Abstract
AIM: To optimize the experimental protocols for a simple, sensitive and accurate bleeding assay.
METHODS: Bleeding assay was performed in mice by tail tip amputation, immersing the tail in saline at 37 °C, continuously monitoring bleeding patterns and measuring bleeding volume from changes in the body weight. Sensitivity and extent of variation of bleeding time and bleeding volume were compared in mice treated with the P2Y receptor inhibitor prasugrel at various doses or in mice deficient of FcRγ, a signaling protein of the glycoprotein VI receptor.
RESULTS: We described details of the bleeding assay with the aim of standardizing this commonly used assay. The bleeding assay detailed here was simple to operate and permitted continuous monitoring of bleeding pattern and detection of re-bleeding. We also reported a simple and accurate way of quantifying bleeding volume from changes in the body weight, which correlated well with chemical assay of hemoglobin levels (r2 = 0.990, P < 0.0001). We determined by tail bleeding assay the dose-effect relation of the anti-platelet drug prasugrel from 0.015 to 5 mg/kg. Our results showed that the correlation of bleeding time and volume was unsatisfactory and that compared with the bleeding time, bleeding volume was more sensitive in detecting a partial inhibition of platelet’s haemostatic activity (P < 0.01). Similarly, in mice with genetic disruption of FcRγ as a signaling molecule of P-selectin glycoprotein ligand-1 leading to platelet dysfunction, both increased bleeding volume and repeated bleeding pattern defined the phenotype of the knockout mice better than that of a prolonged bleeding time.
CONCLUSION: Determination of bleeding pattern and bleeding volume, in addition to bleeding time, improved the sensitivity and accuracy of this assay, particularly when platelet function is partially inhibited.
Keywords: Mouse or mice, Tail bleeding assay, Prasugrel, Platelets, Hemostasis, FcRγ
INTRODUCTION
Bleeding assay is widely used as an in vivo assessment of haemostatic action of platelets in rodents, particularly in genetically modified mice or following treatment with anti-platelet drugs[1]. However, the protocol of the bleeding assay varies considerably among laboratories[2]. This assay involves a longitudinal incision or transverse amputation of the tip of the tail. Bleeding time is largely determined by the interaction between platelets and damaged vessel wall leading to hemostatic plug formation. The bleeding monitoring is usually conducted at room temperature either by blotting with filter paper every few seconds until bleeding ceases[3-6] or dipping the tail into tubes in a preset time-frame to determine the duration of bleeding[7,8]. Bleeding time is usually defined as the time of the first cessation of bleeding although recurrence of bleeding is known to occur[9], and accumulated bleeding time is also commonly used[9-11]. It is also common to immerse the tail in isotonic solution at 37 °C[9,12-17].
Whilst the current protocols permit a valid in vivo bleeding assay, significant limitations exist. Notably, the within-group variation is usually rather large[3-5,12,14-21], making this assay less sensitive in identifying between-group differences. Further, the amount of bleeding was not determined in a majority of studies apparently due to the notion that bleeding time itself is sufficient. However, we recently observed in mice treated with clopidogrel at different doses that while bleeding time was similar, bleeding volume differed significantly between groups, indicating that bleeding time alone was insufficient in differentiating the dose-dependent effect of anti-platelet interventions. Thus, it is necessary to improve and standardize the bleeding assay method[2]. For this purpose, we described here our experimental details of the assay for assessment of (1) dose-dependent effect of an anti-platelet drug prasugrel and (2) phenotype of FcRγ knockout (FcRγ-/-) mice with controversy reports on bleeding time[5,20]. In comparison with bleeding time measurement only, our method does show improved accuracy and sensitivity and yet simple to operate without requiring special equipment.
MATERIALS AND METHODS
Animals and drug treatment
Male C57Bl/6 mice at 12-17 wk of age were used. We also studied bleeding time and volume in FcRγ-/- mice (in C57Bl/6 background) a GPVI receptor signaling molecule. Previous studies showed that FcRγ-/- mice either had unchanged or prolonged bleeding time[4,18]. FcRγ-/- mice were kindly provided by Prof. Shaun Jackson (Australian Blood Disease Centre). All experimental procedures were approved by a local animal ethics committee and were in accordance with the Australian code of practice for the care and use of animals for scientific purposes as described by the National Health and Medical Research Council of Australia.
Using the bleeding assay, the dose-dependent effect of the purinergic receptor antagonist prasugrel was studied. Prasugrel tablets of 5 mg each (Eli Lilly, United States) were crushed to fine power, suspended in 10% methyl cellulous and given orally by daily gavage (0.2 mL/mouse) for 3 d. Six doses of prasugrel were tested at 0.015, 0.05, 0.15, 0.5, 1.5 and 5 mg/kg per day, respectively. This range of dosages was determined according to previous studies at a high dose of 5 mg/kg in mice[10,22,23] and clinically relevant doses (5-10 mg/d per person) of ≤ 0.15 mg/kg[24]. Bleeding assay was conducted on day-3 at 5-6 h after the last dose. Control mice received no treatment. For positive control, thrombocytopenia was induced in one group of mice (n = 6) by treatment with CD41 antibody (BD Biosciences) injection daily at 0.5 mg/kg (ip) for 3 doses[10]. This regime reduces circulating platelets by 90%[25].
Tail bleeding assay
Animals were anesthetized with a mixture of ketamine, xylazine and atropine (at 100, 10 and 1.2 mg/kg, respectively) and body weight (accurate to mg) was obtained. Animals were placed in prone position. A distal 10-mm segment of the tail was amputated with a scalpel. The tail was immediately immersed in a 50-mL Falcon tube containing isotonic saline pre-warmed in a water bath to 37 °C (Figure 1A). The position of the tail was vertical with the tip positioned about 2 cm below the body horizon. Each animal was monitored for 20 min even if bleeding ceased, in order to detect any re-bleeding. Bleeding time was determined using a stop clock. If bleeding on/off cycles occurred, the sum of bleeding times within the 20-min period was used. The experiment was terminated at the end of 20 min to avoid lethality during the experiment as required by the local animal ethics committee. Body weight, including the tail tip, was measured again, and the volume of blood loss during the experimental period was estimated from the reduction in body weight. At the end of experiment, animals were killed by anesthesia overdose.
Figure 1.
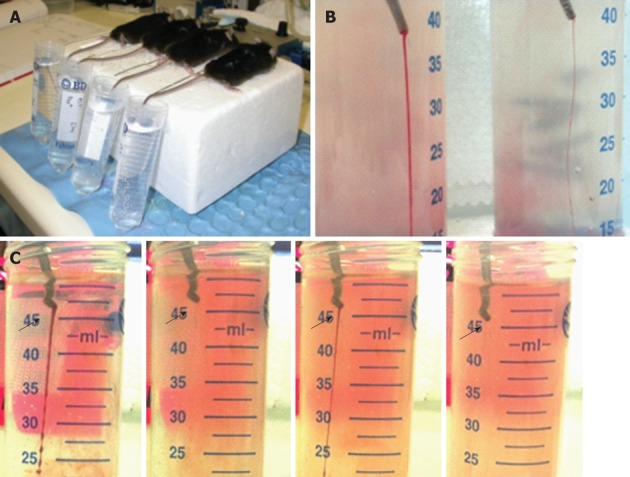
Setting-up of tail bleeding assay in mice. A: Bleeding assay was performed by amputation of the tail tip which was immediately merged in saline (37°C); B: Photo showing difference in the thickness of blood streams in animals receiving prasugrel at 5 (left) or 0.5 (right) mg/kg per day; C: Starting/stopping bleeding cycles (arrows) observed in a FcRγ-/- mouse.
Hemoglobin assay
To validate the accuracy of measurement of bleeding volume by changes in the body weight, in a separate batch of mice, blood cells were separated by centrifuge at 4000 r/min for 5 min at room temperature. The supernatant was removed and erythrocytes were re-suspended in 2 mL of lysis buffer (BD Pharm Lyse). After 10 min incubation in the lysis buffer, tubes were centrifuged at 10 000 r/min for 5 min. Concentrations of hemoglobin were measured spectrophotometrically using a Micro plate spectrophotometer at 550 nm (BioRad). OD readings for hemoglobin were plotted against respective changes in body weight (accurate to in mg).
Statistical analysis
Results are presented as mean ± SE or otherwise specified. Correlation analysis was performed using the least-square method. Between-group comparison was made by analysis of variance followed by the Newman-Keuls multiple-comparison test or unpaired t test. P < 0.05 was considered statistically significant.
RESULTS
Body weights ranged from 23 to 29 g and the averaged group means were similar among the groups (24 to 26 g). In our experimental setting, untreated mice had an average bleeding time of 2 min and bleeding volume of 0.05 mL. None of the control and untreated mice showed re-bleeding. Platelet depletion, induced by treatment with CD41 antibody, markedly prolonged bleeding time up to 20 min in all mice with an average bleeding volume of 1.07 mL. Difference in the severity of bleeding was easily detected based on the thickness of tail bleeding stream (Figure 1B). Re-bleeding was found in 40% of mice receiving prasugrel at doses ≤ 0.5 mg/kg and in 6/8 FcRγ-/- mice, and was readily visible (Figure 1C). This usually occurred with progressive thinning of the bleeding stream till complete stop for a period (usually a few minutes), followed by abrupt restart of bleeding.
An excellent correlation between changes in body weight and corresponding OD readings by spectrophotometry for hemoglobin concentrations (r2 = 0.990, P < 0.0001, Figure 2), validating the accuracy of evaluation of bleeding volume from changes in body weight in our experimental setting.
Figure 2.
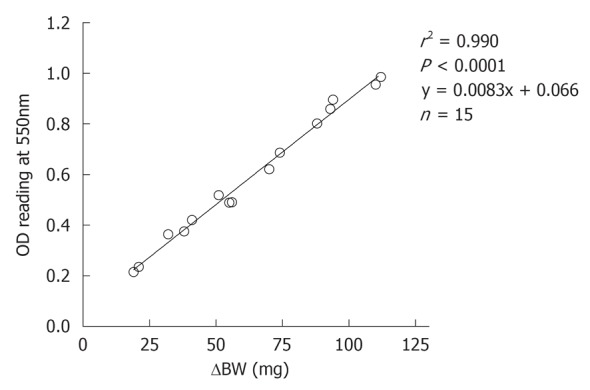
Correlation between changes in the body weight and OD readings for hemoglobin concentrations. Blood was collected in saline and blood cells were separated by centrifugation and erythrocytes re-suspended in lysis buffer. Concentrations of hemoglobin were measured spectrophotometrically.
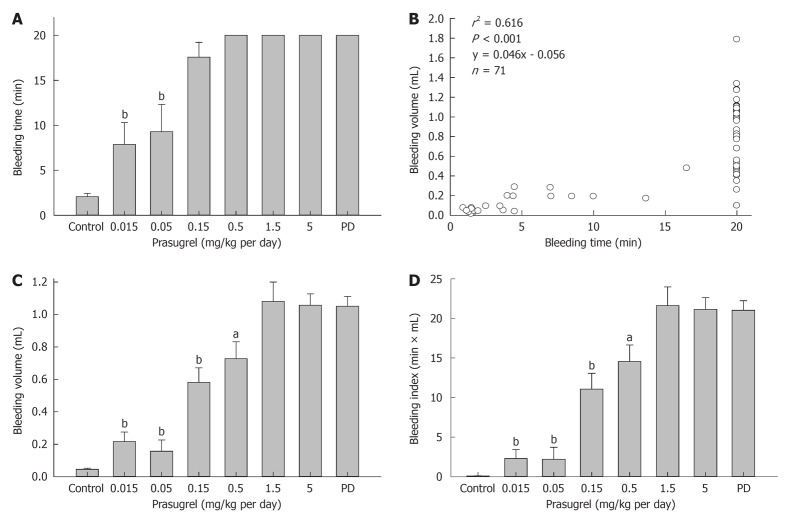
All animals treated with prasugrel at higher doses (i.e., 1.5 or 5 mg/kg per day) had a bleeding time over 20 min and bleeding volume of 0.7 to 1.8 mL each (1.06 ± 0.22 mL, mean ± SD). In animals receiving prasugrel at medium doses (i.e., 0.5 or 0.15 mg/kg), while bleeding time was over 20 min in all animals except one in 0.15 mg/kg group (Figure 3A), bleeding volume was significantly lower compared with either the 5 mg/kg prasugrel group or PD group (Figure 3B). This was in contrast to a lack of difference in bleeding time at intermediate dosages of prasugrel (0.15 or 0.5 mg/kg, Figure 3A and B).
Figure 3.
Dose-dependent reduction by the anti-platelet drug prasugrel on bleeding parameters. Bleeding time failed to detect dose-effect relation only at two lowest doses (0.015 and 0.05 mg/kg per day, A), whereas bleeding volume was significantly lower vs 5 mg/kg per day starting from 0.15 mg/kg per day (B). Panel C shows the correlation between bleeding time and volume. Note the wide variation of bleeding volume in animals with bleeding time exceeding 20 min (C). The bleeding index was derived as the product of time (min) and volume (mL, D). Parameters of all treated groups were significantly higher than the untreated control. Platelet depletion (PD) group served as a positive control. aP < 0.02, bP < 0.001 vs 5 mg/kg prasugrel group. A total of 8 groups of mice (n = 6-13) were studied.
Combined data from prasugrel experiment showed a significant correlation between bleeding time and bleeding volume (r2 = 0.613, P < 0.01, y = 0.046x - 0.056). However, there was a wide range of bleeding volumes in animals with bleeding time exceeding 20 min (Figure 3C), suggesting a dissociation of bleeding time and bleeding volume during the period studied. Thus, there was virtually lack of good association between bleeding volume and time. Considering the significance of both bleeding time and volume in the bleeding assay, we calculated from this set of data “bleeding index” as the product of time (in min) and volume (in mL, Figure 3D). This index was similar to that of bleeding volume in detecting dose-dependent inhibition of hemostasis by prasugrel. However, the variation of this index is greater than that of bleeding volume.
Under our modified experimental conditions and use of the tail amputation method, two FcRγ-/- mice had continuous bleeding up to 20 min and the rest of 6 mice showed recurrent bleeding episodes of 3 to 6 times within the 20 min period (Figure 4A). Bleeding volume in FcRγ-/- mice was significantly greater than that of wild-type controls (Figure 4B). This phenotype of FcRγ-/- mice was identified better by bleeding volume than by bleeding time.
Figure 4.
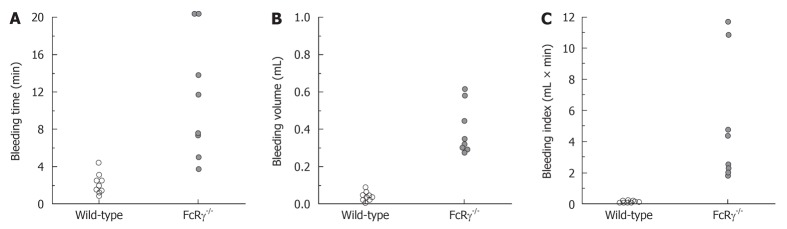
Tail bleeding time and volume measured in wild-type and FcRγ-/- mice. Bleeding assay was performed by tail tip amputation and then immersing the tail in saline at 37°C. FcRγ-/- mice showed a significant increase in both bleeding time and volume (P < 0.01 vs wild-type mice) and development of recurrent bleeding in 6/8 of animals studied. Note that the better separation of FcRγ-/- and control mice by bleeding volume or index than that by bleeding time.
DISCUSSION
The bleeding assay is a widely used test to explore hemostatic function of platelets. However, the details of the assay method have never been standardized. In the current study, we performed the assay by transecting the tip of the tail, immersing the tail in 37 °C saline, continuous monitoring of bleeding pattern, and determining bleeding volume from changes in body weight. Our results suggest that the details applied in the assay yielded a simple and sensitive determination of a partially inhibited hemostatic function of platelets. We emphasize that rather than describing novel techniques, by providing our experimental details we aimed to contribute to standardizing this in vivo assay.
The reported methods of estimating bleeding volume involve collection of blood into a fixed volume of saline and measurement of concentrations of hemoglobin spectrophotometrically or quantification of red blood cell density[9,11,17,18]. We found that loss of the body weight at the end of the assay was a valid approach to determining bleeding volume. During the 20-min experimental period, there was no loss of body weight due to excreting urine or feces. While increase in weights of saline containing tubes should also reflect the amount of blood loss, we found inconsistency of changes in weights of Falcon tubes containing 50 mL saline and pre-soaked in water bath, apparently due to evaporation of surface moisture, water loss due to trace amount left with the cap or attached to mouse tail. By measuring bleeding time and volume, we tested the effect of prasugrel over a wide range of dosages. The bleeding time was able to show a dose-dependent inhibition of platelet activity only at high doses. A significant but partial inhibition by prasugrel at medium doses (e.g., 0.5 and 0.15 mg/kg per day) was detected by a reduced bleeding volume without concomitant change in bleeding time. Further, those animals with bleeding time over 20 min showed a considerable variation in the bleeding volume over the assay period, indicating the limitation of bleeding time as a hemostatic index. By comparison with the bleeding time alone, our results show that either bleeding volume or bleeding index is more sensitive in detecting prasugrel dose-related inhibition of platelet function. This view is in keeping with a recent study showing a significant increase in the bleeding volume in mice deficient of Rab27b while bleeding time was found unreliable[26].
Johansen et al[11] described an automated system that included a vision camera and specially designed software for monitoring bleeding time. Volume of blood loss was derived from measurement of haemoglobin concentration[11]. Such a set-up represents an accurate bleeding assay for detection of multiple cycles of bleeding as well as blood loss, which is desirable to laboratory practice. In our setting, we visually and easily observed recurrence of bleeding in mice with either genetic (FcRγ-/-) or pharmacological (prasugrel) intervention leading to partial inhibition of platelet activity. Re-bleeding pattern is mostly likely due to some residual capability in these animals in formation of platelet thrombi to stop bleeding. However, a partial inhibition of platelets renders an unstable structure and poor adhesion to vessel wall of thrombi that can be washed away under arterial blood flow, similar to that described by Folts as a cyclic variation of blood flow of a stenotic artery with endothelial damage[27].
The location and the way of inducing tail vascular injury are also critical for the outcomes of the assay. Whereas Sarratt et al[20] reported a prolonged but variable bleeding time in FcRγ-/- mice with tail amputation, Mangin et al[5] observed no change in the bleeding time in FcRγ-/- mice using a longitudinal cutting method. Our finding of a prolonged bleeding time in FcRγ-/- mice using the amputation method was consistent with a previous report[20]. Further, we documented a significant increase in the bleeding volume in FcRγ-/- mice. Our findings in FcRγ-/- mice clearly indicate a defect in GPVI signaling and hemostatic action of platelets, as one would have expected[24,28,29]. One potential variable of the bleeding assay comes from different ambient environment by either exposing the tip of the tail to room temperature and air (causing dryness) or immersing the tail in 37 °C saline. Dejana et al[12] determined the tail blood flow by radioisotope labeled microsphere method and found a 3-fold higher tail flow under 37 °C vs that under the room temperature. However, bleeding time was actually shorter at 37 °C than that at 23 °C[12]. Thus, to prevent peripheral hypothermia and resultant vasoconstriction, a regional temperature of 37 °C and moisture are the optimal environment for hemostatic testing relative to that of room temperature and dryness. Other factors potentially affecting the assay might include body temperature, type of anesthetics used and hemodynamic conditions of animals. However, we observed in a previous study that there was no difference in bleeding time between mice with and without myocardial infarction[10].
Whereas tail bleeding is a useful and commonly used in vivo assay without requirement for specialized equipment, there is a need to standardize the details of the assay. We recommend the following: First, tail amputation appears better than longitudinal tail incision. Second, instead of exposure to the air and room temperature, it is recommended to submerge the amputated tail in saline at 37 °C. In addition to keeping constant temperature and moisture at the cut surface, this also allows for continuous monitoring of bleeding stream without disturbance of the wound. Third, visual monitoring of bleeding should continue even after bleeding ceases for likely re-bleeding, a phenomenon that is common when hemostatic action of platelets is partially inhibited[29-31]. Fourth, the volume of bleeding can be easily determined by change in body weight at the end of the assay. Whilst “bleeding index” derived from our two sets of data does not appear to provide further information than that by the bleeding volume, this index takes into consideration of both bleeding time and volume.
In conclusion, the details of bleeding assay described here are easy to perform and allow for detection of recurrent bleeding and bleeding volume, both parameters being useful in assessment of the haemostatic action of platelets.
COMMENTS
Background
Determination of haemeostatic activity of platelets in vivo is important for phenotypic determination of genetically altered mice or assessment of anti-platelet drugs. Tail bleeding assay has been widely adopted for this purpose with the bleeding time as the most commonly used parameter. This index, however, suffers from limitations of a large individual variation making this assay an insensitive one based on many reports. Furthermore there is considerable inconsistency among laboratories in performing the assay rendering data comparison difficult.
Research frontiers
There is an urgent need of standardizing and optimizing the current method of bleeding assay. In the current study, effort was given to establish a simple protocol for this assay and to compare bleeding time and bleeding volume as separate measures.
Innovations and breakthroughs
This paper provides detailed description of the tail bleeding assay by continuously monitoring bleeding pattern and measurement of bleeding volume. The results from drug dose-effect determination and from a genetically modified mouse strain all showed improvement in sensitivity and reduction in individual variation using the method described and by determination of the bleeding volume.
Applications
The detailed description of the assay method in this paper would be helpful towards standardizing and optimizing the tail bleeding assay. In vivo determination of the anti-platelet agent prasugrel using this assay provides useful reference information for future studies testing this class of agents in mice. The authors provide explanation for contradictory reports by different groups on bleeding assay of FcRγ knockout mice.
Terminology
The genetically modified mouse strains have been widely used for research on platelet activity. Mice are also commonly used for assessment of anti-platelet drugs. The only in vivo measure of platelet activity is tail bleeding assay. The study would contribute to the optimization and standardization of this assay.
Peer review
This study addresses an area of interest with obvious importance to the hematologists. The manuscript presents an interesting assay of platelet haemostatic activity in mice. It is clearly written.
Footnotes
Supported by Project and Fellowship Grants from the National Health and Medical Research Council of Australia
Peer reviewer: Isabel Andia, PhD, Departamento de Investigación, Hospital de Zamudio, Bº Arteaga 107, 48016 Zamudio Vizcaya, Spain
S- Editor Li JY L- Editor A E- Editor Zheng XM
References
- 1.Day SM, Reeve JL, Myers DD, Fay WP. Murine thrombosis models. Thromb Haemost. 2004;92:486–494. [PubMed] [Google Scholar]
- 2.Greene TK, Schiviz A, Hoellriegl W, Poncz M, Muchitsch EM. Towards a standardization of the murine tail bleeding model. J Thromb Haemost. 2010;8:2820–2822. doi: 10.1111/j.1538-7836.2010.04084.x. [DOI] [PubMed] [Google Scholar]
- 3.Nieswandt B, Schulte V, Bergmeier W, Mokhtari-Nejad R, Rackebrandt K, Cazenave JP, Ohlmann P, Gachet C, Zirngibl H. Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in mice. J Exp Med. 2001;193:459–469. doi: 10.1084/jem.193.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grüner S, Prostredna M, Aktas B, Moers A, Schulte V, Krieg T, Offermanns S, Eckes B, Nieswandt B. Anti-glycoprotein VI treatment severely compromises hemostasis in mice with reduced alpha2beta1 levels or concomitant aspirin therapy. Circulation. 2004;110:2946–2951. doi: 10.1161/01.CIR.0000146341.63677.3C. [DOI] [PubMed] [Google Scholar]
- 5.Mangin P, Yap CL, Nonne C, Sturgeon SA, Goncalves I, Yuan Y, Schoenwaelder SM, Wright CE, Lanza F, Jackson SP. Thrombin overcomes the thrombosis defect associated with platelet GPVI/FcRgamma deficiency. Blood. 2006;107:4346–4353. doi: 10.1182/blood-2005-10-4244. [DOI] [PubMed] [Google Scholar]
- 6.Liu JY, Li N, Yang J, Li N, Qiu H, Ai D, Chiamvimonvat N, Zhu Y, Hammock BD. Metabolic profiling of murine plasma reveals an unexpected biomarker in rofecoxib-mediated cardiovascular events. Proc Natl Acad Sci USA. 2010;107:17017–17022. doi: 10.1073/pnas.1011278107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broze GJ, Yin ZF, Lasky N. A tail vein bleeding time model and delayed bleeding in hemophiliac mice. Thromb Haemost. 2001;85:747–748. [PubMed] [Google Scholar]
- 8.Gushiken FC, Han H, Li J, Rumbaut RE, Afshar-Kharghan V. Abnormal platelet function in C3-deficient mice. J Thromb Haemost. 2009;7:865–870. doi: 10.1111/j.1538-7836.2009.03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.André P, DeGuzman F, Haberstock-Debic H, Mills S, Pak Y, Inagaki M, Pandey A, Hollenbach S, Phillips DR, Conley PB. Thienopyridines, but not elinogrel, result in off-target effects at the vessel wall that contribute to bleeding. J Pharmacol Exp Ther. 2011;338:22–30. doi: 10.1124/jpet.110.178574. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Gao XM, Fang L, Jennings NL, Su Y, Q X, Samson AL, Kiriazis H, Wang XF, Shan L, et al. Novel role of platelets in mediating inflammatory responses and ventricular rupture or remodeling following myocardial infarction. Arterioscler Thromb Vasc Biol. 2011;31:834–841. doi: 10.1161/ATVBAHA.110.220467. [DOI] [PubMed] [Google Scholar]
- 11.Johansen PB, Henriksen L, Andresen PR, Lauritzen B, Jensen KL, Juhl TN, Tranholm M. Automated registration of tail bleeding in rats. Thromb Haemost. 2008;99:956–962. doi: 10.1160/TH07-12-0738. [DOI] [PubMed] [Google Scholar]
- 12.Dejana E, Callioni A, Quintana A, de Gaetano G. Bleeding time in laboratory animals. II - A comparison of different assay conditions in rats. Thromb Res. 1979;15:191–197. doi: 10.1016/0049-3848(79)90064-1. [DOI] [PubMed] [Google Scholar]
- 13.Robertson JO, Li W, Silverstein RL, Topol EJ, Smith JD. Deficiency of LRP8 in mice is associated with altered platelet function and prolonged time for in vivo thrombosis. Thromb Res. 2009;123:644–652. doi: 10.1016/j.thromres.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheli Y, Jensen D, Marchese P, Habart D, Wiltshire T, Cooke M, Fernandez JA, Ware J, Ruggeri ZM, Kunicki TJ. The Modifier of hemostasis (Mh) locus on chromosome 4 controls in vivo hemostasis of Gp6-/- mice. Blood. 2008;111:1266–1273. doi: 10.1182/blood-2007-09-111369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subramaniam M, Frenette PS, Saffaripour S, Johnson RC, Hynes RO, Wagner DD. Defects in hemostasis in P-selectin-deficient mice. Blood. 1996;87:1238–1242. [PubMed] [Google Scholar]
- 16.Kato K, Kanaji T, Russell S, Kunicki TJ, Furihata K, Kanaji S, Marchese P, Reininger A, Ruggeri ZM, Ware J. The contribution of glycoprotein VI to stable platelet adhesion and thrombus formation illustrated by targeted gene deletion. Blood. 2003;102:1701–1707. doi: 10.1182/blood-2003-03-0717. [DOI] [PubMed] [Google Scholar]
- 17.Bolliger D, Szlam F, Suzuki N, Matsushita T, Tanaka KA. Heterozygous antithrombin deficiency improves in vivo haemostasis in factor VIII-deficient mice. Thromb Haemost. 2010;103:1233–1238. doi: 10.1160/TH09-10-0732. [DOI] [PubMed] [Google Scholar]
- 18.Carmeliet P, Stassen JM, Schoonjans L, Ream B, van den Oord JJ, De Mol M, Mulligan RC, Collen D. Plasminogen activator inhibitor-1 gene-deficient mice. II. Effects on hemostasis, thrombosis, and thrombolysis. J Clin Invest. 1993;92:2756–2760. doi: 10.1172/JCI116893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas GM, Panicot-Dubois L, Lacroix R, Dignat-George F, Lombardo D, Dubois C. Cancer cell-derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo. J Exp Med. 2009;206:1913–1927. doi: 10.1084/jem.20082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarratt KL, Chen H, Zutter MM, Santoro SA, Hammer DA, Kahn ML. GPVI and alpha2beta1 play independent critical roles during platelet adhesion and aggregate formation to collagen under flow. Blood. 2005;106:1268–1277. doi: 10.1182/blood-2004-11-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Smith PL, Hsu MY, Tamasi JA, Bird E, Schumacher WA. Deficiency in thrombin-activatable fibrinolysis inhibitor (TAFI) protected mice from ferric chloride-induced vena cava thrombosis. J Thromb Thrombolysis. 2007;23:41–49. doi: 10.1007/s11239-006-9009-4. [DOI] [PubMed] [Google Scholar]
- 22.Duggan ST, Keating GM. Prasugrel: a review of its use in patients with acute coronary syndromes undergoing percutaneous coronary intervention. Drugs. 2009;69:1707–1726. doi: 10.2165/10484190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto M, Sugidachi A, Isobe T, Niitsu Y, Ogawa T, Jakubowski JA, Asai F. The influence of P2Y12 receptor deficiency on the platelet inhibitory activities of prasugrel in a mouse model: evidence for specific inhibition of P2Y12 receptors by prasugrel. Biochem Pharmacol. 2007;74:1003–1009. doi: 10.1016/j.bcp.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 24.Moroi M, Jung SM. Platelet glycoprotein VI: its structure and function. Thromb Res. 2004;114:221–233. doi: 10.1016/j.thromres.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 25.van der Heyde HC, Gramaglia I, Sun G, Woods C. Platelet depletion by anti-CD41 (alphaIIb) mAb injection early but not late in the course of disease protects against Plasmodium berghei pathogenesis by altering the levels of pathogenic cytokines. Blood. 2005;105:1956–1963. doi: 10.1182/blood-2004-06-2206. [DOI] [PubMed] [Google Scholar]
- 26.Tolmachova T, Abrink M, Futter CE, Authi KS, Seabra MC. Rab27b regulates number and secretion of platelet dense granules. Proc Natl Acad Sci USA. 2007;104:5872–5877. doi: 10.1073/pnas.0609879104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folts J. An in vivo model of experimental arterial stenosis, intimal damage, and periodic thrombosis. Circulation. 1991;83:IV3–I14. [PubMed] [Google Scholar]
- 28.Goto S, Tamura N, Ishida H, Ruggeri ZM. Dependence of platelet thrombus stability on sustained glycoprotein IIb/IIIa activation through adenosine 5'-diphosphate receptor stimulation and cyclic calcium signaling. J Am Coll Cardiol. 2006;47:155–162. doi: 10.1016/j.jacc.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 29.Séverin S, Gratacap MP, Lenain N, Alvarez L, Hollande E, Penninger JM, Gachet C, Plantavid M, Payrastre B. Deficiency of Src homology 2 domain-containing inositol 5-phosphatase 1 affects platelet responses and thrombus growth. J Clin Invest. 2007;117:944–952. doi: 10.1172/JCI29967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andre P, Delaney SM, LaRocca T, Vincent D, DeGuzman F, Jurek M, Koller B, Phillips DR, Conley PB. P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J Clin Invest. 2003;112:398–406. doi: 10.1172/JCI17864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, Lachowicz JE, Zhang FL, Gustafson E, Monsma FJ, Wiekowski MT, et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107:1591–1598. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]