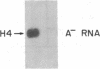
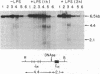
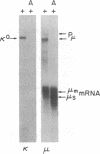
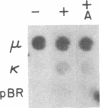
Abstract
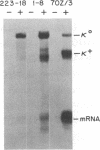
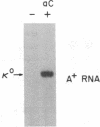
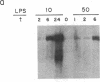
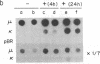
Transcription of unrearranged kappa constant region (kappa 0) loci is dramatically induced in pre-B cells transformed by the Abelson murine leukemia virus when the cells are exposed to bacterial lipopolysaccharide (LPS). Transcriptional activity, detected both by accumulation of the 8-kilobase kappa 0 RNA product and by nuclear run-on measurements, is evident within a few hours after exposure to LPS and continues to increase over a 24-hr period. During this time, transcription of rearranged mu heavy-chain loci remains at the basal constitutive level. In accord with previous studies of the B-cell lymphoma 70Z/3, this transcriptional activation is accompanied by the appearance of a DNase I-hypersensitive site in the kappa enhancer region but not by any detectable hypomethylation of the locus. Moreover, the present studies demonstrate that induction of kappa transcription can occur in the absence of DNA or protein synthesis. These results have led us to propose a model in which an external signal such as LPS or a functionally equivalent lymphokine may initiate kappa transcription in pre-B cells by modifying or overriding the activity of an enhancer-specific factor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Rosenberg N., Casanova R. J., Thomas E., Baltimore D. Immunoglobulin heavy-chain expression and class switching in a murine leukaemia cell line. Nature. 1982 Mar 25;296(5855):325–331. doi: 10.1038/296325a0. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F., Rosenberg N., Lewis S., Thomas E., Baltimore D. Organization and reorganization of immunoglobulin genes in A-MULV-transformed cells: rearrangement of heavy but not light chain genes. Cell. 1981 Dec;27(2 Pt 1):381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- Borun T. W., Scharff M. D., Robbins E. Rapidly labeled, polyribosome-associated RNA having the properties of histone messenger. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1977–1983. doi: 10.1073/pnas.58.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. Y., Folsom V., Wooley J. DNase I-hypersensitive sites in the chromatin of immunoglobulin kappa light chain genes. Proc Natl Acad Sci U S A. 1983 May;80(9):2427–2431. doi: 10.1073/pnas.80.9.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleclough C., Perry R. P., Karjalainen K., Weigert M. Aberrant rearrangements contribute significantly to the allelic exclusion of immunoglobulin gene expression. Nature. 1981 Apr 2;290(5805):372–378. doi: 10.1038/290372a0. [DOI] [PubMed] [Google Scholar]
- Ephrussi A., Church G. M., Tonegawa S., Gilbert W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985 Jan 11;227(4683):134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- Gerondakis S., Boyd A., Bernard O., Webb E., Adams J. M. Activation of immunoglobulin mu gene expression involves stepwise demethylation. EMBO J. 1984 Dec 1;3(12):3013–3021. doi: 10.1002/j.1460-2075.1984.tb02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri J. G., Kincade P. W., Mizel S. B. Interleukin 1-mediated induction of kappa-light chain synthesis and surface immunoglobulin expression on pre-B cells. J Immunol. 1984 Jan;132(1):223–228. [PubMed] [Google Scholar]
- Grollman A. P. Inhibitors of protein biosynthesis. II. Mode of action of anisomycin. J Biol Chem. 1967 Jul 10;242(13):3226–3233. [PubMed] [Google Scholar]
- Harrison J. J., Schwoch G., Schweppe J. S., Jungmann R. A. Phosphorylative modification of histone H1 subspecies following isoproterenol and N6,O2'-dibutyryl cyclic AMP stimulation of rat C6 glioma cells. J Biol Chem. 1982 Nov 25;257(22):13602–13609. [PubMed] [Google Scholar]
- Iwasa Y., Takai Y., Kikkawa U., Nishizuka Y. Phosphorylation of calf thymus H1 histone by calcium-activated, phospholipid-dependent protein kinase. Biochem Biophys Res Commun. 1980 Sep 16;96(1):180–187. doi: 10.1016/0006-291x(80)91198-5. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Marcu K. B., Banerji J., Penncavage N. A., Lang R., Arnheim N. 5' flanking region of immunoglobulin heavy chain constant region genes displays length heterogeneity in germlines of inbred mouse strains. Cell. 1980 Nov;22(1 Pt 1):187–196. doi: 10.1016/0092-8674(80)90167-1. [DOI] [PubMed] [Google Scholar]
- Mather E. L., Perry R. P. Methylation status and DNase I sensitivity of immunoglobulin genes: changes associated with rearrangement. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4689–4693. doi: 10.1073/pnas.80.15.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather E. L., Perry R. P. Transcriptional regulation of immunoglobulin V genes. Nucleic Acids Res. 1981 Dec 21;9(24):6855–6867. doi: 10.1093/nar/9.24.6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K. J., Haimovich J., Perry R. P. Characterization of productive and sterile transcripts from the immunoglobulin heavy-chain locus: processing of micron and muS mRNA. Mol Cell Biol. 1983 Jul;3(7):1317–1332. doi: 10.1128/mcb.3.7.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K. J., Mather E. L., Perry R. P. Lipopolysaccharide-induced transcription of the kappa immunoglobulin locus occurs on both alleles and is independent of methylation status. Nucleic Acids Res. 1984 Feb 24;12(4):1911–1923. doi: 10.1093/nar/12.4.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige C. J., Kincade P. W., Ralph P. Murine B cell leukemia line with inducible surface immunoglobulin expression. J Immunol. 1978 Aug;121(2):641–647. [PubMed] [Google Scholar]
- Palacios R., Henson G., Steinmetz M., McKearn J. P. Interleukin-3 supports growth of mouse pre-B-cell clones in vitro. Nature. 1984 May 10;309(5964):126–131. doi: 10.1038/309126a0. [DOI] [PubMed] [Google Scholar]
- Parslow T. G., Granner D. K. Chromatin changes accompany immunoglobulin kappa gene activation: a potential control region within the gene. Nature. 1982 Sep 30;299(5882):449–451. doi: 10.1038/299449a0. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Coleclough C., Kearney J. F. Organization and expression of immunoglobulin genes in fetal liver hybridomas. Proc Natl Acad Sci U S A. 1981 Jan;78(1):247–251. doi: 10.1073/pnas.78.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P. What controls the transcription of immunoglobulin genes? Nature. 1984 Jul 5;310(5972):14–15. doi: 10.1038/310014a0. [DOI] [PubMed] [Google Scholar]
- Picard D., Schaffner W. A lymphocyte-specific enhancer in the mouse immunoglobulin kappa gene. Nature. 1984 Jan 5;307(5946):80–82. doi: 10.1038/307080a0. [DOI] [PubMed] [Google Scholar]
- Premkumar E., Potter M., Singer P. A., Sklar M. D. Synthesis, surface deposition, and secretion of immunoglobulins by Abelson virus-transformed lymphosarcoma cell lines. Cell. 1975 Oct;6(2):149–159. doi: 10.1016/0092-8674(75)90005-7. [DOI] [PubMed] [Google Scholar]
- Queen C., Stafford J. Fine mapping of an immunoglobulin gene activator. Mol Cell Biol. 1984 Jun;4(6):1042–1049. doi: 10.1128/mcb.4.6.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler-Tuyns A., Birnstiel M. L. Structure and expression in L-cells of a cloned H4 histone gene of the mouse. J Mol Biol. 1981 Oct 5;151(4):607–625. doi: 10.1016/0022-2836(81)90426-5. [DOI] [PubMed] [Google Scholar]
- Sidman C. L., Paige C. J., Schreier M. H. B cell maturation factor (BMF): a lymphokine or family of lymphokines promoting the maturation of B lymphocytes. J Immunol. 1984 Jan;132(1):209–222. [PubMed] [Google Scholar]
- Storb U., O'Brien R. L., McMullen M. D., Gollahon K. A., Brinster R. L. High expression of cloned immunoglobulin kappa gene in transgenic mice is restricted to B lymphocytes. Nature. 1984 Jul 19;310(5974):238–241. doi: 10.1038/310238a0. [DOI] [PubMed] [Google Scholar]
- Taylor M. V., Metcalfe J. C., Hesketh T. R., Smith G. A., Moore J. P. Mitogens increase phosphorylation of phosphoinositides in thymocytes. 1984 Nov 29-Dec 5Nature. 312(5993):462–465. doi: 10.1038/312462a0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Van Ness B. G., Weigert M., Coleclough C., Mather E. L., Kelley D. E., Perry R. P. Transcription of the unrearranged mouse C kappa locus: sequence of the initiation region and comparison of activity with a rearranged V kappa-C kappa gene. Cell. 1981 Dec;27(3 Pt 2):593–602. doi: 10.1016/0092-8674(81)90401-3. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Histone-H1-dependent chromatin superstructures and the suppression of gene activity. Cell. 1984 Aug;38(1):17–27. doi: 10.1016/0092-8674(84)90522-1. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985 Feb;40(2):271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- Yuan D., Tucker P. W. Effect of lipopolysaccharide stimulation on the transcription and translation of messenger RNA for cell surface immunoglobulin M. J Exp Med. 1982 Oct 1;156(4):962–974. doi: 10.1084/jem.156.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]