., Reynolds F. H., Jr, Stephenson J. R. Transformation induced by Abelson murine leukemia virus involves production of a polypeptide growth factor. Science. 1982 May 21;216(4548):894–897. doi: 10.1126/science.6177040. [DOI] [PubMed] [Google Scholar]
- Venkatesan S., Gershowitz A., Moss B. Complete nucleotide sequences of two adjacent early vaccinia virus genes located within the inverted terminal repetition. J Virol. 1982 Nov;44(2):637–646. doi: 10.1128/jvi.44.2.637-646.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zoelen E. J., Twardzik D. R., van Oostwaard T. M., van der Saag P. T., de Laat S. W., Todaro G. J. Neuroblastoma cells produce transforming growth factors during exponential growth in a defined hormone-free medium. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4085–4089. doi: 10.1073/pnas.81.13.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]


