Abstract
Background
Cannabinoid, particularly hashish and WIN 55212-2 (WIN), consumption during embryonic period may affect fetal growth, and the development of motor functioning, memory and cognitive functions. Therefore, the present study aimed to evaluate the effects of WIN 55212-2 during embryonic period on behavioral responses, as well as tissue and memory changes among neonatal rats.
Methods
WIN treated groups subcutaneously received daily doses of 0.5 or 1 mg/kg WIN suspended in 1% Tween-80-saline (1 ml/kg) from days 5 to 20 of pregnancy. The vehicle group received 1% Tween-80-saline from days 5 to 20 of pregnancy. Three, five and seven weeks after birth, the effects of maternal WIN consumption on infants' body weight, mortality, histological changes, motor functioning, and memory function were assessed.
Findings
Prenatal WIN consumption was associated with atrophy of cerebellum cortex in granular and Purkinje cells layers. WIN treatment of pregnant rats produced a significant decrease in the rearing frequency of the offspring, but significantly increased the grooming frequency at 22, 36 and 50 days of age. During the acquisition trials, approach latencies were not significantly different between all groups of rats (50 days old). When the trial was repeated 24 hours and seven days later (retention trial), the avoidance latencies of the WIN-exposed group were significantly shorter than those of the control and vehicle animals. The mortality percent was increased significantly and litter size was decreased significantly in WIN (1 mg/kg) treated rats compared to the control, vehicle and WIN (0.5 mg/kg) treatment groups.
Conclusion
These findings suggested that prenatal exposure to WIN probably induces long-term alterations in histological, motor functioning, and learning and memory parameters.
Keywords: Hashish, WIN 55212-2, Cerebellum, Prenatal exposure, Memory, Motor functioning
Introduction
Cannabis derivatives such as hashish and marijuana are widely consumed for medical and recreational purposes, and also in some religions.1,2 In the late 20th century, most Western governments banned the medical use of cannabis. This decision might have been raised result of believing cannabis to be a dangerous and risky drug for mental and physical health. However, this view has significantly changed lately and efforts have been made to legalize and remove the legal sanctions of using medical and therapeutic properties of cannabis.1,2 Cannabinoids are greatly consumed in Western communities particularly by women at the reproductive age and during pregnancy. Human and animal researches have shown that cannabinoids easily cross the placental barrier and transmit to the infant through breast milk. Due to the effects they can have on fetal growth, marijuana and hashish consumption during the embryonic period causes problems in health.1,3-6 Consumption of such drugs during the embryonic period has a significant impact on several neurotransmitter systems including dopaminergic, serotonergic, gamma-Aminobutyric acid (GABA)-ergic, glutamatergic and opioidergic systems. They can thus cause major and long-term changes in behavioral patterns.1-7 The cannabinoid system has a strong modulatory action on synaptic transmission in the mammalian brain. Endogenous cannabinoids such as anandamide can inhibit the release of glutamate and GABA in large areas of brain including the cerebellum, hippocampus and cerebral cortex.8-11 This modulatory role is applied by the cannabinoid receptors type I and II which are presynaptically accumulated on the axonic structure in the central nervous system (CNS), particularly in cerebellum and hippocampus.11,12 Mammalian cerebellar cortex is one of the CNS regions with very large density of cannabinoid receptors type I.3 Density and differentiation of cannabinoid receptors in brain in different stages of growth and development before and after birth and reflection of this differentiation in adulthood or maturity period suggest the likelihood of a particular vulnerability in long-term consumption of cannabis during certain phases of growth.1 Adult animals exposed to cannabinoid agonists during their pregnancy or infancy suffer from stable changes in behavioral tests such as behaviors in animal mating, learning ability, sensitivity to pain, sexuality and social behavior as well as neuroendocrine abnormalities.13-17 Recent studies have shown that cannabis consumption is associated with increased irritability, impaired memory and learning, ataxia and defects in making decisions about future life.18,19 According to what we know, there has been very few behavioral and histological studies identifying the effects of chronic cannabinoid consumption on the fetus of pregnant mothers. Due to presence of cannabinoid receptors on synapses in the cerebellar cortex and hippocampus, consumption of exogenous agonists will probably interfere with the normal process of signaling. Particularly, the probable effects of these agonists on memory process, balance, motor activity, and functioning can be taken into account. Therefore, the present study aimed to review the effects of exogenous cannabinoid WIN 55212-2 on the behavioral responses of the embryonic period, histological changes, and memory of neonatal rats. WIN was selected due to its high tendency and affinity to connect to cannabinoid receptors. It can also mimic most of marijuana and hashish effects.7,19
Methods
Study Design and Methods:
In this study, female primiparous Wistar rats, weighed 200-250 grams, were used. The animals were provided by the Animal Reproduction and Breeding Center of Pasteur Institute. They were kept in a 12/12 light/dark cycle at 20-22°C in animal rooms of Shahid Beheshti School of Medicine, Tehran, Iran. They had easy access to food and water. Two female and one male rats were placed in a cage for mating in the last hours of the afternoon. Vaginal smear was performed the next morning at 9. The day of observing sperm was considered as day zero. The pregnant rats were randomly divided into control, vehicle and WIN treated (at least 8 rats in each group) groups. The pregnant rats in daily treatment groups received 0.5 or 1 ml/kg WIN powder (Sigma) from fifth to twentieth days of pregnancy (these doses were according to the previous studies).7,19 In the treatment group, the drug was dissolved in Tween 80 and saline solution (1% Tween/saline) and subcutaneously injected to the rats (1 ml/kg). The vehicle group on the other hand was subcutaneously injected with 1% Tween/saline alone (1 ml/kg). In order to prevent the effect of circadian rhythm on animals, all the tests were conducted in daylight between 8 A.M. to 4 P.M. In order to make rats accustomed to laboratory conditions, male neonates of all the groups were transferred to the laboratory 1 hour before starting the tests.
Histological Researches
The male neonates of rats in the control, vehicle and WIN groups were deeply anesthetized and killed at the fifth week after birth using ether vapor. Thereafter, their heads were cut off and their cerebellum was sunk in formalin solution in an appropriate time for better fixing. In the next stage, cerebellar vermis tissue of the control and WIN groups were fixed and cryopreserved in a fixation solution plus sucrose overnight. The next day, the tissues were cut at a thickness of 25 micrometers. In order to evaluate pathologic changes, the slices were stained by Fluoro-Jade (a perfect fluorescein marker to diagnose degenerated neurons).20,21
Behavioral Studies
Open-field behavioral test using EthoVision video tracking system:
Open-field test was used to assess motor behaviors of animals at the end of the third, fifth and seventh weeks after birth (at least 15 young rats in each group). EthoVision software is a video detector system for automation of behavioral tests. It performs all stages from designing the test to analyzing the obtained data. It thus can be used for most behavioral tests in a wide in vitro range. The most general application of EthoVision is to measure behavioral impacts of drugs, treatments, and surgeries. In addition, it is increasingly used for behavioral phenotype of genetically modified rats. This device allows monitoring many social reactions tests. EthoVision Pro was used for the tests of the present study in which the behaviors of rats were detected in 16 areas. In order to do so, each rat was put in the center of a defined area and their motor behaviors were recorded through a camera for five minutes. The open-field space was cleaned and dried with alcohol between every two tests.22
Passive-avoidance learning test:
In this test, Shuttle Box Device was used. The device consisted of two dark and light compartments of equal size with a controllable door between them. At the end of the fifth week, in the adaptability stage, the rats (at least 15 young rats in each group) were placed in the light part with their head in the dark part. After 10 seconds, the door between the two compartments was opened and the rats were freely allowed to enter the dark part. The delayed time for leaving the light part was recorded. If this time was more than 60 seconds, it indicated that the rat did not desire to enter the dark environment. Such rats were excluded from the study. Two hours after re-adaptability, rats were placed in the light compartment again. The learning stage was conducted similar to the adaptability stage. However, the door was immediately closed after the rats entered the dark compartment and the rats' paws were shocked (0.5 ma for 2 seconds). The rats were kept inside the device for 20 minutes before they were taken out. Two minutes later, the learning stage was repeated and if the rat entered the dark part, it was shocked. The number of times the rat was socked was recorded. In retrieval phase, avoidance responses were measured one and seven days after the learning stage. Each rat was placed in the light compartment and the delayed time for re-entrance into the dark compartment was recorded. In this stage, the time of entrance, staying duration and the number of entrance times into the dark zone were measured.18
Statistical Data Analysis:
The results are reported as mean ± SEM (standard error of the mean). In order to review the normal distribution of data, Kolmogorov-Smirnov (K-S) test was conducted in each group. One way analysis of variance (ANOVA) was used to compare the motor and retrieval activity. Kruskal-Wallis non-parametric test was used for non-normally distributed data. A P < 0.05 indicated statistical significance.
Results
The impacts of cannabis extract consumption during embryonic period on reproductive factors:
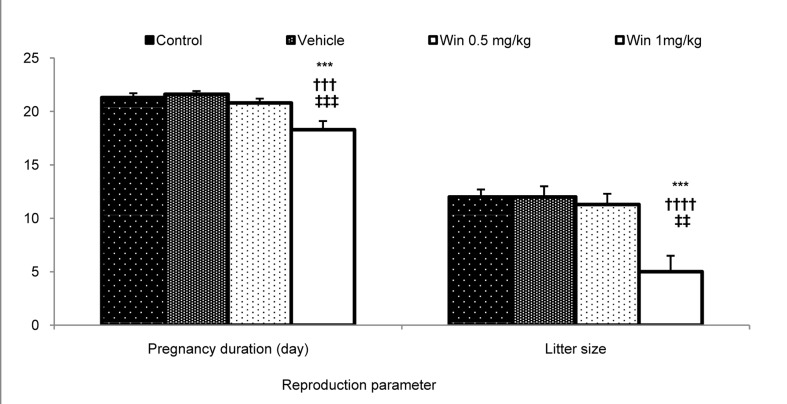
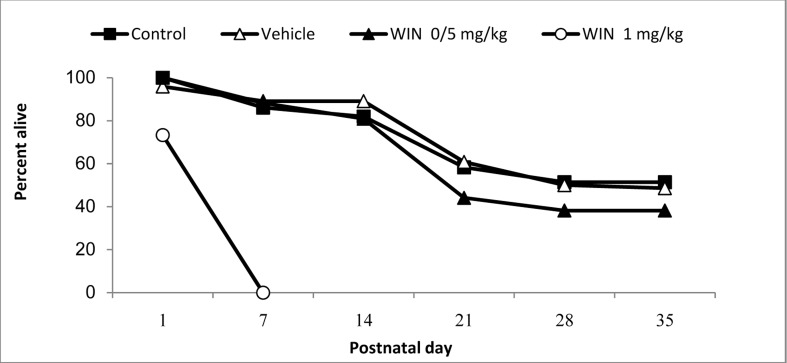
In this study, the effects of WIN consumption during embryonic period on reproductive factors such as pregnancy duration, the number of neonates, neonatal weight and the percentage of infant survival were assessed. In analysis, the number of infants and duration of pregnancy were significantly less in the WIN 1 mg/kg group compared to the control, vehicle, and WIN 0.5 mg/kg groups (P < 0.001) (Figure 1). The survival rate was also significantly reduced in the WIN 1 mg/kg group compared to other groups. In fact, most neonate rats died during the first days after birth (Figure 2).
Figure 1.
Comparison of pregnancy duration and number of litter size for born rats in the studied groups; Results are illustrated as mean ± SEM (n = at least 8) ***: Significant difference with the control group (P < 0.001) †††, ††††: Significant differences with the vehicle group (P < 0.001 and P< 0.0001, respectively) ‡‡, ‡‡‡: Significant differences with the 0.5 mg/kg and 1 mg/kg WIN treated groups, respectively (P < 0.01 and P < 0.001, respectively)
Figure 2.
The survival rate of born rats in the studied groups on day 1 and at the end of first to fifth postnatal weeks (The numbers were different: from 35 neonate rats in WIN 1 mg/kg group and maximum 61 rats in the control group)
The WIN 1 mg/kg group was excluded from the study due to major changes observed in the reproductive and birth factors. In the evaluation of weights at the first day, and at the first to fifth postnatal weeks were significantly different between WIN 0.5 mg/kg group and control and vehicle groups only at the end of the first week (Table 1).
Table 1.
Comparison of neonatal weights (g) in the studied groups on the first day, and at the end of the first to fifth postnatal weeks
| Experimental Days |
1 | 8 | 15 | 22 | 29 | 36 |
|---|---|---|---|---|---|---|
| Group | ||||||
| Control | 0.10 ± 5.51 | 0.22 ± 10.71 | 0.50 ± 19.11 | 0.84 ± 27.78 | 1.68 ± 44.76 | 2.58 ± 74.00 |
| Vehicle | 0.06 ± 5.23 | 0.22 ± 10.09 | 0.40 ± 18.41 | 0.63 ± 27.53 | 2.45 ± 42.04 | 1.03 ± 71.66 |
| WIN 0.5 mg/kg | 0.13 ± 5.50 | 0.33 ± 9.71*†† | 0.39 ± 18.10 | 0.76 ± 26.08 | 1.56 ± 39.66 | 2.05 ± 69.58 |
Significant difference with the control group (P < 0.01);
Significant difference with the vehicle group (P < 0.01) (at least n = 30)
Findings of Histological Studies:
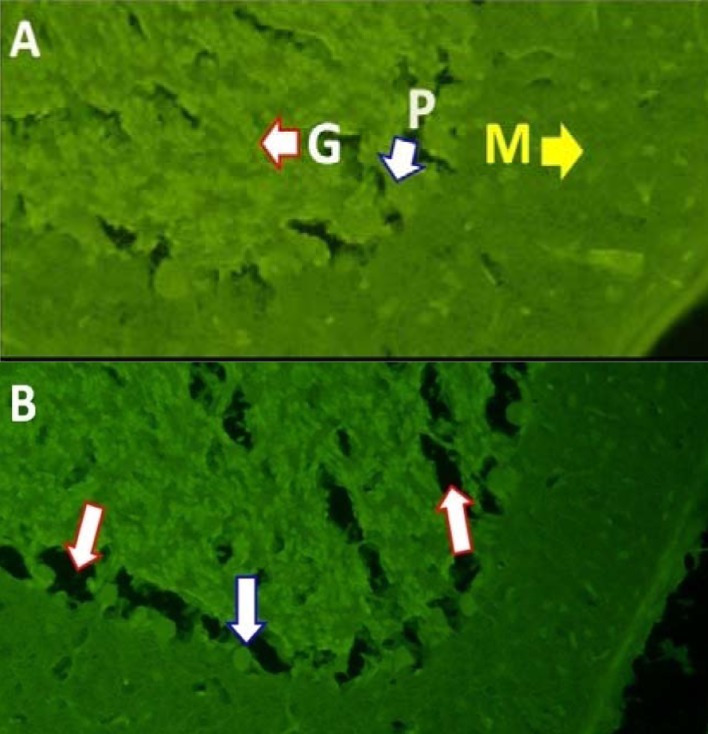
The effects of hashish extract consumption during embryonic period on cerebellar cortex: Fluoro-Jade staining was used to evaluate the destructive effects and probable pathologic changes on Purkinje neurons resulted by WIN (0.5 mg/kg) during embryonic period. The normal cerebellar cortex with ordered granular and Purkinje cells layers was observed after Fluoro-Jade staining in the three control, vehicle and treatment groups (Figure 3A). In WIN treated rats, the damaged areas were clearly detectable as black and hollow dots both in Purkinje cell layers and in layers containing granular cells. In the WIN group, Purkinje neurons were stained as irregular and shattered dots in vermis layer with high intensity. In addition, the apparent density of Purkinje neurons in this group (Figure 3B) was lower than the control group (Figure 3A). Therefore, Purkinje neuron cell body with lower density in the treated group was clearer (more visible) than Purkinje neuron cells of the control group with high density.
Figure 3.
Figure A illustrates the order of cerebellar cortical layers and high density of Purkinje cells in the control rats (molecular layer (M), Purkinje cell layer (P) and granular layer (G). Figure B illustrates different cerebellar cortex layers 30 days after birth of rats treated with WIN 55212-2 during embryonic period. In the treated group, Purkinje neuron cells are irregular and shattered and with lower density than the control group. The red arrowhead indicates the places damaged and the blue arrowhead indicates cell bodies from healthy Purkinje cells.
Findings of Behavioral Studies:
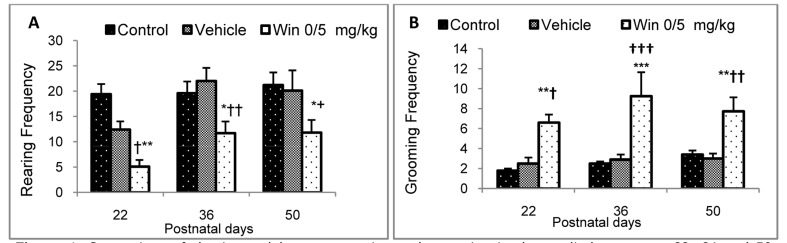
In the present study, in the open-field test at the end of the third, fifth and seventh postnatal weeks, the number of rearing in neonate rats whose mothers were treated with WIN during pregnancy, had a significant reduction compared to the control and vehicle groups (Figure 4A). On the other hand, grooming behavior had a significant increase in the WIN group compared to the control and vehicle groups, i.e. rats in the first group were often sitting alone in a corner grooming themselves and had the highest frequency of grooming behavior (Figure B4).
Figure 4.
Comparison of the interval between rearing and grooming in the studied groups on 22, 36 and 50 postnatal days in figures A and B, respectively (n = 8) (Values are expressed as mean ± SEM.) *, **, ***, **** indicate significant differences with the control group (P < 0.05, P < 0.01, P < 0.001, and P < 0.0001, respectively) †, ††, †††, †††† indicate significant differences with the vehicle group (P < 0.05, P < 0.01, P < 0.001, and P < 0.0001, respectively)
The effects of cannabis extract consumption during embryonic period on memory and learning:
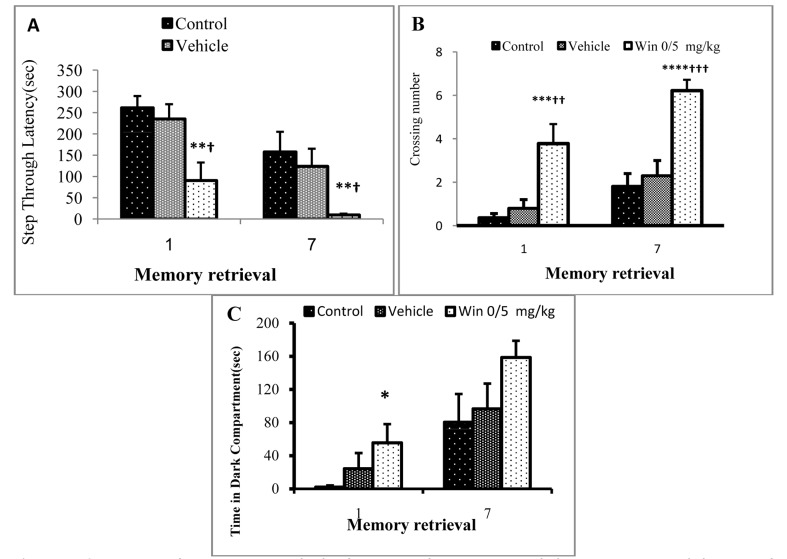
In this study, the effects of chronic administration of WIN on acquisition and retrieval abilities of neonate rats whose mothers were treated with WIN 0.5 mg/kg during pregnancy were evaluated. There were no significant differences between the groups in terms of the number of times necessary for learning. Likewise, there were no significant differences between the control and vehicle groups in the duration and frequency of entrance into the dark room on days 1 and 7 after the acquisition. However, there was a significant difference between WIN treated and vehicle groups on day 1 (P < 0.01) and day 7 (P < 0.05) (Figures 5A and 5B). The duration of staying in the dark zone on day 1 after the acquisition increased significantly in the WIN treated group compared to the control group (P < 0.05). However, the duration of staying in the dark on day 7 after the acquisition was not significantly different between groups (Figure 5C).
Figure 5.
Comparison of memory retrieval, the frequency of entrance into dark compartment and duration of staying in dark zone in the studied groups on days 1 and 7 after acquisition, and at the end of the seventh week after birth in Figures A, B, and C, respectively (n = 8) (Values are expressed as mean ± SEM.) *, **, ***, **** indicate significant differences with the control group (P < 0.05, P < 0.01, P < 0.001, and P < 0.0001, respectively) †, ††, ††† indicate significant differences with the vehicle group (P < 0.05, P < 0.01, and P < 0.001, respectively)
Discussion
Cannabinoids are the active components of hashish or marijuana and are their endogenous analogues which cause extensive central and peripheral effects by connecting to their receptors. In the past few centuries, marijuana and hashish used to be consumed greatly by women at reproductive age and during pregnancy.23 Human and animal studies have shown that cannabinoids easily cross the placental barrier and can be transmitted to infant through breast milk.23 Among the WIN 55212-2 treated rats in the present study, the damaged areas were clearly detectable as black and hollow dots both in Purkinje cell layers and in layers containing granular cells. In this group, Purkinje neurons were stained as irregular and shattered dots in vermis layer with high intensity. Moreover, the apparent density of Purkinje neurons in this group (Figure 3B) was lower than the control group (Figure 3A).
Consumption of marijuana particularly influences posterior cerebral blood circulation and may be considered as a risk factor for stroke in children. It also has acute effects such as dizziness, disordered balance due to orthostatic hypotension. It is associated with bradycardia and general hypotension in high doses. Unlike most cerebellar ischemic strokes which can be seen in a blood vessel, in cerebellar stroke resulted from marijuana, several blood vessels have infarction. Cerebellar damage due to lack of strong peripheral circuits in the blood circulation have higher risk for infarction in marijuana consumption. Marijuana results in vascular spasm, hypotension, impaired self-regulatory system, and vasomotor reflex and can cause infarction and cerebellar damage.24 Often, a large area of cortex and cerebellum in the brain suffers from atrophy in drugs, particularly cannabis and heroin and alcohol, abusers. Severe atrophy of cerebellum and frontal vermis lobe have specifically been reported.25 There is a high density of CB1 receptors at the end of axons which synapse with Purkinje cells. These receptors usually apply their effects through connecting to G proteins. Overexpression of these receptors in the cerebellum and other brain regions are associated with defects in motor control system.26-28 Chronic treatment with cannabinoid leads to insensitivity and reduction of cannabinoid receptors in brain and significantly increases the cyclic adenosine monophosphate (cAMP) levels and protein kinase A (PKA) activity.29 Chronic consumption of cannabinoids can reduce mesolimbic system activity and is thus considered as a high and significant risk factor for progression and development of schizophrenia in adults.30 Change in the number of rearing is a sign of alterations in spontaneous motor activity and searching behavior of animal. On the other hand, changes in the number of grooming indicate the probable damage, obsessive behavior and decreased locomotor activity. Decreased number of rearing in the present study was in accordance with the results reported by Miller et al. who indicated chronic WIN consumption during pregnancy to cause decreased locomotor activity and seeking behavior of neonates after birth.31 They also reported reductions in two motor criteria, i.e. ambulation and rearing, in tetrahydrocannabinol (THC) consumption.31 Meanwhile, Mereu et al. found no significant differences in these two parameters following chronic consumption of WIN during embryonic period at 40 and 80 days after birth compared to the control group. However, increased locomotor activity and deficits in memory retrieval were reported as a result of chronic cannabis consumption.32 Consumption of these substances during embryonic period can bring about major health problems to the fetus.3,7,9,19,23 Pregnancy is a critical period in development of the nervous system. During the fetal development, each region, system and circuit of the brain develops rapidly. Therefore, any incomplete stage would remain uncompensated in the later stages. Subsequently, any change in the development of the nervous system in this period would be associated with deficits in CNS functioning including motor and cognitive activities.33 In a study by Masur et al., reductions in rearing, grooming, and the frequency of excretion were reported following 25 mg/kg THC consumption.34 Vardaris et al. evaluated the effects of THC by open-field and observed a reduction in invasive behavior.35 Navarro et al. found that fetal exposure to cannabis extract reduced motor activity in male rats. However, they discovered increased motor activity in female rats born from mothers exposed to hashish extract during pregnancy.36 In this study, WIN consumption during pregnancy reduced the time of entrance and increased the frequency of the dark zone in neonate rats at young ages (Figure 5). This can indicate impairments in the memory retrieval process due to the effects of CB1 cannabinoid receptor agonist. In learning and acquisition stages, there were no significant differences between the WIN, vehicle and control groups. Vardaris et al. administered THC in pregnant rats and reported the active ingredient in cannabis to cause impairments in acquisition and retrieval processes in neonate rats 21 days after birth.35 However, Miller et al. found no impacts on acquisition and retrieval processes after administration of 5 and 15 mg/kg THC.31 Similar to the present study, Mereu et al. reported impairments in retrieval in neonate rats after cannabis consumption during pregnancy.32 On the other hand, Luo et al. discussed that due to the activation of calcineurin and effects on long-term potentiation (LTP) and long-term depression (LTD), cannabis can improve the induced impairments in memory and learning by chemicals.37 Hippocampus, especially in contortion areas and CA3, is one of the regions with the highest density of cannabinoid receptors.38 Cannabinoids consumption influences CB1 receptors, activates several signaling paths inside the cell, inhibits adenyl cyclase activity and voltage-dependent calcium channels, activates potassium channels (specially inward rectifier fast type), and causes irreversible changes on cannabinoid system in the body. It can thus trigger changes in emotional status and mood and interfer in short-term memory.9,39 Cannabinoid receptor density varies during different stages of growth. In long-term consumption and abuse of cannabis, their receptors are affected by the reduction of sensitivity. Such a finding has particularly been reported in hippocampus of adult rats.38
Available evidence indicates cannabis consumption to make changes in morphology of cannabinoids in hippocampus neurons and to reduce the density of gray matter in hippocampus.40 Special imaging methods have proved that blood circulation in the prefrontal cortex and also parahippocampal activity during verbal memory and learning reduce among cannabis consumers.41,42
Conclusion
In this study, the observed changes in locomotor activity, learning and memory along with histological examinations in neonates of rats treated with cannabis during pregnancy confirmed the hypothesis of fundamental and long-term changes in behavioral patterns due to cannabis consumption. Such changes could have been caused by the effects of cannabis on neurotransmitter systems including dopaminergic, serotonergic, GABAergic, glutamatergic and opioidergic systems.1,7
Footnotes
Conflicts of Interest
The Authors have no conflict of interest.
REFERENCES
- 1.Castelli MP, Paola PA, D'Agostino A, Pibiri F, Perra S, Gessa GL, et al. Dysregulation of the endogenous cannabinoid system in adult rats prenatally treated with the cannabinoid agonist WIN 55,212-2. Eur J Pharmacol. 2007;573(1-3):11–9. doi: 10.1016/j.ejphar.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 2.Arnold JC. The behavioural and neural effects of cannabinoids: Studies using Lewis and Wistar strain rats. Sydney, Australia: University of Sydney; 2000. [Google Scholar]
- 3.Benagiano V, Lorusso L, Flace P, Girolamo F, Rizzi A, Sabatini R, et al. Effects of prenatal exposure to the CB-1 receptor agonist WIN 55212-2 or CO on the GABAergic neuronal systems of rat cerebellar cortex. Neuroscience. 2007;149(3):592–601. doi: 10.1016/j.neuroscience.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 4.Bortolato M, Frau R, Orru M, Casti A, Aru GN, Fa M, et al. Prenatal exposure to a cannabinoid receptor agonist does not affect sensorimotor gating in rats. Eur J Pharmacol. 2006;531(1-3):166–70. doi: 10.1016/j.ejphar.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Ebrahim SH, Gfroerer J. Pregnancy-related substance use in the United States during 1996-1998. Obstet Gynecol. 2003;101(2):374–9. doi: 10.1016/s0029-7844(02)02588-7. [DOI] [PubMed] [Google Scholar]
- 6.Hutchings DE, Martin BR, Gamagaris Z, Miller N, Fico T. Plasma concentrations of delta-9-tetrahydrocannabinol in dams and fetuses following acute or multiple prenatal dosing in rats. Life Sci. 1989;44(11):697–701. doi: 10.1016/0024-3205(89)90380-9. [DOI] [PubMed] [Google Scholar]
- 7.Viveros MP, Llorente R, Moreno E, Marco EM. Behavioural and neuroendocrine effects of cannabinoids in critical developmental periods. Behav Pharmacol. 2005;16(5-6):353–62. doi: 10.1097/00008877-200509000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Brown SP, Brenowitz SD, Regehr WG. Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat Neurosci. 2003;6(10):1048–57. doi: 10.1038/nn1126. [DOI] [PubMed] [Google Scholar]
- 9.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83(3):1017–66. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22(23):10182–91. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galante M, Diana MA. Group I Metabotropic Glutamate Receptors Inhibit GABA Release at Interneuron-Purkinje Cell Synapses through Endocannabinoid Production. The Journal of Neuroscience. 2004;24(20):4865–74. doi: 10.1523/JNEUROSCI.0403-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diana MA, Levenes C, Mackie K, Marty A. Short-term retrograde inhibition of GABAergic synaptic currents in rat Purkinje cells is mediated by endogenous cannabinoids. J Neurosci. 2002;22(1):200–8. doi: 10.1523/JNEUROSCI.22-01-00200.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalterio SL. Cannabinoid exposure: effects on development. Neurobehav Toxicol Teratol. 1986;8(4):345–52. [PubMed] [Google Scholar]
- 14.Fernandez-Lopez J, Martinez-Org D, Nunez E, Romero J, Lorenzo P, Moro MA, et al. Characterization of the neuroprotective effect of the cannabinoid agonist WIN-55212 in an in vitro model of hypoxic-ischemic brain damage in newborn rats. Pediatr Res. 2006;60(2):169–73. doi: 10.1203/01.pdr.0000228839.00122.6c. [DOI] [PubMed] [Google Scholar]
- 15.Fried PA, Smith AM. A literature review of the consequences of prenatal marihuana exposure. An emerging theme of a deficiency in aspects of executive function. Neurotoxicol Teratol. 2001;23(1):1–11. doi: 10.1016/s0892-0362(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 16.Moreno M, Escuredo L, Munoz R, Rodriguez de FF, Navarro M. Long-term behavioural and neuroendocrine effects of perinatal activation or blockade of CB1 cannabinoid receptors. Behav Pharmacol. 2005;16(5-6):423–30. doi: 10.1097/00008877-200509000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Dey SK, Maccarrone M. Jekyll and hyde: two faces of cannabinoid signaling in male and female fertility. Endocr Rev. 2006;27(5):427–48. doi: 10.1210/er.2006-0006. [DOI] [PubMed] [Google Scholar]
- 18.Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2003;25(4):427–36. doi: 10.1016/s0892-0362(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 19.Richardson GA, Ryan C, Willford J, Day NL, Goldschmidt L. Prenatal alcohol and marijuana exposure: effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol. 2002;24(3):309–20. doi: 10.1016/s0892-0362(02)00193-9. [DOI] [PubMed] [Google Scholar]
- 20.Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874(2):123–30. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- 21.Schmued LC, Albertson C, Slikker W. Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751(1):37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- 22.Dursun I, Jakubowska-Dogru E, Uzbay T. Effects of prenatal exposure to alcohol on activity, anxiety, motor coordination, and memory in young adult Wistar rats. Pharmacol Biochem Behav. 2006;85(2):345–55. doi: 10.1016/j.pbb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Benagiano V, Roncali L, Virgintino D, Flace P, Errede M, Rizzi A, et al. GABA immunoreactivity in the human cerebellar cortex: a light and electron microscopical study. Histochem J. 2001;33(9-10):537–43. doi: 10.1023/a:1014903908500. [DOI] [PubMed] [Google Scholar]
- 24.Geller T, Loftis L, Brink DS. Cerebellar infarction in adolescent males associated with acute marijuana use. Pediatrics. 2004;113(4):e365–e370. doi: 10.1542/peds.113.4.e365. [DOI] [PubMed] [Google Scholar]
- 25.Cala LA, Mastaglia FL. Computerized axial tomography in the detection of brain damage: 1. Alcohol, nutritional deficiency and drugs of addiction. Med J Aust. 1980;2(4):193–8. [PubMed] [Google Scholar]
- 26.Dowie MJ, Bradshaw HB, Howard ML, Nicholson LF, Faull RL, Hannan AJ, et al. Altered CB1 receptor and endocannabinoid levels precede motor symptom onset in a transgenic mouse model of Huntington's disease. Neuroscience. 2009;163(1):456–65. doi: 10.1016/j.neuroscience.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Herkenham M, Groen BG, Lynn AB, De Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors and second messengers in mutant mouse cerebellum. Brain Res. 1991;552(2):301–10. doi: 10.1016/0006-8993(91)90096-e. [DOI] [PubMed] [Google Scholar]
- 28.Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83(2):393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 29.Rubino T, Vigano' D, Massi P, Spinello M, Zagato E, Giagnoni G, et al. Chronic delta-9-tetrahydrocannabinol treatment increases cAMP levels and cAMP-dependent protein kinase activity in some rat brain regions. Neuropharmacology. 2000;39(7):1331–6. doi: 10.1016/s0028-3908(99)00196-3. [DOI] [PubMed] [Google Scholar]
- 30.Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ. 2002;325(7374):1199. doi: 10.1136/bmj.325.7374.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller LL, Drew WG, Joyce P. 9 -THC: effect on acquisition and retention of a one-trial passive avoidance response. Behav Biol. 1973;8(3):421–6. doi: 10.1016/s0091-6773(73)80082-3. [DOI] [PubMed] [Google Scholar]
- 32.Mereu G, Fa M, Ferraro L, Cagiano R, Antonelli T, Tattoli M, et al. Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long-term potentiation and glutamate release. Proc Natl Acad Sci U S A. 2003;100(8):4915–20. doi: 10.1073/pnas.0537849100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev. 2006;30(1):24. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Masur J, Martz RM, Carlini EA. Effects of acute and chronic administration of cannabis sativa and (-) delta9-trans-tetrahydrocannabinol on the behavior of rats in an open-field arena. Psychopharmacologia. 1971;19(4):388–97. doi: 10.1007/BF00404383. [DOI] [PubMed] [Google Scholar]
- 35.Vardaris RM, Weisz DJ, Fazel A, Rawitch AB. Chronic administration of delta-9-tetrahydrocannabinol to pregnant rats: studies of pup behavior and placental transfer. Pharmacol Biochem Behav. 1976;4(3):249–54. doi: 10.1016/0091-3057(76)90236-7. [DOI] [PubMed] [Google Scholar]
- 36.Navarro M, Rodriguez de FF, Hernandez ML, Ramos JA, Fernandez-Ruiz JJ. Motor behavior and nigrostriatal dopaminergic activity in adult rats perinatally exposed to cannabinoids. Pharmacol Biochem Behav. 1994;47(1):47–58. doi: 10.1016/0091-3057(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 37.Luo J, Yin JH, Wu HZ, Wei Q. Extract from Fructus cannabis activating calcineurin improved learning and memory in mice with chemical drug-induced dysmnesia. Acta Pharmacol Sin. 2003;24(11):1137–42. [PubMed] [Google Scholar]
- 38.Nestor L, Roberts G, Garavan H, Hester R. Deficits in learning and memory: parahippocampal hyperactivity and frontocortical hypoactivity in cannabis users. Neuroimage. 2008;40(3):1328–39. doi: 10.1016/j.neuroimage.2007.12.059. [DOI] [PubMed] [Google Scholar]
- 39.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87(5):1932–6. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matochik JA, Eldreth DA, Cadet JL, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug Alcohol Depend. 2005;77(1):23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Block RI, O'Leary DS, Hichwa RD, Augustinack JC, Boles Ponto LL, Ghoneim MM, et al. Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacol Biochem Behav. 2002;72(1-2):237–50. doi: 10.1016/s0091-3057(01)00771-7. [DOI] [PubMed] [Google Scholar]
- 42.Jager G, Van Hell HH, De Win MM, Kahn RS, Van Den Brink W, Van Ree JM, et al. Effects of frequent cannabis use on hippocampal activity during an associative memory task. Eur Neuropsychopharmacol. 2007;17(4):289–97. doi: 10.1016/j.euroneuro.2006.10.003. [DOI] [PubMed] [Google Scholar]