Abstract
Cancer prevention research has drawn much attention worldwide. It is believed that some types of cancer can be prevented by following a healthy life style. Cancer chemoprevention by either natural or synthetic agents is a promising route towards lowering cancer incidence. In recent years, the concept of cancer chemoprevention has evolved greatly. Experimental studies in animal models demonstrate that the reversal or suppression of premalignant lesions by chemopreventive agents is achievable. Natural occurring agents such as dietary phytochemicals, tea polyphenols and resveratrol show chemopreventive activity in animal models. Moreover, clinical trials for testing the safety and efficacy of a variety of natural agents in preventing or treating human malignancy have been ongoing. Here, we summarize experimental data on the chemopreventive or tumor suppressive effects of several natural compounds including curcumin, (-)-epigallocatechin-3-gallate, resveratrol, indole-3-carbinol, and vitamin D.
Keywords: Cancer, Chemoprevention, Natural agents, (-)-Epigallocatechin-3-gallate, Resveratrol, Curcumin, Vitamin D, Indole-3-carbinol
INTRODUCTION
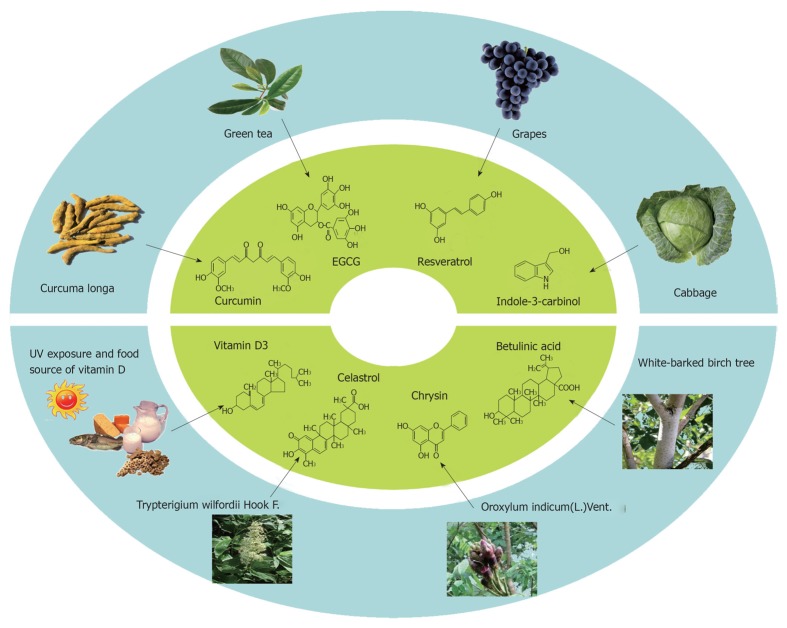
Cancer is common disease that limits lifespan. Many factors including life style, genetic variation, virus infection and chronic inflammation may affect the susceptibility to cancer. In the past decades, both the diagnosis and treatment of malignant tumors are improving. In addition to traditional treatments such as chemotherapy and radiotherapy, molecular targeted therapy is emerging as a promising trend for cancer therapeutics. For those who are at high risk for cancer, chemoprevention may be an alternative intervention to inhibit or delay carcinogenesis. While a number of chemotherapeutic agents have been administered in the clinic for many years, there is still long way to go for chemopreventive agents to be safely and effectively administered in human populations. The identification of chemopreventive targets and biomarkers that can help monitoring their effectiveness are a huge challenge. In addition to synthetic compounds, many natural products have been found to be able to inhibit carcinogenesis, at least in animal models. There are many ongoing clinical trails to test the safety and efficacy of natural agents in preventing or treating cancer (Table 1). It is highly likely that natural agents can be used for cancer prevention without recognizable adverse effects. Here, we highlight the experimental evidence concerning some natural agents that exhibit protective effect against cancer. The sources of some natural compounds are shown in Figure 1.
Table 1.
Selected ongoing clinical trials with natural anti-cancer compounds
| Agent | Phase | Status | Trial type | Conditions/cancer type | Combinati-on | Trial No. |
| Curcumin | Phase II | Recruiting | Prevention | Familial adenomatous polyposis | NCT00641147 | |
| Phase I | Completed | Prevention | Healthy | NCT00027495 | ||
| Phase I | Recruiting | Treatment | Advanced osteosarcoma | NCT00689195 | ||
| Phase II | ||||||
| Phase II | Recruiting | Treatment | Advanced pancreatic cancer | NCT00094445 | ||
| Tea polyphenols | Phase II | Recruiting | Prevention | Postmenopausal women with high breast density | NCT00917735 | |
| Phase II | Recruiting | Prevention | Tobacco use disorder | NCT00611650 | ||
| Phase I | Recruiting | Prevention | Premalignant lesions of the head and neck | Erlotinib | NCT01116336 | |
| Phase I | Recruiting | Treatment | Small cell lung carcinoma | NCT01317953 | ||
| Phase II | Recruiting | Treatment | Multiple myeloma and plasma cell neoplasm | NCT00942422 | ||
| Phase II | Completed | Treatment | Bladder cancer | NCT00088946 | ||
| Indole-3-carbinol/3,3-diindolylmethane | Phase II | Completed | Prevention | Healthy men at risk for prostate cancer progression | NCT00579332 | |
| Phase III | ||||||
| Phase I | Recruiting | Prevention | Women with a BRCA1 mutation | NCT01022333 | ||
| Phase I | Completed | Treatment | Prostate cancer | Radical prostatectomy | NCT00450229 | |
| Phase II | ||||||
| Phase II | Recruiting | Treatment | Breast cancer | NCT01391689 | ||
| Phase III | ||||||
| Vitamin D | Completed | Observation of the relationship between vitamin D level and thyroid cancer | Thyroid cancer | NCT00719615 | ||
| Phase II | Recruiting | Prevention | Postmenopausal women at high risk for breast cancer | NCT00859651 | ||
| Phase III | Enrolling by invitation | Prevention | Adenomatous colon polyps | Calcium | NCT00399607 | |
| Phase I | Recruiting | Treatment | Metastatic melanoma | Temozolomide | NCT00301067 | |
| Phase II | ||||||
| Phase II | Completed | Treatment | Metastatic or locally advanced pancreatic cancer | Docetaxel | NCT00238199 | |
| Phase IV | Completed | Prevention of osteoporosis | Postmenopausal breast cancer survivors | Risedronate; calcium | NCT00567606 |
Data from the United States National Institutes of Health, http://www.clinicaltrials.gov/.
Figure 1.
The sources of natural anti-cancer agents, including curcumin, (-)-epigallocatechin-3-gallate, resveratrol, indole-3-carbinol, and vitamin D, chrysin, celastrol and betulinic acid.
CURCUMIN
Curcumin, a polyphenolic molecule isolated from the roots (rhizomes) of the plant Curcuma longa, is a promising compound for cancer chemoprevention and therapy[1,2]. Although Curcuma longa and its chemical components have been used in Chinese and Hindu medicine for thousands of years, curcumin has attracted much attention in recent decades because of its anticancer activity. The beneficial effects of curcumin include anti-oxidant, anti-inflammatory, anti-proliferative, and anti-angiogenic properties[1,3]. Many preclinical studies of curcumin have shown anti-carcinogenic and therapeutic effects in various tumor cell lines and xenograft models. The anticancer efficacy of curcumin is well established in a range of animal cancer models including those associated with colon, breast, pancreas, lung, kidney, bladder, blood and skin[1]. It is worth mentioning that curcumin has the ability to kill cancer cells selectively without apparent toxicity to nonmalignant cells, a good property for a cancer-preventive candidate[4]. Extensive toxicological screening and preclinical investigation showed minimal adverse effects of curcumin administration in mice, rats, dogs, and monkeys[5]. Phase I and phase II clinical trials have already demonstrated the safety of curcumin even at high doses (8-12 g/d) over several months. Adverse events were mainly nausea and diarrhea[6-9].
Regardless of its excellent safety profile, the poor solubility and low bioavailability of curcumin are obstacles to therapeutic drug development. Data on the pharmacokinetics, metabolites, and systemic bioavailability of curcumin in rodents and humans show that curcumin is poorly absorbed, rapidly metabolized, and may have limited systemic bioavailability[10,11]. In fact, after oral administration of curcumin, very low concentrations of curcumin or corresponding metabolites are found in patient serum and tissues outside the gastrointestinal tract[8,12,13]. Compared to the effective concentrations in vitro (5-50 μmol/L), the poor absorption and bioavailability of curcumin suggest that its anticancer effects may be limited in vivo. Some approaches to improving the bioavailability of curcumin have been investigated, including the combination with adjuvants, the use of chemical analogues and novel delivery methods[14,15].
It is interesting that studies in animals still show curcumin is an effective agent for several cancer models, in spite of its limited bioavailability. It is not clear whether this efficacy comes from unmeasured curcumin metabolites, or for other unknown indirect effects. The pharmacodynamic data in humans is still limited. Currently, several phase I and phase II clinical trials are ongoing to investigate the benefits of curcumin as a chemopreventive and chemotherapeutic agent in a variety of cancers, including multiple myeloma, pancreatic cancer, breast cancer and colon cancer (www.clinicaltrials.gov). According to reported data from these ongoing studies, some results are promising. Results from a phase I clinical study of twenty-five patients with various pre-malignant or high-risk lesions suggested that oral curcumin may have chemopreventive effects on these lesions (histological improvement)[8]. In another study from the Cleveland Clinic in Florida, five patients with familial adenomatous polyposis were treated with a combination of curcumin and quercetin three times a day for a mean duration of 6 mo. The numbers and sizes of polyps were reduced in all patients compared to baseline values[16]. A recent phase II clinical trial reported by Carroll and colleagues investigated curcumin’s potential activities for prevention of colorectal neoplasia in smokers with aberrant crypt foci (ACF). The data showed a significant reduction of ACF number by curcumin at the 4-g dose level and indicated that curcumin may have cancer-preventive effects in the setting of early pre-invasive neoplastic lesions. Interpretation of this study, however, was limited by the remaining controversy around ACF as a biomarker of colon carcinogenesis, nonrandomization and lack of placebo group[17].
Curcumin efficacy in the treatment of human pancreatic cancer has been reported in a phase II clinical trial in patients with advanced disease. Patients received 8 g curcumin by mouth daily until disease progression, with restaging every 2 mo. Twenty-one of twenty-five patients were evaluable for response. Low concentrations of curcumin were able to elicit a biological effect by downregulating the expression of nuclear factor κB (NF-κB), cyclooxygenase (COX)-2 and phosphorylated signal transducer and activator of transcription 3 in peripheral blood mononuclear cells derived from patients[9]. A further phase II clinical studies suggested that a combination of gemcitabine and curcumin is a feasible treatment for patients with pancreatic cancer[18]. Bayet-Robert et al[19] treated 14 advanced and metastatic breast cancer patients with a combination of curcumin and docetaxel. The study demonstrated that the combination therapy decreased the vascular endothelial grow factor (VEGF) levels and showed encouraging efficacy. Hopefully, there will soon be more data to demonstrate the anticancer effects of curcumin, especially measurements which confirm the mechanism-based molecular targets which are really implicated in vivo.
(-)-EPIGALLOCATECHIN-3-GALLATE
Tea, from the plant Camellia sinensis, is one of the most popular beverages consumed worldwide. The most abundant chemical compound in green tea is catechins, which include (-)-epigallocatechin-3-gallate (EGCG), (-)-epigallocalechin, (-)-epicatechin-3-gallate and (-)-epicatechin. Among these, EGCG accounts for more than 50% of the total catechins, and appears to be the most effective and best-studied constituent of green tea[20].
EGCG holds considerable promise for chemoprevention according to epidemiological, cell culture, animal and clinical studies. EGCG has been shown to cause growth inhibition and apoptosis in a number of human cancer cell lines in vitro and inhibit tumor incidence and multiplicity in animal models, such as liver, colon, prostate, pancreas, mammary glands, lung and skin cancer models[21]. The mechanisms underlying EGCG anticancer effects include anti-oxidant activities, modification of carcinogen metabolism, prevention of DNA damage, induction of cell cycle arrest and apoptosis, inhibition of metastasis, proteasome inhibition and modulation of multiple signal transduction pathways [epidermal growth factor receptor, human epidermal growth factor receptor 2, VEGF receptor, insulin-like growth factor (IGF)1R, phosphoinositide 3-kinase/AKT, mitogen-activated protein kinase and NF-κB signaling][22]. Abundant evidence from animal cancer models has demonstrated the strong chemopreventive effects of EGCG. As early as 1987, Yoshizawa et al[23] reported that the application of EGCG suppressed 7,12-dimethylbenz[a]anthracene (DMBA) plus teleocidin-initiated carcinogenesis. EGCG significantly decreased tumor incidence and burden per mouse compared with controls. The subsequent studies showed EGCG to have a broad spectrum against carcinogens. In the animal model, EGCG effectively inhibited (4-methylnitro-samino)-1-(3-pyridyl)-1-butanon (NNK) and benzo(a)pyrene-induced lung cancer, azoxymethane-induced ACF and colon tumors, NNK and diethylnitrosamine-induced liver tumors, UV-induced skin cancer, and N-butyl-N-(4-hydroxybuty1)-nitrosamine-induced urinary bladder tumors[24].
Despite the impressing anti-tumor effect of EGCG in animal, the available epidemiological evidence on tea consumption and cancer prevention in humans has not yielded conclusive results. The inconsistent results of epidemiological studies were probably due to various confounding factors. The quantity and quality of the tea consumed will definitely affect the outcome of epidemiological studies. In addition, the effect of caffeine in tea, large intersubject and intrasubject variability could be additional contributing factors to the inconsistency[24]. Furthermore, the concentrations of EGCG in plasma and tissue after simply drinking green tea is low compared to the effective concentrations used in cell culture experiments (10-100 μmol/L)[20]. To minimalize the confounding effects, more potent tools were used in later well-designed clinical intervention studies, including a defined green tea catechin (GTC) extract, EGCG-enriched fractions such as Polyphenone E (the EGCG content about 70%) and highly purified EGCG provided by a pharmaceutical company.
Several studies of the systemic bioavailability of orally administered catechins in human volunteers have been conducted. Chow et al[25,26] conducted several studies to examine the safety, tolerability, and pharmacokinetic properties of single and multiple dose administration of EGCG and Polyphenon E from 200 to 800 mg. Their studies showed that the oral bioavailability of tea polyphenols in humans was low. Oral administration of EGCG and Polyphenon E at the same dose level (based on EGCG content) resulted in similar plasma EGCG levels. However, the repeated administration of 800 mg of green tea polyphenols once daily for 4 wk resulted in a 60% increase in the systemic availability of free EGCG, which may be due to inhibition of presystemic elimination of this catechin. The majority of clinical studies demonstrate the safety and limited side effects of EGCG. However, a recent review suggested a causal association between green tea and liver damage. This hepatotoxicity may be attributed to EGCG or its metabolites which, under particular conditions related to the patient’s metabolism, can induce oxidative stress in the liver[27].
Some data are currently available from EGCG chemoprevetion and chemotherapy trials, which offer us more details of EGCG action in the human body. Ahn and coworkers reported that oral treatment of polyphenon E or purified EGCG, 200 mg daily for 12 wk, was effective in patients with human papilloma virus (HPV)-infected cervical lesions[28]. A pilot study conducted in Japan, investigated the effect of green tea extract (GTE) on metachronous colorectal adenomas. Oral administration of GTE, 1.5 g/d for 12 mo, in addition to a tea drinking life-style, showed efficacy in preventing the incidence of metachronous adenoma in patients 1 year postpolypectomy. The incidence of metachronous colorectal adenomas at end-point colonoscopy was 31% (20 of 65) in the control group and 15% (9 of 60) in the GTE group[29]. Another encouraging clinical trial investigated possible prostate cancer chemoprevention with oral GTCs. Sixty volunteers with high-grade prostate intraepithelial neoplasia received either 600 mg of GTCs or placebo daily for 12 mo. The primary end point was prevalence of prostate cancer. After 1 year of follow-up, only 3% of the patients in the treatment group developed prostate cancer, compared with 30% in the placebo group[30]. In a 2-year follow-up, despite the high drop-out rate (57% in the placebo-arm and 55% in the GTCs-arm), three further cancer diagnoses appeared. One prostate cancer was diagnosed among 13 GTC-treated patients and 2 among 9 placebo-treated patients. Overall, treatment with GTCs led to almost 80% reduction in prostate cancer diagnosis. These results suggest that the inhibition of prostate cancer progression after 1 year of GTCs administration was long-lasting and no adverse effect was associated with the treatment[31].
The results from clinical trials with EGCG are not all positive. A phase I study performed in 49 patients with various tumours reported no major antitumor responses when using GTE at the maximum-tolerated dose of 4.2 g/m2 once daily or 1.0 g/m2 three times day[32]. In a Phase II study, green tea showed minimal anti-neoplastic activity, as defined by a decline in prostate specific antigen (PSA) levels, among 42 patients with androgen independent prostate carcinoma. Only a single patient manifested a 50% decrease in PSA level from baseline and this response was not sustained beyond 2 mo. Green tea toxicity occurred in 69% of patients and included nausea, emesis, insomnia, fatigue, diarrhea, abdominal pain, and confusion[33]. On the other hand, other therapeutic trials with EGCG have shown promising results. A recent phase II clinical trial demonstrated the effects of short-term supplementation with Polyphenon E on serum biomarkers in prostate cancer patients. Twenty-six men were given daily doses of Polyphenon E (total of 800 mg EGCG) before radical prostatectomy (average drug administration of 6 wk). Polyphenon E administration significantly reduced serum levels of hepatocyte growth factor, VEGF, PSA, IGF-I, IGF binding protein (IGFBP)-3, and the IGF-I/IGFBP-3 ratio with no adverse effects on liver function. These findings support a potential role for Polyphenon E in the treatment or prevention of prostate cancer[34]. Shanafelt et al[35] reported that EGCG induced apoptotic cell death in the leukemic B-cells from a majority of patients with chronic lymphocytic leukemia (CLL), and four patients with low grade B-cell malignancies developed positive responses shortly after self-initiating EGCG therapy by oral ingestion of EGCG containing products. Based on this evidence, the same group conducted a phase I trial to define the clinical benefit of Polyphenon E. Thirty-three previously untreated patients with asymptomatic Rai stage 0 to II CLL received Polyphenon E treatment. Declines in absolute lymphocyte count (ALC) and/or lymphadenopathy were observed in the majority of patients. One patient achieved partial remission and more than 50% of study patients attained a sustained decline in ALC of 20% and a 50% reduction in lymphadenopathy at some point during treatment. No differences in response were observed based on IgVH, ZAP-70, or CD38 gene mutation status except for trisomy 12[36]. Furthermore, this research group is conducting an ongoing phase II trial to evaluate efficacy of Polyphenon E at 2000 mg dose, twice a day in patients with asymptomatic Rai stage 0 to II CLL (www.clinicaltrials.gov).
While EGCG alone is active in suppressing cancer, combination of EGCG with other agents may be more promising. EGCG reportedly exhibits synergistic effects with other anti-cancer drugs, such as curcumin, chrysin, tamoxifen, etoposide, 5-fluorouracil, temozolomide, taxane, erlotinib[37-43]. Consequently, several clinical trials of EGCG in combination with other drugs for cancer treatment are now ongoing. On the other hand, recent studies indicate that EGCG may be able to block the therapeutic efficacy of some anticancer agents such as bortezomib and other boronic acid-based proteasome inhibitors. This should be highly relevant for clinical considerations[44,45].
RESVERATROL
Resveratrol, a polyphenol, was first isolated in 1940 as an ingredient of the roots of white hellebore (Veratrum grandiflorum O.Loes) and has since been found in various food sources including red wine, grapes, mulberries, peanuts[46,47]. Resveratrol was identified in 1963 as the active constituent of the roots of Polygonum cuspidatum, a plant used in Chinese and Japanese traditional medicine[46]. Jang et al[48] reported the ability of resveratrol to inhibit carcinogenesis at multiple stages, including initiation, promotion and progression. Subsequent studies demonstrated the strong chemopreventive efficacy of resveratrol in many different animal models of carcinogenesis. Oral or local application of resveratrol in mice or rats significantly reduced DMBA-initiated and 12-O-tetradecanoylphorbol-13-acetate-promoted skin tumors, suppressed DMBA-induced mammary carcinogenesis, inhibited 1,2-dimethylhydrazine-induced colon carcinogenesis and N-nitrosomethylbenzylamine-induced esophageal tumors. Overall, the majority of these studies strongly support the chemopreventive effect of resveratrol, although there are exceptions in which a lack of in vivo benefit has been observed[49-53].
Besides its chemopreventive effects, extensive study over the past decade has suggested that resveratrol might be a promising candidate for cancer therapy by interfering with many signaling pathways that regulate cell proliferation, apoptosis, inflammation, angiogenesis and metastasis[46,47]. It suppresses the proliferation in a wide variety of human tumor cells in vitro and in xenograft models. Resveratrol was also reported to exhibit synergistic chemopreventive effects with other anti-cancer drugs, such as cisplatin, doxorubicin and vinorelbine[54-56]. However, a recent study indicated that resveratrol can significantly attenuate the efficacy of paclitaxel’s anticancer actions in certain human breast cancer cell lines both in vitro and in vivo[57], suggesting that concomitant use of resveratrol with paclitaxel may be detrimental in certain types of human cancers. More preclinical and clinical testing of the potential benefits and risks of using resveratrol as an anticancer adjuvant in cancer patients is warranted.
The bioavailability and the pharmacokinetics of resveratrol have been studied in experimental animals and humans. These studies showed that resveratrol was rapidly absorbed after oral intake, and rapidly metabolized to glucuronide and sulphate conjugates which result in the low concentrations of resveratrol observed in plasma[47]. The low bioavailability led to uncertainty over whether oral resveratrol can reach the bioactive concentrations in target tissues. The limited data from research about the tissue distribution of resveratrol and its metabolites offers us some clues. Data from mice demonstrated the significant accumulation of resveratrol in the intestine, stomach, liver, kidney and bile[58,59]. In a clinical study, a level of resvertrol was found in colon tissue in excess of that required for activity in vitro, which supported the colon as a target organ for oral resveratrol in humans[60].
A wide range of doses of resveratrol (0.1-1500 mg/kg) was used in animal studies with various effecicacy and low toxicity, although more human studies are needed to establish the relevant dose for human use. To date, limited data was obtained from human studies performed with resveratrol, and it is difficult to compare the results concerning the safety and tolerability of resveratrol because of variations in conditions of administration (e.g., pure resveratrol formulation, or other non-pure resveratrol samples in various matrices). It was generally agreed by the expert panel of Resverarol 2010 that at least some portion of the population is likely take 1-2 mg of resveratrol per day from dietary sources and at this amount is almost certain to be safe for chronic consumption[61].
Two clinical studies in healthy volunteers investigated the cancer-preventive effect of resveratrol through examination of related biomarkers. A phase I study carried out in forty healthy volunteers showed that ingesting a range of doses of resveratrol (0.5, 1.0, 2.5 or 5.0 g daily) for 29 d caused a decrease in circulating IGF-1 and IGFBP-3, respectively, compared to pre-dosing values. At the 2.5 g dose level, the decrease was most marked. The observed decrease in circulating IGF-1 and IGFBP-3 may contribute to cancer chemopreventive activity. Several other potential markers of activity were also investigated in blood samples from the volunteers. Resveratrol neither significantly affected circulating levels of prostaglandin E-2 (PGE-2), reflecting perturbation of the arachidonic acid cascade, nor influenced leukocyte levels of the malondialdehyde-DNA adduct M1dG, reflecting DNA oxidation[62]. Consistent with the evidence in vitro and in animal models, another clinical study performed in healthy volunteers showed that resveratrol intervention inhibited the phenotypic indices of CYP3A4, CYP2D6, and CYP2C9 and induced the phenotypic index of 1A2. In addition, in individuals with low baseline GST-π levels and UGT1A1 activity, intervention was associated with a significant increase in enzyme activity. Modulation of enzyme systems involved in carcinogen activation and detoxification could be one of the mechanisms responsible for the cancer preventive effect of resveratrol. However, such activities may also alter the pharmacokinetics of other drugs. Therefore, the authors suggested that further clinical studies should consider evaluation of lower doses of resveratrol to minimize adverse metabolic drug interactions[63].
Based on the evidence from animal studies which showed oral administration of resveratrol can efficiently induced apoptosis in colon cancer with high levels achievable in local tissue, most of the clinical therapeutic trials have focused on investigating the effects of resveratrol on colon cancer. The first reported clinical trial of resveratrol in patients with colon cancer was conducted to assess the effects of a low dose of a plant-derived resveratrol formulation and resveratrol-containing freeze-dried grape powder (GP) on biomarkers related to the Wnt pathway, a key signaling pathway activated in over 85% of colon cancers[64]. Eight patients received 14 d of treatment until the day prior to surgery for colon cancer resection. Resveratrol and GP had significant activity in inhibiting Wnt targets on normal colonic mucosa, such as cyclin D1 and axinII. However, GP treatment increased the expression of some Wnt target genes in colon cancer, including myc and cyclin D1. Resveratrol may have more clinical utility for colon cancer prevention rather than for treatment of established colon cancer[64]. In another clinical study, twenty patients with histologically confirmed colorectal cancer consumed 8 daily doses of resveratrol at 0.5 or 1.0 g prior to surgical resection. Resveratrol was found to be well tolerated. With respect to its activity in target tissues, resveratrol slightly inhibited cell proliferation in colorectal cancer tissue after ingestion, as assessed by Ki-67 immunostaining[60]. A recent report of a phase I, randomised, double-blind clinical trial, described the effects of SRT501 (micronized resveratrol) in patients with colorectal cancer and hepatic metastases[65]. Cleaved caspase-3, a marker of apoptosis, was significantly increased by 39% in malignant hepatic tissue following SRT501 treatment, compared to tissue from placebo-treated patients. However, SRT501 failed to change the levels of several other biomarkers associated with cell survival and apoptosis in plasma or in tumor tissues, including PGE-2, VEGF, IGF-1, Ki67, phosphor-Akt (ser473), Akt1, phospho-GSK3, GSK3, phospho-extracellular signal-regulated kinase (ERK), ERK, phospho-JNK, JNK, β-catenin, survivin, BCL2, Bax or PARP[65].
Cellular senescence is an anticancer mechanism that our organism may implement to arrest cancer cells. The arrest of senescence and inhibition of cancer growth appear to be two antagonistic activities. However, it is well documented that resveratrol has both anti-senescence and anti-cancer activities, indicating that it has complex roles in preventing ageing and carcinogenesis. In addition, the numerous formulations of resveratrol used in clinical research and its potential interactions with other drugs make it difficult to recommend an optimal dosage for clinical usage. Long-term clinical trials are needed to validate the anti-cancer effect of resveratrol when used as a drug or as food supplement.
INDOLE-3-CARBINOL
Indole-3-carbinol (I3C), an indole compound, is naturally found in many plants, particularly in cruciferous vegetables such as broccoli, cabbage, cauliflower, Brussels sprouts, and bok choy[66,67]. Glucobrassicin, a major component of cruciferous vegetables, is hydrolyzed in acidic conditions to give I3C[68]. I3C is chemically unstable in aqueous and gastric acidic environments, such as those encountered under cell culture conditions and the acidic environment of the stomach in vivo. In acidic conditions, I3C is rapidly converted to numerous condensation products, of which 3,3-diindolylmethane (DIM) is the most active and effective metabolite[66,69]. The effect of I3C in vivo might, at least in part, be attributable to the formation of DIM.
To date, I3C and its metabolite, DIM, have been demonstrated in numerous epidemiological and preclinical studies to possess cancer preventive properties. In vitro studies demonstrated that both I3C and DIM inhibit growth of most types of hormone-dependent and -independent cancer cells (breast, prostate, liver, lung, colon, cervix, and ovarian cancers)[66,70-72]. In addition, in in vivo studies, I3C and DIM have been shown to have pronounced chemopreventive effects against growth of both spontaneous and chemically induced cancers in various animal models[73-77]. The anti-cancer properties of I3C is attributable to its ability to modulate multiple signaling pathways which control DNA repair, hormonal regulation, inflammation, cell division and growth, apoptosis, angiogenesis, and multiple drug resistance[66,78,79]. I3C has been to shown to induce phase 1 and phase 2 enzymes that metabolize carcinogens, prevent carcinogen-DNA adduct formation, regulate several nuclear transcription factors [such as estrogen receptor (ER), aryl hydrocarbon receptors (AhR, Sp1 and NF-κB)], modulate anti-apoptotic and pro-apoptotic factors, repress extracellular matrix-degrading proteases, and reverse the process of epithelial mesenchymal transition via regulation of key miRNAs[66,67,80-82]. Among these multiple mechanisms, the most important effect of I3C and DIM is modulation of estrogen metabolism. I3C and DIM has received special attention as an effective chemopreventive agent against hormone-dependent cancers such as breast, cervical and prostate cancers, for the most part, due to its ability to negatively regulate ERα signaling and alter cytochrome P450-mediated estrogen metabolism[83,84]. Despite the low affinity of I3C for ERα, it significantly inhibits ERα activity thereby diminishing estrogen-mediated cellular and biochemical effects in estrogen-responsive cells and tissues[85]. I3C could also induce ER protein ubiquitination and degradation in a process requiring AhR, which binds to a wide range of ligands including DIM[86,87]. In addition, a recent study has identified DIM as a ligand-independent activator of ERβ, a molecule associated with antiproliferative activity in breast cancer cells[88]. Moreover, I3C and DIM may reverse the metabolism of estradiol to a more beneficial pathway, thus reducing levels of toxic 16α-OHE1 and increasing levels of protective 2-OHE1, which correlates with reduced risk of breast cancer and other cancers including cervical and prostate cancer[89].
Several studies have been conducted to detect the pharmacokinetics of I3C. Upon oral administration of 250 mg/kg I3C to female CD-1 mice, I3C and its acid condensation products were absorbed and distributed systemically into a number of well-perfused tissues[90]. In contrast, in human testing, no I3C was found in the plasma after giving a single dose of up to 1200 mg or multiple-doses at 400 mg administered twice daily for 4 wk, and DIM was the only detectable I3C-derived compound in plasma[91]. These results support the concept that I3C may serve as the prodrug rather than the actual therapeutic agent. Most clinical data on I3C and DIM indicate a good safety profile and only minor adverse effects. Rosen et al[92] reported a long term clinical study using I3C for the treatment of recurrent respiratory papillomatosis. Among 11 patients having a complete response to I3C, the average number of months on I3C was 50.2 mo, and no immediate or long-term side effects were found. However, I3C also has been found to promote cancer of the liver in rats, raising some doubt its use[93,94].
Both I3C and DIM have already undergone human clinical trials, most of them focused on investigating effects on hormone responsive cancers, including cervical dysplasia, breast cancer, vulvar intraepithelial neoplasia (VIN), and prostate cancer. Similar to the effects observed in animal models, several studies showed that I3C and DIM strongly affect estradiol metabolism in healthy humans[95,96]. They were able to induce the activity of cytochrome P450 isoenzyme CYP1A2, and increase the ratio of 2-OHE1:16α-OHE1. A similar beneficial shift in estrogen metabolites was also observed in early studies on women with increased risk for breast cancer[97-99] and a more detailed study on postmenopausal women with a history of early-stage breast cancer[100]. Based on the preclinical evidence which indicated that I3C and DIM may offer benefit for diseases caused by HPV, two studies explored the effect on cervical intraepithelial neoplasia (CIN). Bell and colleagues used I3C administered orally to treat women for CIN. Thirty patients with biopsy-proven CIN II-III were randomized to receive placebo or 200 or 400 mg/d I3C for 12 wk. There was a statistically significant regression of CIN in patients treated with oral I3C compared with placebo. In addition, the 2/16α-hydroxyestrone ratio changed in a dose-dependent fashion[101]. In another randomized clinical trial, oral DIM at a dose of 2 mg/kg per day for 12 wk was well tolerated with no significant toxicity and clinically significant improvement was demonstrated in patients with grade 2 or 3 CIN[102]. Naik et al[103] reported the results of a randomized phase II trial of I3C in the treatment of VIN. In this study, 12 women were randomized to receive 200 or 400 mg/d of I3C, following histological confirmation of high-grade VIN. I3C administration led to a significant reduction in symptoms, lesion size, and severity as well as significant improvement in estrogen metabolism. Recently, in a phase I clinical trial, Rajoria et al[104] recommended DIM as an anti-estrogenic dietary supplement to help reduce the risk of developing thyroid proliferative disease based on the fact that DIM was detected in thyroid tissue and that DIM supplementation significantly improved estrogen metabolism.
VITAMIN D
In addition to its originally identified role on calcium homeostasis and bone metabolism, vitamin D is being recognized as a steroid hormone which exerts a wide range of biological activities related to various clinical disorders including cancer[105,106]. Vitamin D2 is mainly produced by the irradiation of yeast or plant ergosterol[106]. In humans, vitamin D3 (cholecalciferol) is synthesized naturally in skin cell by exposing to ultraviolet B radiation in sunlight, the major source of vitamin D for most humans. In the skin, 7-dehydrocholesterol, a cholesterol precursor is converted to vitamin D3. Vitamin D3 is hydroxylated to 25-hydroxyvitamin D3 (25-OH-D3) in the liver by 27-hydroxylase and further converted to 1α,25-dihydroxycholecalciferol (1,25-(OH)2D3,calcitriol) by 1α-hydroxylase in the kidney and other tissues. Calcitriol is mainly catabolized by 24-hydroxylase (CYP24A1) to 1α,24,25-(OH)2D3, removing its bioactivity[107].
Most of the anticancer effects of 1,25-(OH)2D3 are mediated through binding to its specific receptor, the vitamin D receptor (VDR). In the cell nucleus, 1,25-(OH)2D3 binds to VDR, which subsequently heterodimerizes with another nuclear receptor, the retinoid X receptor[106,108]. The heterodimer binds to vitamin D responsive element in target genes and initiates the regulation of specific genes, including those involved in the regulation of cell growth, differentiation, apoptosis, and inflammation, the key mechanisms underlying the development and progression of cancer[109-112]. 1,25-(OH)2D3 has been shown to have significant anticancer effect on prostate, colon, breast, lung, liver, skin and pancreatic cancer cells, which express VDR[105,112]. In addition, through a so-called non-genomic mechanism, 1,25-(OH)2D3 may have rapid effects on cellular functions[113,114].
Substantial experimental studies, in vitro and in animals, showed the significant antitumor action of vitamin D and thoroughly investigated mechanisms at cellular and molecular levels[115-117]. Furthermore, a large number of epidemiological studies explored the relationships between cancer incidence and geographic location, ultraviolet irradiation, and circulating levels of 25-OH-D3[118-125]. Despite abundant experimental evidence in support of an inverse association between vitamin D status and cancer risk, the available epidemiological evidence has not demonstrated consistently positive results to date. With some exceptions, most epidemiological studies have reported that populations in areas with low UV exposure have an increased risk of various types of cancers such as prostate, colon and breast[119-125].
Serum 25-OH-D3 level is the most widely used indicator of vitamin D status in relation to other vitamin D metabolites[126]. The result of a large population-based case-control study supported by the Long Island Breast Cancer Study Project showed that plasma 25-OH-D3 level was inversely associated with breast cancer risk in a concentration-dependent fashion. Women with circulating 25-OH-D3 above 40 ng/mL had approximately a 40% reduction in breast cancer risk compared with those who were vitamin D deficient[127]. A large population-based case-control study from Germany reported similar findings[128]. However, results from prospective studies did not support an association between vitamin D status and breast cancer. The inconsistent results also extend to studies of other types of cancer[129]. A recent meta-analysis suggested that, in well-fed populations, an inverse relationship between serum 25-OH-D3 levels and colorectal cancer existed (2630 cases in 9 studies), while no association was found for breast (6175 cases in 10 studies) and prostate cancer (3956 cases in 11 studies)[130].
Excessive vitamin D intake was associated with additional toxic effects, such as hyperphosphatemia and hypercalciuria with clinical symptoms including nausea and vomiting, dehydration, muscle weakness, lethargy and confusion. The toxicity generally occurred when the daily dose exceeds 10 000 IU of vitamin D on a chronic basis[131,132]. In this respect, several vitamin D analogs were synthesized with an attempt to minimize these side effects[117,133]. In addition, recent investigations have followed the approach of intermittent administration of calcitriol in very high doses which elicits its antiproliferative effects with only transient hypercalcemia[134,135]. There are still many unresolved questions regarding the maximum tolerated dose, optimal biologic dose and the optimal schedule for available vitamin D formulations.
A number of cancer intervention trials in humans have been conducted using vitamin D and its metabolites, as well as analogs alone or in combination with other anticancer drugs for the prevention or treatment of various cancers, especially prostate cancer, breast cancer and colorectal cancer. Similar to the results of the epidemiological studies, the outcomes of the clinical trials are inconclusive. The largest number of clinical studies have attempted to assess the efficacy of vitamin D in prostate cancer patients. Several phase II studies with calcitriol in combination with various chemotherapies showed a decrease in prostate-specific antigen levels. Additionally, in a large randomized, double-blinded, phase II trial (called ASCENT I) in patients with advanced prostate cancer (n = 250), the administration of a high dose (45 mg) of calcitriol (DN101) in combination with docetaxel caused a significant improvement in overall survival while there were no changes in the PSA response. Unfortunately, the 900 patient phase III study (ASCENT II) was stopped early because of inferior survival in the DN101 group, thus failing to confirm the improved survival seen in the ASCENT I trial[136]. In a recent phase II study, high-dose intravenous calcitriol at a dose of 74 μg weekly in combination with dexamethasone failed to produce a clinical or PSA response in men with advanced prostate cancer[137]. As regards breast cancer, the large intervention trial in 36 282 postmenopausal women conducted by the Women’s Health Initiative showed calcium plus vitamin D supplementation for an average of 7 years did not reduce the incidence of invasive breast cancer compared with placebo[138]. In contrast, another randomized study of vitamin D supplementation (cholecalciferol) indicated that diagnosis of all invasive cancers including breast cancer and colon cancer was substantially reduced in the vitamin D replacement group[139]. For colorectal cancer, Fedirko et al[140] conducted a double-blind, 2 × 2 factorial clinical trial to test the effects of calcium and vitamin D3 alone or in combination on markers of apoptosis in the normal colorectal mucosa. Bax expression along the full length of the crypts increased by 56% in the vitamin D group (vs placebo). However, another randomized trial indicated that calcium with vitamin D supplementation had no detectable effect on the incidence of colorectal cancer[141].
OTHER PROMISING NATURAL AGENTS
Besides the natural agents mentioned above, there are other natural compounds with chemopreventive and chemotherapeutic potential. Chrysin, a natural flavonoid found in many plants, honey and propolis, possesses strong anti-inflammatory and anti-oxidant activity, and exhibits anti-cancer activity against leukemia, malignant glioma, breast carcinoma, cervical cancer, prostate cancer, lung cancer and colon cancer[142]. Chrysin may inhibit cell proliferation and induce apoptosis by cell cycle arrest, inactivation of Akt signaling, activation of caspases, suppression of COX-2 and NF-κB activation, and inhibition of proteasome activity[142,143]. Chrysin has also been shown to reverse multidrug resistance of cancer cells via inhibition of P-glycoprotein and breast cancer resistance protein (BCRP/ABCG2)[144,145]. However, there are few clinical study of the activity of chrysin against human cancers. Celastrol, a quinone methide triterpene, is the major active compound derived from the root of Trypterigium wilfordii Hook F. (the Chinese Thunder of God Vine), and generally used for the treatment of inflammatory and auto-immune diseases[146]. Celastrol has attracted considerable attention recently, for its potential anti-cancer effects, with a broad spectrum of activity against multiple cancer types in both cell culture and animal models. Several molecular mechanisms of celastrol have been identified, including inhibition of IKK-NF-κB signaling, disruption of the Cdc37/Hsp90 interaction, proteasome inhibition and heat shock response activation[147,148]. However, no systematic clinical trials in human subjects have been carried out with celastrol to date. Betulinic acid is a natural product that is present in a variety of plants, especially the white-barked birch tree. The molecule is a member of the triterpene family of compounds that exhibit a variety of biological activities, especially, potent anti-HIV-1 and antitumor properties[149,150]. This triterpene has been found to inhibit the proliferation of a variety tumor cells and suppress tumor growth in animal studies. Numerous molecular targets for betulinic acid have been reported including enzymes (kinases, aminopeptidase N, acetyl-CoA acetyltransferase, topoisomerase I/II), the proteasome, NF-κB and cell cycle regulation[150,151]. In future studies, more anticancer mechanisms of betulinic acid will be delineated and clinical studies are needed to confirm the therapeutic value and exact anticancer target of betulinic acid in the human body.
Footnotes
Supported by National Natural Science Foundation of China, No. 81001587
Peer reviewer: Umberto Galderisi, PhD, Associate Professor of Molecular Biology, Department Experimental Medicine, Second University of Naples, Via L. De Crecchio 7, 80138 Napoli, Italy
S- Editor Li JY L- Editor Hughes D E- Editor Zheng XM
References
- 1.Epstein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr. 2010;103:1545–1557. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- 2.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14:141–153. [PubMed] [Google Scholar]
- 3.Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively. AAPS J. 2009;11:495–510. doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical development plan: curcumin. J Cell Biochem Suppl. 1996;26:72–85. [PubMed] [Google Scholar]
- 6.Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, Pirmohamed M, Marnett LJ, Gescher AJ, Steward WP. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- 7.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 8.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 9.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 10.Asai A, Miyazawa T. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci. 2000;67:2785–2793. doi: 10.1016/s0024-3205(00)00868-7. [DOI] [PubMed] [Google Scholar]
- 11.Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M, et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61:1058–1064. [PubMed] [Google Scholar]
- 12.Garcea G, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, Steward WP, Gescher AJ, Berry DP. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer. 2004;90:1011–1015. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcea G, Berry DP, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, Steward WP, Gescher AJ. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev. 2005;14:120–125. [PubMed] [Google Scholar]
- 14.Padhye S, Chavan D, Pandey S, Deshpande J, Swamy KV, Sarkar FH. Perspectives on chemopreventive and therapeutic potential of curcumin analogs in medicinal chemistry. Mini Rev Med Chem. 2010;10:372–387. doi: 10.2174/138955710791330891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarkar FH, Li Y, Wang Z, Padhye S. Lesson learned from nature for the development of novel anti-cancer agents: implication of isoflavone, curcumin, and their synthetic analogs. Curr Pharm Des. 2010;16:1801–1812. doi: 10.2174/138161210791208956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, Giardiello FM. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, Kakarala M, Carpenter PM, McLaren C, Meyskens FL, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila) 2011;4:354–364. doi: 10.1158/1940-6207.CAPR-10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanai M, Yoshimura K, Asada M, Imaizumi A, Suzuki C, Matsumoto S, Nishimura T, Mori Y, Masui T, Kawaguchi Y, et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol. 2011;68:157–164. doi: 10.1007/s00280-010-1470-2. [DOI] [PubMed] [Google Scholar]
- 19.Bayet-Robert M, Kwiatkowski F, Leheurteur M, Gachon F, Planchat E, Abrial C, Mouret-Reynier MA, Durando X, Barthomeuf C, Chollet P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther. 2010;9:8–14. doi: 10.4161/cbt.9.1.10392. [DOI] [PubMed] [Google Scholar]
- 20.Nagle DG, Ferreira D, Zhou YD. Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives. Phytochemistry. 2006;67:1849–1855. doi: 10.1016/j.phytochem.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan N, Mukhtar H. Cancer and metastasis: prevention and treatment by green tea. Cancer Metastasis Rev. 2010;29:435–445. doi: 10.1007/s10555-010-9236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan N, Mukhtar H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008;269:269–280. doi: 10.1016/j.canlet.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshizawa S, Horiuchi T, Fujiki H, Yoshida T, Okuda T, Sugimura T. Antitumor promoting activity of (-)-epigallocatechin gallate, the main constituent of “Tannin” in green tea. Phytother Res. 1987;1:44–47. [Google Scholar]
- 24.Yang CS, Ju J, Lu G, Xiao H, Hao X, Sang S, Lambert JD. Cancer prevention by tea and tea polyphenols. Asia Pac J Clin Nutr. 2008;17 Suppl 1:245–248. [PMC free article] [PubMed] [Google Scholar]
- 25.Chow HH, Cai Y, Alberts DS, Hakim I, Dorr R, Shahi F, Crowell JA, Yang CS, Hara Y. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev. 2001;10:53–58. [PubMed] [Google Scholar]
- 26.Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, Dorr RT, Hara Y, Alberts DS. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–3319. [PubMed] [Google Scholar]
- 27.Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, Mastrangelo S. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol. 2009;65:331–341. doi: 10.1007/s00228-008-0610-7. [DOI] [PubMed] [Google Scholar]
- 28.Ahn WS, Yoo J, Huh SW, Kim CK, Lee JM, Namkoong SE, Bae SM, Lee IP. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur J Cancer Prev. 2003;12:383–390. doi: 10.1097/00008469-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu M, Fukutomi Y, Ninomiya M, Nagura K, Kato T, Araki H, Suganuma M, Fujiki H, Moriwaki H. Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiol Biomarkers Prev. 2008;17:3020–3025. doi: 10.1158/1055-9965.EPI-08-0528. [DOI] [PubMed] [Google Scholar]
- 30.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 31.Brausi M, Rizzi F, Bettuzzi S. Chemoprevention of human prostate cancer by green tea catechins: two years later. A follow-up update. Eur Urol. 2008;54:472–473. doi: 10.1016/j.eururo.2008.03.100. [DOI] [PubMed] [Google Scholar]
- 32.Pisters KM, Newman RA, Coldman B, Shin DM, Khuri FR, Hong WK, Glisson BS, Lee JS. Phase I trial of oral green tea extract in adult patients with solid tumors. J Clin Oncol. 2001;19:1830–1838. doi: 10.1200/JCO.2001.19.6.1830. [DOI] [PubMed] [Google Scholar]
- 33.Jatoi A, Ellison N, Burch PA, Sloan JA, Dakhil SR, Novotny P, Tan W, Fitch TR, Rowland KM, Young CY, et al. A phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer. 2003;97:1442–1446. doi: 10.1002/cncr.11200. [DOI] [PubMed] [Google Scholar]
- 34.McLarty J, Bigelow RL, Smith M, Elmajian D, Ankem M, Cardelli JA. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev Res (Phila) 2009;2:673–682. doi: 10.1158/1940-6207.CAPR-08-0167. [DOI] [PubMed] [Google Scholar]
- 35.Shanafelt TD, Lee YK, Call TG, Nowakowski GS, Dingli D, Zent CS, Kay NE. Clinical effects of oral green tea extracts in four patients with low grade B-cell malignancies. Leuk Res. 2006;30:707–712. doi: 10.1016/j.leukres.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Shanafelt TD, Call TG, Zent CS, LaPlant B, Bowen DA, Roos M, Secreto CR, Ghosh AK, Kabat BF, Lee MJ, et al. Phase I trial of daily oral Polyphenon E in patients with asymptomatic Rai stage 0 to II chronic lymphocytic leukemia. J Clin Oncol. 2009;27:3808–3814. doi: 10.1200/JCO.2008.21.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Somers-Edgar TJ, Scandlyn MJ, Stuart EC, Le Nedelec MJ, Valentine SP, Rosengren RJ. The combination of epigallocatechin gallate and curcumin suppresses ER alpha-breast cancer cell growth in vitro and in vivo. Int J Cancer. 2008;122:1966–1971. doi: 10.1002/ijc.23328. [DOI] [PubMed] [Google Scholar]
- 38.Sun X, Huo X, Luo T, Li M, Yin Y, Jiang Y. The anticancer flavonoid chrysin induces the unfolded protein response in hepatoma cells. J Cell Mol Med. 2011;15:2389–2398. doi: 10.1111/j.1582-4934.2010.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sartippour MR, Pietras R, Marquez-Garban DC, Chen HW, Heber D, Henning SM, Sartippour G, Zhang L, Lu M, Weinberg O, et al. The combination of green tea and tamoxifen is effective against breast cancer. Carcinogenesis. 2006;27:2424–2433. doi: 10.1093/carcin/bgl066. [DOI] [PubMed] [Google Scholar]
- 40.Ermakova SP, Kang BS, Choi BY, Choi HS, Schuster TF, Ma WY, Bode AM, Dong Z. (-)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006;66:9260–9269. doi: 10.1158/0008-5472.CAN-06-1586. [DOI] [PubMed] [Google Scholar]
- 41.Pyrko P, Schönthal AH, Hofman FM, Chen TC, Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67:9809–9816. doi: 10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- 42.Stearns ME, Wang M. Synergistic Effects of the Green Tea Extract Epigallocatechin-3-gallate and Taxane in Eradication of Malignant Human Prostate Tumors. Transl Oncol. 2011;4:147–156. doi: 10.1593/tlo.10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Zhang H, Tighiouart M, Lee JE, Shin HJ, Khuri FR, Yang CS, Chen ZG, Shin DM. Synergistic inhibition of head and neck tumor growth by green tea (-)-epigallocatechin-3-gallate and EGFR tyrosine kinase inhibitor. Int J Cancer. 2008;123:1005–1014. doi: 10.1002/ijc.23585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah JJ, Kuhn DJ, Orlowski RZ. Bortezomib and EGCG: no green tea for you. Blood. 2009;113:5695–5696. doi: 10.1182/blood-2009-03-204776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golden EB, Lam PY, Kardosh A, Gaffney KJ, Cadenas E, Louie SG, Petasis NA, Chen TC, Schönthal AH. Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors. Blood. 2009;113:5927–5937. doi: 10.1182/blood-2008-07-171389. [DOI] [PubMed] [Google Scholar]
- 46.Signorelli P, Ghidoni R. Resveratrol as an anticancer nutrient: molecular basis, open questions and promises. J Nutr Biochem. 2005;16:449–466. doi: 10.1016/j.jnutbio.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 47.Shankar S, Singh G, Srivastava RK. Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front Biosci. 2007;12:4839–4854. doi: 10.2741/2432. [DOI] [PubMed] [Google Scholar]
- 48.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 49.Kalra N, Roy P, Prasad S, Shukla Y. Resveratrol induces apoptosis involving mitochondrial pathways in mouse skin tumorigenesis. Life Sci. 2008;82:348–358. doi: 10.1016/j.lfs.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Whitsett T, Carpenter M, Lamartiniere CA. Resveratrol, but not EGCG, in the diet suppresses DMBA-induced mammary cancer in rats. J Carcinog. 2006;5:15. doi: 10.1186/1477-3163-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sengottuvelan M, Deeptha K, Nalini N. Resveratrol attenuates 1,2-dimethylhydrazine (DMH) induced glycoconjugate abnormalities during various stages of colon carcinogenesis. Phytother Res. 2009;23:1154–1158. doi: 10.1002/ptr.2770. [DOI] [PubMed] [Google Scholar]
- 52.Li ZG, Hong T, Shimada Y, Komoto I, Kawabe A, Ding Y, Kaganoi J, Hashimoto Y, Imamura M. Suppression of N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by resveratrol. Carcinogenesis. 2002;23:1531–1536. doi: 10.1093/carcin/23.9.1531. [DOI] [PubMed] [Google Scholar]
- 53.Bove K, Lincoln DW, Tsan MF. Effect of resveratrol on growth of 4T1 breast cancer cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;291:1001–1005. doi: 10.1006/bbrc.2002.6554. [DOI] [PubMed] [Google Scholar]
- 54.Rezk YA, Balulad SS, Keller RS, Bennett JA. Use of resveratrol to improve the effectiveness of cisplatin and doxorubicin: study in human gynecologic cancer cell lines and in rodent heart. Am J Obstet Gynecol. 2006;194:e23–e26. doi: 10.1016/j.ajog.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 55.Scifo C, Milasi A, Guarnera A, Sinatra F, Renis M. Resveratrol and propolis extract: an insight into the morphological and molecular changes induced in DU145 cells. Oncol Res. 2006;15:409–421. doi: 10.3727/096504005776568255. [DOI] [PubMed] [Google Scholar]
- 56.Harikumar KB, Kunnumakkara AB, Sethi G, Diagaradjane P, Anand P, Pandey MK, Gelovani J, Krishnan S, Guha S, Aggarwal BB. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. Int J Cancer. 2010;127:257–268. doi: 10.1002/ijc.25041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fukui M, Yamabe N, Zhu BT. Resveratrol attenuates the anticancer efficacy of paclitaxel in human breast cancer cells in vitro and in vivo. Eur J Cancer. 2010;46:1882–1891. doi: 10.1016/j.ejca.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sale S, Verschoyle RD, Boocock D, Jones DJ, Wilsher N, Ruparelia KC, Potter GA, Farmer PB, Steward WP, Gescher AJ. Pharmacokinetics in mice and growth-inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans 3,4,5,4’-tetramethoxystilbene. Br J Cancer. 2004;90:736–744. doi: 10.1038/sj.bjc.6601568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vitrac X, Desmoulière A, Brouillaud B, Krisa S, Deffieux G, Barthe N, Rosenbaum J, Mérillon JM. Distribution of [14C]-trans-resveratrol, a cancer chemopreventive polyphenol, in mouse tissues after oral administration. Life Sci. 2003;72:2219–2233. doi: 10.1016/s0024-3205(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 60.Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA, et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70:7392–7399. doi: 10.1158/0008-5472.CAN-10-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smoliga JM, Vang O, Baur JA. Challenges of translating basic research into therapeutics: resveratrol as an example. J Gerontol A Biol Sci Med Sci. 2012;67:158–167. doi: 10.1093/gerona/glr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chow HH, Garland LL, Hsu CH, Vining DR, Chew WM, Miller JA, Perloff M, Crowell JA, Alberts DS. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev Res (Phila) 2010;3:1168–1175. doi: 10.1158/1940-6207.CAPR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen AV, Martinez M, Stamos MJ, Moyer MP, Planutis K, Hope C, Holcombe RF. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag Res. 2009;1:25–37. [PMC free article] [PubMed] [Google Scholar]
- 65.Howells LM, Berry DP, Elliott PJ, Jacobson EW, Hoffmann E, Hegarty B, Brown K, Steward WP, Gescher AJ. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases--safety, pharmacokinetics, and pharmacodynamics. Cancer Prev Res (Phila) 2011;4:1419–1425. doi: 10.1158/1940-6207.CAPR-11-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4:1201–1215. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- 67.Kim YS, Milner JA. Targets for indole-3-carbinol in cancer prevention. J Nutr Biochem. 2005;16:65–73. doi: 10.1016/j.jnutbio.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 68.Verhoeven DT, Verhagen H, Goldbohm RA, van den Brandt PA, van Poppel G. A review of mechanisms underlying anticarcinogenicity by brassica vegetables. Chem Biol Interact. 1997;103:79–129. doi: 10.1016/s0009-2797(96)03745-3. [DOI] [PubMed] [Google Scholar]
- 69.Grose KR, Bjeldanes LF. Oligomerization of indole-3-carbinol in aqueous acid. Chem Res Toxicol. 1992;5:188–193. doi: 10.1021/tx00026a007. [DOI] [PubMed] [Google Scholar]
- 70.Kim EJ, Park SY, Shin HK, Kwon DY, Surh YJ, Park JH. Activation of caspase-8 contributes to 3,3’-Diindolylmethane-induced apoptosis in colon cancer cells. J Nutr. 2007;137:31–36. doi: 10.1093/jn/137.1.31. [DOI] [PubMed] [Google Scholar]
- 71.Kandala PK, Srivastava SK. Activation of checkpoint kinase 2 by 3,3’-diindolylmethane is required for causing G2/M cell cycle arrest in human ovarian cancer cells. Mol Pharmacol. 2010;78:297–309. doi: 10.1124/mol.110.063750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ahmad A, Kong D, Sarkar SH, Wang Z, Banerjee S, Sarkar FH. Inactivation of uPA and its receptor uPAR by 3,3’-diindolylmethane (DIM) leads to the inhibition of prostate cancer cell growth and migration. J Cell Biochem. 2009;107:516–527. doi: 10.1002/jcb.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bradlow HL, Michnovicz J, Telang NT, Osborne MP. Effects of dietary indole-3-carbinol on estradiol metabolism and spontaneous mammary tumors in mice. Carcinogenesis. 1991;12:1571–1574. doi: 10.1093/carcin/12.9.1571. [DOI] [PubMed] [Google Scholar]
- 74.Kojima T, Tanaka T, Mori H. Chemoprevention of spontaneous endometrial cancer in female Donryu rats by dietary indole-3-carbinol. Cancer Res. 1994;54:1446–1449. [PubMed] [Google Scholar]
- 75.Oganesian A, Hendricks JD, Williams DE. Long term dietary indole-3-carbinol inhibits diethylnitrosamine-initiated hepatocarcinogenesis in the infant mouse model. Cancer Lett. 1997;118:87–94. doi: 10.1016/s0304-3835(97)00235-8. [DOI] [PubMed] [Google Scholar]
- 76.Kim YH, Kwon HS, Kim DH, Shin EK, Kang YH, Park JH, Shin HK, Kim JK. 3,3’-diindolylmethane attenuates colonic inflammation and tumorigenesis in mice. Inflamm Bowel Dis. 2009;15:1164–1173. doi: 10.1002/ibd.20917. [DOI] [PubMed] [Google Scholar]
- 77.Kassie F, Kalscheuer S, Matise I, Ma L, Melkamu T, Upadhyaya P, Hecht SS. Inhibition of vinyl carbamate-induced pulmonary adenocarcinoma by indole-3-carbinol and myo-inositol in A/J mice. Carcinogenesis. 2010;31:239–245. doi: 10.1093/carcin/bgp174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weng JR, Tsai CH, Kulp SK, Wang D, Lin CH, Yang HC, Ma Y, Sargeant A, Chiu CF, Tsai MH, et al. A potent indole-3-carbinol derived antitumor agent with pleiotropic effects on multiple signaling pathways in prostate cancer cells. Cancer Res. 2007;67:7815–7824. doi: 10.1158/0008-5472.CAN-07-0794. [DOI] [PubMed] [Google Scholar]
- 79.Kunimasa K, Kobayashi T, Kaji K, Ohta T. Antiangiogenic effects of indole-3-carbinol and 3,3’-diindolylmethane are associated with their differential regulation of ERK1/2 and Akt in tube-forming HUVEC. J Nutr. 2010;140:1–6. doi: 10.3945/jn.109.112359. [DOI] [PubMed] [Google Scholar]
- 80.Marconett CN, Sundar SN, Poindexter KM, Stueve TR, Bjeldanes LF, Firestone GL. Indole-3-carbinol triggers aryl hydrocarbon receptor-dependent estrogen receptor (ER)alpha protein degradation in breast cancer cells disrupting an ERalpha-GATA3 transcriptional cross-regulatory loop. Mol Biol Cell. 2010;21:1166–1177. doi: 10.1091/mbc.E09-08-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hung WC, Chang HC. Indole-3-carbinol inhibits Sp1-induced matrix metalloproteinase-2 expression to attenuate migration and invasion of breast cancer cells. J Agric Food Chem. 2009;57:76–82. doi: 10.1021/jf802881d. [DOI] [PubMed] [Google Scholar]
- 82.Li Y, VandenBoom TG, Kong D, Wang Z, Ali S, Philip PA, Sarkar FH. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Auborn KJ, Fan S, Rosen EM, Goodwin L, Chandraskaren A, Williams DE, Chen D, Carter TH. Indole-3-carbinol is a negative regulator of estrogen. J Nutr. 2003;133:2470S–2475S. doi: 10.1093/jn/133.7.2470s. [DOI] [PubMed] [Google Scholar]
- 84.Meng Q, Yuan F, Goldberg ID, Rosen EM, Auborn K, Fan S. Indole-3-carbinol is a negative regulator of estrogen receptor-alpha signaling in human tumor cells. J Nutr. 2000;130:2927–2931. doi: 10.1093/jn/130.12.2927. [DOI] [PubMed] [Google Scholar]
- 85.Ashok BT, Chen YG, Liu X, Garikapaty VP, Seplowitz R, Tschorn J, Roy K, Mittelman A, Tiwari RK. Multiple molecular targets of indole-3-carbinol, a chemopreventive anti-estrogen in breast cancer. Eur J Cancer Prev. 2002;11 Suppl 2:S86–S93. [PubMed] [Google Scholar]
- 86.Okino ST, Pookot D, Basak S, Dahiya R. Toxic and chemopreventive ligands preferentially activate distinct aryl hydrocarbon receptor pathways: implications for cancer prevention. Cancer Prev Res (Phila) 2009;2:251–256. doi: 10.1158/1940-6207.CAPR-08-0146. [DOI] [PubMed] [Google Scholar]
- 87.Wang TT, Milner MJ, Milner JA, Kim YS. Estrogen receptor alpha as a target for indole-3-carbinol. J Nutr Biochem. 2006;17:659–664. doi: 10.1016/j.jnutbio.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 88.Vivar OI, Saunier EF, Leitman DC, Firestone GL, Bjeldanes LF. Selective activation of estrogen receptor-beta target genes by 3,3’-diindolylmethane. Endocrinology. 2010;151:1662–1667. doi: 10.1210/en.2009-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Czapski J. Cancer preventing properties of cruciferous vegetables. Veg Crops Res Bull. 2009;70:5–18. [Google Scholar]
- 90.Anderton MJ, Manson MM, Verschoyle RD, Gescher A, Lamb JH, Farmer PB, Steward WP, Williams ML. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin Cancer Res. 2004;10:5233–5241. doi: 10.1158/1078-0432.CCR-04-0163. [DOI] [PubMed] [Google Scholar]
- 91.Reed GA, Arneson DW, Putnam WC, Smith HJ, Gray JC, Sullivan DK, Mayo MS, Crowell JA, Hurwitz A. Single-dose and multiple-dose administration of indole-3-carbinol to women: pharmacokinetics based on 3,3’-diindolylmethane. Cancer Epidemiol Biomarkers Prev. 2006;15:2477–2481. doi: 10.1158/1055-9965.EPI-06-0396. [DOI] [PubMed] [Google Scholar]
- 92.Rosen CA, Bryson PC. Indole-3-carbinol for recurrent respiratory papillomatosis: long-term results. J Voice. 2004;18:248–253. doi: 10.1016/j.jvoice.2003.05.005. [DOI] [PubMed] [Google Scholar]
- 93.Kim DJ, Lee KK, Han BS, Ahn B, Bae JH, Jang JJ. Biphasic modifying effect of indole-3-carbinol on diethylnitrosamine-induced preneoplastic glutathione S-transferase placental form-positive liver cell foci in Sprague-Dawley rats. Jpn J Cancer Res. 1994;85:578–583. doi: 10.1111/j.1349-7006.1994.tb02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stoner G, Casto B, Ralston S, Roebuck B, Pereira C, Bailey G. Development of a multi-organ rat model for evaluating chemopreventive agents: efficacy of indole-3-carbinol. Carcinogenesis. 2002;23:265–272. doi: 10.1093/carcin/23.2.265. [DOI] [PubMed] [Google Scholar]
- 95.Michnovicz JJ, Bradlow HL. Altered estrogen metabolism and excretion in humans following consumption of indole-3-carbinol. Nutr Cancer. 1991;16:59–66. doi: 10.1080/01635589109514141. [DOI] [PubMed] [Google Scholar]
- 96.Michnovicz JJ, Adlercreutz H, Bradlow HL. Changes in levels of urinary estrogen metabolites after oral indole-3-carbinol treatment in humans. J Natl Cancer Inst. 1997;89:718–723. doi: 10.1093/jnci/89.10.718. [DOI] [PubMed] [Google Scholar]
- 97.Wong GY, Bradlow L, Sepkovic D, Mehl S, Mailman J, Osborne MP. Dose-ranging study of indole-3-carbinol for breast cancer prevention. J Cell Biochem Suppl. 1997;28-29:111–116. doi: 10.1002/(sici)1097-4644(1997)28/29+<111::aid-jcb12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 98.Bradlow HL, Michnovicz JJ, Halper M, Miller DG, Wong GY, Osborne MP. Long-term responses of women to indole-3-carbinol or a high fiber diet. Cancer Epidemiol Biomarkers Prev. 1994;3:591–595. [PubMed] [Google Scholar]
- 99.Reed GA, Peterson KS, Smith HJ, Gray JC, Sullivan DK, Mayo MS, Crowell JA, Hurwitz A. A phase I study of indole-3-carbinol in women: tolerability and effects. Cancer Epidemiol Biomarkers Prev. 2005;14:1953–1960. doi: 10.1158/1055-9965.EPI-05-0121. [DOI] [PubMed] [Google Scholar]
- 100.Dalessandri KM, Firestone GL, Fitch MD, Bradlow HL, Bjeldanes LF. Pilot study: effect of 3,3’-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr Cancer. 2004;50:161–167. doi: 10.1207/s15327914nc5002_5. [DOI] [PubMed] [Google Scholar]
- 101.Bell MC, Crowley-Nowick P, Bradlow HL, Sepkovic DW, Schmidt-Grimminger D, Howell P, Mayeaux EJ, Tucker A, Turbat-Herrera EA, Mathis JM. Placebo-controlled trial of indole-3-carbinol in the treatment of CIN. Gynecol Oncol. 2000;78:123–129. doi: 10.1006/gyno.2000.5847. [DOI] [PubMed] [Google Scholar]
- 102.Del Priore G, Gudipudi DK, Montemarano N, Restivo AM, Malanowska-Stega J, Arslan AA. Oral diindolylmethane (DIM): pilot evaluation of a nonsurgical treatment for cervical dysplasia. Gynecol Oncol. 2010;116:464–467. doi: 10.1016/j.ygyno.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 103.Naik R, Nixon S, Lopes A, Godfrey K, Hatem MH, Monaghan JM. A randomized phase II trial of indole-3-carbinol in the treatment of vulvar intraepithelial neoplasia. Int J Gynecol Cancer. 2006;16:786–790. doi: 10.1111/j.1525-1438.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- 104.Rajoria S, Suriano R, Parmar PS, Wilson YL, Megwalu U, Moscatello A, Bradlow HL, Sepkovic DW, Geliebter J, Schantz SP, et al. 3,3’-diindolylmethane modulates estrogen metabolism in patients with thyroid proliferative disease: a pilot study. Thyroid. 2011;21:299–304. doi: 10.1089/thy.2010.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Holick MF. Vitamin D: its role in cancer prevention and treatment. Prog Biophys Mol Biol. 2006;92:49–59. doi: 10.1016/j.pbiomolbio.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 106.Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364:248–254. doi: 10.1056/NEJMcp1009570. [DOI] [PubMed] [Google Scholar]
- 107.Anaizi N. Rediscovering vitamin D. Libyan J Med. 2010;5 doi: 10.3402/ljm.v5i0.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Plum LA, DeLuca HF. The functional metabolism and molecular biology of vitamin D action. Clin Rev Bone Miner Metab. 2009;7:20–41. [Google Scholar]
- 109.Ingraham BA, Bragdon B, Nohe A. Molecular basis of the potential of vitamin D to prevent cancer. Curr Med Res Opin. 2008;24:139–149. doi: 10.1185/030079908x253519. [DOI] [PubMed] [Google Scholar]
- 110.Krishnan AV, Feldman D. Molecular pathways mediating the anti-inflammatory effects of calcitriol: implications for prostate cancer chemoprevention and treatment. Endocr Relat Cancer. 2010;17:R19–R38. doi: 10.1677/ERC-09-0139. [DOI] [PubMed] [Google Scholar]
- 111.Chung I, Han G, Seshadri M, Gillard BM, Yu WD, Foster BA, Trump DL, Johnson CS. Role of vitamin D receptor in the antiproliferative effects of calcitriol in tumor-derived endothelial cells and tumor angiogenesis in vivo. Cancer Res. 2009;69:967–975. doi: 10.1158/0008-5472.CAN-08-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Norman AW, Bouillon R. Vitamin D nutritional policy needs a vision for the future. Exp Biol Med (Maywood) 2010;235:1034–1045. doi: 10.1258/ebm.2010.010014. [DOI] [PubMed] [Google Scholar]
- 113.Fleet JC. Rapid, membrane-initiated actions of 1,25 dihydroxyvitamin D: what are they and what do they mean. J Nutr. 2004;134:3215–3218. doi: 10.1093/jn/134.12.3215. [DOI] [PubMed] [Google Scholar]
- 114.Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147:5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 115.Ordonez-Moran P, Larriba MJ, Pendas-Franco N, Aguilera O, Gonzalez-Sancho JM, Munoz A. Vitamin D and cancer: an update of in vitro and in vivo data. Front Biosci. 2005;10:2723–2749. doi: 10.2741/1731. [DOI] [PubMed] [Google Scholar]
- 116.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Masuda S, Jones G. Promise of vitamin D analogues in the treatment of hyperproliferative conditions. Mol Cancer Ther. 2006;5:797–808. doi: 10.1158/1535-7163.MCT-05-0539. [DOI] [PubMed] [Google Scholar]
- 118.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Grant WB. Geographic variation of prostate cancer mortality rates in the United States: Implications for prostate cancer risk related to vitamin D. Int J Cancer. 2004;111:470–471; author reply 472. doi: 10.1002/ijc.20220. [DOI] [PubMed] [Google Scholar]
- 120.Waltz P, Chodick G. International comparisons of prostate cancer mortality rates with dietary practices and sunlight levels. Urol Oncol. 2007;25:85. doi: 10.1016/j.urolonc.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 121.Waltz P, Chodick G. Assessment of ecological regression in the study of colon, breast, ovary, non-Hodgkin’s lymphoma, or prostate cancer and residential UV. Eur J Cancer Prev. 2008;17:279–286. doi: 10.1097/CEJ.0b013e3282b6fd0f. [DOI] [PubMed] [Google Scholar]
- 122.Gupta D, Lammersfeld CA, Trukova K, Lis CG. Vitamin D and prostate cancer risk: a review of the epidemiological literature. Prostate Cancer Prostatic Dis. 2009;12:215–226. doi: 10.1038/pcan.2009.7. [DOI] [PubMed] [Google Scholar]
- 123.John EM, Koo J, Schwartz GG. Sun exposure and prostate cancer risk: evidence for a protective effect of early-life exposure. Cancer Epidemiol Biomarkers Prev. 2007;16:1283–1286. doi: 10.1158/1055-9965.EPI-06-1053. [DOI] [PubMed] [Google Scholar]
- 124.Cui Y, Rohan TE. Vitamin D, calcium, and breast cancer risk: a review. Cancer Epidemiol Biomarkers Prev. 2006;15:1427–1437. doi: 10.1158/1055-9965.EPI-06-0075. [DOI] [PubMed] [Google Scholar]
- 125.Spina C, Tangpricha V, Yao M, Zhou W, Wolfe MM, Maehr H, Uskokovic M, Adorini L, Holick MF. Colon cancer and solar ultraviolet B radiation and prevention and treatment of colon cancer in mice with vitamin D and its Gemini analogs. J Steroid Biochem Mol Biol. 2005;97:111–120. doi: 10.1016/j.jsbmb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 126.Heaney RP. The Vitamin D requirement in health and disease. J Steroid Biochem Mol Biol. 2005;97:13–19. doi: 10.1016/j.jsbmb.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 127.Crew KD, Gammon MD, Steck SE, Hershman DL, Cremers S, Dworakowski E, Shane E, Terry MB, Desai M, Teitelbaum SL, et al. Association between plasma 25-hydroxyvitamin D and breast cancer risk. Cancer Prev Res (Phila) 2009;2:598–604. doi: 10.1158/1940-6207.CAPR-08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Abbas S, Linseisen J, Slanger T, Kropp S, Mutschelknauss EJ, Flesch-Janys D, Chang-Claude J. Serum 25-hydroxyvitamin D and risk of post-menopausal breast cancer--results of a large case-control study. Carcinogenesis. 2008;29:93–99. doi: 10.1093/carcin/bgm240. [DOI] [PubMed] [Google Scholar]
- 129.Freedman DM, Chang SC, Falk RT, Purdue MP, Huang WY, McCarty CA, Hollis BW, Graubard BI, Berg CD, Ziegler RG. Serum levels of vitamin D metabolites and breast cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2008;17:889–894. doi: 10.1158/1055-9965.EPI-07-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, Mullie P, Autier P. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128:1414–1424. doi: 10.1002/ijc.25439. [DOI] [PubMed] [Google Scholar]
- 131.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85:6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- 132.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582S–586S. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 133.Ma Y, Khalifa B, Yee YK, Lu J, Memezawa A, Savkur RS, Yamamoto Y, Chintalacharuvu SR, Yamaoka K, Stayrook KR, et al. Identification and characterization of noncalcemic, tissue-selective, nonsecosteroidal vitamin D receptor modulators. J Clin Invest. 2006;116:892–904. doi: 10.1172/JCI25901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Beer TM, Lemmon D, Lowe BA, Henner WD. High-dose weekly oral calcitriol in patients with a rising PSA after prostatectomy or radiation for prostate carcinoma. Cancer. 2003;97:1217–1224. doi: 10.1002/cncr.11179. [DOI] [PubMed] [Google Scholar]
- 135.Trump DL, Potter DM, Muindi J, Brufsky A, Johnson CS. Phase II trial of high-dose, intermittent calcitriol (1,25 dihydroxyvitamin D3) and dexamethasone in androgen-independent prostate cancer. Cancer. 2006;106:2136–2142. doi: 10.1002/cncr.21890. [DOI] [PubMed] [Google Scholar]
- 136.Scher HI, Jia X, Chi K, de Wit R, Berry WR, Albers P, Henick B, Waterhouse D, Ruether DJ, Rosen PJ, et al. Randomized, open-label phase III trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancer. J Clin Oncol. 2011;29:2191–2198. doi: 10.1200/JCO.2010.32.8815. [DOI] [PubMed] [Google Scholar]
- 137.Chadha MK, Tian L, Mashtare T, Payne V, Silliman C, Levine E, Wong M, Johnson C, Trump DL. Phase 2 trial of weekly intravenous 1,25 dihydroxy cholecalciferol (calcitriol) in combination with dexamethasone for castration-resistant prostate cancer. Cancer. 2010;116:2132–2139. doi: 10.1002/cncr.24973. [DOI] [PubMed] [Google Scholar]
- 138.Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, Rohan T, Rossouw J, Lane D, O’Sullivan MJ, Yasmeen S, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. 2008;100:1581–1591. doi: 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 140.Fedirko V, Bostick RM, Flanders WD, Long Q, Shaukat A, Rutherford RE, Daniel CR, Cohen V, Dash C. Effects of vitamin D and calcium supplementation on markers of apoptosis in normal colon mucosa: a randomized, double-blind, placebo-controlled clinical trial. Cancer Prev Res (Phila) 2009;2:213–223. doi: 10.1158/1940-6207.CAPR-08-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O’Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 142.Khoo BY, Chua SL, Balaram P. Apoptotic effects of chrysin in human cancer cell lines. Int J Mol Sci. 2010;11:2188–2199. doi: 10.3390/ijms11052188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bonfili L, Cecarini V, Amici M, Cuccioloni M, Angeletti M, Keller JN, Eleuteri AM. Natural polyphenols as proteasome modulators and their role as anti-cancer compounds. FEBS J. 2008;275:5512–5526. doi: 10.1111/j.1742-4658.2008.06696.x. [DOI] [PubMed] [Google Scholar]
- 144.Bansal T, Jaggi M, Khar RK, Talegaonkar S. Emerging significance of flavonoids as P-glycoprotein inhibitors in cancer chemotherapy. J Pharm Pharm Sci. 2009;12:46–78. doi: 10.18433/j3rc77. [DOI] [PubMed] [Google Scholar]
- 145.Wang X, Morris ME. Effects of the flavonoid chrysin on nitrofurantoin pharmacokinetics in rats: potential involvement of ABCG2. Drug Metab Dispos. 2007;35:268–274. doi: 10.1124/dmd.106.011684. [DOI] [PubMed] [Google Scholar]
- 146.Liu Z, Ma L, Zhou GB. The main anticancer bullets of the Chinese medicinal herb, thunder god vine. Molecules. 2011;16:5283–5297. doi: 10.3390/molecules16065283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Salminen A, Lehtonen M, Paimela T, Kaarniranta K. Celastrol: Molecular targets of Thunder God Vine. Biochem Biophys Res Commun. 2010;394:439–442. doi: 10.1016/j.bbrc.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 148.Kannaiyan R, Shanmugam MK, Sethi G. Molecular targets of celastrol derived from Thunder of God Vine: potential role in the treatment of inflammatory disorders and cancer. Cancer Lett. 2011;303:9–20. doi: 10.1016/j.canlet.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 149.Aiken C, Chen CH. Betulinic acid derivatives as HIV-1 antivirals. Trends Mol Med. 2005;11:31–36. doi: 10.1016/j.molmed.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 150.Fulda S, Kroemer G. Targeting mitochondrial apoptosis by betulinic acid in human cancers. Drug Discov Today. 2009;14:885–890. doi: 10.1016/j.drudis.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 151.Mullauer FB, Kessler JH, Medema JP. Betulinic acid, a natural compound with potent anticancer effects. Anticancer Drugs. 2010;21:215–227. doi: 10.1097/CAD.0b013e3283357c62. [DOI] [PubMed] [Google Scholar]