Abstract
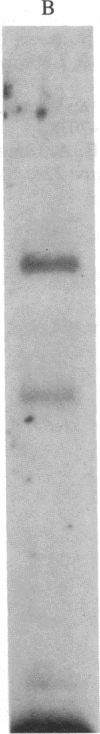
The mechanism by which viruses bind to and infect specific tissues to cause disease has only recently begun to be understood. The mammalian reoviruses provide an especially attractive model for studying the details of cell surface recognition. The cell and tissue tropism of reovirus is determined by a portion of the viral hemagglutinin termed the neutralization domain. We have reported previously on the generation of both monoclonal and polyclonal anti-idiotypic antibodies that mimic the viral hemagglutinin in the specificity of binding to the reovirus receptor. By using these anti-idiotypic antibodies as specific probes, we have successfully isolated the mammalian reovirus receptor from neuronal and lymphoid cells. In the present study, we report that the reovirus receptor is structurally similar to the mammalian beta-adrenergic receptor. This conclusion is based on the following observations: (i) purified beta-adrenergic receptor is immunoprecipitable by anti-reovirus receptor antibody; (ii) purified reovirus receptor obtained from murine thymoma cells and beta-adrenergic receptor obtained from calf lung exhibit identical molecular masses and isoelectric points; (iii) trypsin digests of purified reovirus and beta-adrenergic receptors display indistinguishable fragment patterns; (iv) purified reovirus receptor binds the beta-antagonist [125I]iodohydroxybenzylpindolol and this binding is blocked by the beta-agonist isoproterenol.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benovic J. L., Stiles G. L., Lefkowitz R. J., Caron M. G. Photoaffinity labelling of mammalian beta-adrenergic receptors: metal-dependent proteolysis explains apparent heterogeneity. Biochem Biophys Res Commun. 1983 Jan 27;110(2):504–511. doi: 10.1016/0006-291x(83)91178-6. [DOI] [PubMed] [Google Scholar]
- Co M. S., Gaulton G. N., Fields B. N., Greene M. I. Isolation and biochemical characterization of the mammalian reovirus type 3 cell-surface receptor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1494–1498. doi: 10.1073/pnas.82.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmock N. J. Review article initial stages in infection with animal viruses. J Gen Virol. 1982 Mar;59(Pt 1):1–22. doi: 10.1099/0022-1317-59-1-1. [DOI] [PubMed] [Google Scholar]
- Fields B. N., Greene M. I. Genetic and molecular mechanisms of viral pathogenesis: implications for prevention and treatment. Nature. 1982 Nov 4;300(5887):19–23. doi: 10.1038/300019a0. [DOI] [PubMed] [Google Scholar]
- Fingeroth J. D., Weis J. J., Tedder T. F., Strominger J. L., Biro P. A., Fearon D. T. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4510–4514. doi: 10.1073/pnas.81.14.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade R., Barel M., Ehlin-Henriksson B., Klein G. gp140, the C3d receptor of human B lymphocytes, is also the Epstein-Barr virus receptor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1490–1493. doi: 10.1073/pnas.82.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser C. M., Venter J. C. Monoclonal antibodies to beta-adrenergic receptors: use in purification and molecular characterization of beta receptors. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7034–7038. doi: 10.1073/pnas.77.12.7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Morein B., Fries E., Simons K., Robinson P., Schirrmacher V., Terhorst C., Strominger J. L. Human (HLA-A and HLA-B) and murine (H-2K and H-2D) histocompatibility antigens are cell surface receptors for Semliki Forest virus. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3846–3850. doi: 10.1073/pnas.75.8.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homcy C. J., Rockson S. G., Countaway J., Egan D. A. Purification and characterization of the mammalian beta 2-adrenergic receptor. Biochemistry. 1983 Feb 1;22(3):660–668. doi: 10.1021/bi00272a021. [DOI] [PubMed] [Google Scholar]
- Inada T., Mims C. A. Mouse Ia antigens are receptors for lactate dehydrogenase virus. Nature. 1984 May 3;309(5963):59–61. doi: 10.1038/309059a0. [DOI] [PubMed] [Google Scholar]
- Jondal M., Klein G., Oldstone M. B., Bokish V., Yefenof E. Surface markers on human B and T lymphocytes. VIII. Association between complement and Epstein-Barr virus receptors on human lymphoid cells. Scand J Immunol. 1976;5(4):401–410. doi: 10.1111/j.1365-3083.1976.tb00294.x. [DOI] [PubMed] [Google Scholar]
- Kauffman R. S., Noseworthy J. H., Nepom J. T., Finberg R., Fields B. N., Greene M. I. Cell receptors for the mammalian reovirus. II. Monoclonal anti-idiotypic antibody blocks viral binding to cells. J Immunol. 1983 Nov;131(5):2539–2541. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Stadel J. M., Caron M. G. Adenylate cyclase-coupled beta-adrenergic receptors: structure and mechanisms of activation and desensitization. Annu Rev Biochem. 1983;52:159–186. doi: 10.1146/annurev.bi.52.070183.001111. [DOI] [PubMed] [Google Scholar]
- Lentz T. L., Burrage T. G., Smith A. L., Crick J., Tignor G. H. Is the acetylcholine receptor a rabies virus receptor? Science. 1982 Jan 8;215(4529):182–184. doi: 10.1126/science.7053569. [DOI] [PubMed] [Google Scholar]
- Nepom J. T., Tardieu M., Epstein R. L., Noseworthy J. H., Weiner H. L., Gentsch J., Fields B. N., Greene M. I. Virus-binding receptors: similarities to immune receptors as determined by anti-idiotypic antibodies. Surv Immunol Res. 1982;1(3):255–261. doi: 10.1007/BF02918466. [DOI] [PubMed] [Google Scholar]
- Nepom J. T., Weiner H. L., Dichter M. A., Tardieu M., Spriggs D. R., Gramm C. F., Powers M. L., Fields B. N., Greene M. I. Identification of a hemagglutinin-specific idiotype associated with reovirus recognition shared by lymphoid and neural cells. J Exp Med. 1982 Jan 1;155(1):155–167. doi: 10.1084/jem.155.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseworthy J. H., Fields B. N., Dichter M. A., Sobotka C., Pizer E., Perry L. L., Nepom J. T., Greene M. I. Cell receptors for the mammalian reovirus. I. Syngeneic monoclonal anti-idiotypic antibody identifies a cell surface receptor for reovirus. J Immunol. 1983 Nov;131(5):2533–2538. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Southern P., Rodriquez M., Lampert P. Virus persists in beta cells of islets of Langerhans and is associated with chemical manifestations of diabetes. Science. 1984 Jun 29;224(4656):1440–1443. doi: 10.1126/science.6203172. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Tishon A., Dutko F. J., Kennedy S. I., Holland J. J., Lampert P. W. Does the major histocompatibility complex serve as a specific receptor for Semliki Forest virus? J Virol. 1980 Apr;34(1):256–265. doi: 10.1128/jvi.34.1.256-265.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles G. L., Strasser R. H., Lavin T. N., Jones L. R., Caron M. G., Lefkowitz R. J. The cardiac beta-adrenergic receptor. Structural similarities of beta 1 and beta 2 receptor subtypes demonstrated by photoaffinity labeling. J Biol Chem. 1983 Jul 10;258(13):8443–8449. [PubMed] [Google Scholar]
- Tardieu M., Epstein R. L., Weiner H. L. Interaction of viruses with cell surface receptors. Int Rev Cytol. 1982;80:27–61. doi: 10.1016/S0074-7696(08)60366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]