Abstract
AIM: To evaluate selected factors influencing resting energy expenditure (REE) in obese female subjects.
METHODS: Seventy seven 61 obese Caucasian women [mean age of 52.93 ± 13.45 years, and mean body mass index (BMI) of 41.78 ± 11.54 kg/m2] were enrolled; measurements of resting metabolic rate (RMR) by a ventilated, open-circuit system, indirect calorimeter were performed after an overnight fast. Body composition as well as medications, physical parameters, blood samples, disease pattern, and smoking were considered.
RESULTS: RMR was significantly associated with body weight (r = 0.732, P < 0.001), body height (r = 0.401, P = 0.008), BMI (r = 0.504, P < 0.001), waist circumference (r = 0.602, P < 0.001), mid-upper arm circumference (r = 0.417, P = 0.006), mid-upper arm muscle circumference (r = 0.344, P = 0.028), total body water (r = 0.339, P = 0.035), body temperature (r = 0.409, P = 0.007), smoking (P = 0.031), serum T4 levels (r = 0.331, P = 0.036), obstructive sleep apnoea syndrome (OSAS; P = 0.023), impaired glucose tolerance (IGT; P = 0.017) and impaired glycaemic status, including hyperinsulinism, IGT and diabetes mellitus (P = 0.003).
CONCLUSION: Future research should be prompted to optimize the procedure of indirect calorimetry to achieve clinical benefits in obese subjects.
Keywords: Indirect calorimetry, Obesity, Resting metabolic rate, Resting energy expenditure
INTRODUCTION
An imbalance between energy expenditure (EE) and energy intake (EI) underlies the accumulation of exceeding body fat (BF) in obese subjects[1,2]. Moreover, adiposity represents a common soil at the origin of different disorders such as type 2 diabetes mellitus (T2DM), hypertension, dyslipidemia, coronary heart disease (CHD), metabolic syndrome (MetS), sleep apnoea, as well as cancer[3].
In overweight and obese patients, the exact assessment of basal metabolism plays a pivotal role in order to tailor a balanced nutritional support. Predictive equations have been originally developed from data collected in normal-weight individuals[4,5]; even if they are considered to be a rapid and easy indirect method of resting metabolic rate (RMR) definition, they could not be thoroughly appropriate for obese patients, as a lack of correspondence between predicted values and real metabolic rate has been described in this subset of subjects[6].
The direct measurement of RMR should be performed when the clear- cut definition of energy requirements is needed to address correctly dietary interventions[7]. Indirect calorimetry (IC) is based on the evaluation of O2 consumption (Vo2) and CO2 production (Vco2), and it is the most common method currently used with this purpose[8].
Several factors have been shown to influence RMR measurement by IC. FFM, gender, and age may affect RMR, whereas the role of other factors, such as T2DM, ethnicity, menstrual cycle, hypertension, thyroid function, and smoking, remains to be univocally clarified[9,10].
The aim of this study was the analysis of selected factors potentially affecting the RMR for a better understanding of determinants of RMR, providing evidence that would be helpful in obesity management and prevention of obesity-related comorbidities, optimizing weight loss strategies.
MATERIALS AND METHODS
Subjects
Sixty-one obese Caucasian women (mean age: 52.93 ± 13.45 years, and mean BMI: 41.78 ± 11.54 kg/m2), were enrolled in the study. Secondary diseases and medications at the time of the recruitment were not considered as exclusion criteria. After the IC performance, 19 women were excluded because the steady state criterion was not achieved. Hence, a sample of 42 female obese subjects was considered.
Study design
Examinations were performed under thermo-neutral conditions, from 8 to 10 o’clock in the morning[11]. Participants had to remain in a 12-h fasting state before the examination, refraining from any heavy physical activity in the same period, abstaining from smoking in the preceding 2 h, and without ingesting coffee or water during 4 h prior to examination[12].
RMR measurement
RMR was defined for each subject using an open- circuit indirect computerized calorimeter (stable IC: Quark RMR Cosmed ), equipped with a canopy. Measurements were performed along a 15-min period preceded by a 10-min rest. Thermo-neutral conditions in a darkened room were chosen, leaving subjects in a comfortable environment. Subjects were asked not to move and not to talk during the test performance. Heart rate (HR) was monitored. Excluding the preliminary 5 min of each measurement, the subsequent 10 min were considered for the evaluation of data, expressed in kJ/d; also the steady state criterion was established (5-min stable period of < 10% variation in measured VO2 and VCO2). O2 consumption and CO2 production were standardized for temperature, barometric pressure and humidity[13].
Anthropometric parameters
Body weight (BW) was measured to an accuracy of 0.1 kg through a standard column body scale. Body height (BH) was determined using a rigid stadiometer to an accuracy of 0.5 cm. BMI was calculated as BW/(BH)2. Waist circumference (WC) was gathered using a standard measuring tape, to an accuracy of 0.1 cm. Mid arm circumference (MAC) was gathered using a standard measuring tape, on the non-dominant arm midway between the shoulder and elbow, to an accuracy of 0.1 cm.
Body composition
Bioimpedance analysis was performed on the right body side[14] using a bioimpedance analyser AKERN Bioresearch SRL, Pontassieve, FL, Italy. Skinfold measurements: fat mass (FM) was estimated from the sum of four skinfolds (SF): tricipital (TSF), bicipital, subscapular, and suprailiac, measured on the non-dominant body side. Each measurement was repeated for three times, and the mean value was calculated to reduce the variability in the performance (Harpenden Caliper User manual). Body density (BD) was assessed using the Durnin and Womersley sex- and age- adjusted linear regression equation; from BD, FM was calculated by the Siri equation[15].
Physical parameters
Measurements of blood pressure (BP) were performed on the left upper arm, to an accuracy of 5 mmHg. The body temperature (BT) was measured in the armpit, using a standard clinical thermometer.
Laboratory data/ biochemistry
Blood samples were taken from each subject after an overnight fast. The following biochemical parameters were assayed: glucose, insulin, triglycerides (TG), total cholesterol (TC), LDL-cholesterol, HDL-cholesterol, thyroid stimulating hormone (TSH), thyroxin (T4), albumin, and pre- albumin.
Laboratory tests were performed using a COBAS-MIRA analyser and a Cell-Dyn 1700 Analyser (Abbott), at the Laboratory of the “Villa delle Querce” Clinical Rehabilitation Institute. Serum concentrations of the biological indices were determined by routine methods with conventional commercial kits (ABX, Rome, Italy).
Disorders, diseases, medications and lifestyle habits
These parameters encompassed: T2DM (defined as fasting glucose levels ≥ 126 mg/dL), impaired glucose tolerance (IGT) (fasting glucose levels ranging from 110 to 126 mg/dL), hyperinsulinism, obstructive sleep apnoea syndrome (OSAS), hypothyroidism/dysthyroidism, dyslipidemia, hypertension (BP > 130/85 mmHg), depression. Metformin, levothyroxine, anti- depressants and anti- hypertensive agents were included. Smoking habit (more than ten cigarettes per day), and the menstrual state (regular/irregular menses and postmenopausal period) were also taken into account.
Statistical analysis
Baseline data and subjects’ characteristics were evaluated and collected. Statistical analysis was performed using Excel 2007 and SPSS 10.0 for Windows. Parameters were expressed by mean values and by standard deviation (SD) indicating the minimal and maximal extremity of each range of values. The outcome dependent variable, the RMR, was expressed in kcal/d. Independent variables were expressed as continuous or as cathegorial variables.
Pearson’s correlation coefficient was used to correlate the means of the RMR with parameters. Linear regression models, analysis of variance (ANOVA), and independent t test were used. Differences and correlations were considered statistically significant when P value was < 0.05.
RESULTS
A total of sixty-one women (mean age of 52.93 ± 13.45 years, and mean BMI of 41.78 ± 11.54 kg/m2), were studied. Descriptive data, anthropometric characteristics, and selected parameters are shown in Tables 1 and 2, respectively. Just forty two of the sixty one women achieved the steady state criterion. Thus, for further evaluation, 42 obese women (mean age 52.72 ± 13.96 years and mean BMI 41.35 ± 11.37 kg/m2) were considered only.
Table 1.
Anthropometric characteristics, clinical parametersand body composition
| Variable | mean ± SD |
| Age (yr) | 52.72 ± 13.96 |
| BW1 (kg) | 102.97 ± 26.55 |
| BH1 (cm) | 158.41 ± 6.71 |
| BMI1 (kg/m2) | 41.35 ± 11.37 |
| WC1 (cm) | 115.44 ± 22.08 |
| HR1 (bpm) | 66.61 ± 11.03 |
| SBP1 (mmHg) | 127.32 ± 18.06 |
| DBP1 (mmHg) | 78.04 ± 8.58 |
| CB1 (cm) | 39.01 ± 8.23 |
| CMB1 (cm) | 29.31 ± 5.27 |
| FM12 (%) | 42.86 ± 10.06 |
| FM13 (%) | 45.34 ± 28.07 |
| FFM13 (%) | 54.66 ± 28.07 |
| MM13 (%) | 32.16 ± 25.71 |
| BCM13 (%) | 44.49 ± 8.40 |
| BCM1-Index3 | 8.91 ± 2.43 |
| TBW13 (%) | 41.06 ± 20.42 |
| ECW13 (%) | 52.56 ± 5.37 |
| ICW13 (%) | 47.44 ± 5.37 |
| RMR1 (kcal/d) | 1537.91 ± 281.76 |
Descriptive data in obese women.
See the full text for abbreviations of subjects’ anthropometric parameters;
Estimated by Harpenden caliper;
Estimated by BIA. BW: Body weight; BH: Body height; BMI: Body mass index; WC: Waist circumference; HR: Heart rate; FM: Fat mass; RMR: Resting metabolic rate.
Table 2.
Parameters influencing resting metabolic rate (descriptive data)
| Variable | Subjects (%) |
| T2DM | 33.3 |
| IGT | 18.8 |
| Hyperinsulinism | 26.1 |
| OSAS | 17.4 |
| Dysthyroidism | 31.3 |
| Dyslipidemia | 13.0 |
| Hypertension | 65.2 |
| Depression | 27.5 |
| Metformin | 36.2 |
| Antidepressants | 18.8 |
| Antihypertensive drugs | 10.1 |
| Levothyroxine | 31.9 |
| Smoking habit | 43.8 |
| Postmenopausal state | 0 |
T2DM: Type 2 diabetes mellitus; IGT: Impaired glucose tolerance; OSAS: Obstructive sleep apnoea syndrome.
Factors influencing the RMR
Anthropometric parameters influencing the RMR: The following anthropometric parameters: BW (r = 0.732, P < 0.001), BMI (r = 0.504, P < 0.001), WC (r = 0.602, P < 0.001), MAC (r = 0.417, P = 0.006), BH (r = 0.401, P = 0.008), MAMC (r = 0.344, P = 0.028), and TBW (r = 0.339, P = 0.035), showed a statistically significant correlation with RMR (Table 3).
Table 3.
Correlation analysis among subjects’ anthropometric parameters, body composition and resting metabolic rate (kJ/d) (n = 42)
| Variables | Correlation (r) | P4 |
| Age (yr) | 0.006 | 0.969 |
| BW1 (kg) | 0.732 | < 0.001 |
| BH1 (cm) | 0.401 | 0.008 |
| BMI1 (kg/m2) | 0.504 | < 0.001 |
| WC1 (cm) | 0.602 | < 0.001 |
| MAC1 (cm) | 0.417 | 0.006 |
| MAMC1 (cm) | 0.344 | 0.028 |
| FM12 and FFM12 (%) | 0.169 | 0.290 |
| FM13 and FFM13 (%) | 0.113 | 0.485 |
| MM13 (%) | 0.105 | 0.522 |
| BCM13 (%) | 0.033 | 0.837 |
| BCM1-Index3 | 0.120 | 0.458 |
| TBW13 (%) | 0.339 | 0.035 |
| ECW13 and ICW13 (%) | 0.029 | 0.865 |
See the full text for abbreviations of subjects’ anthropometric parameters;
Estimated by Harpenden caliper;
Estimated by BIA. BW: Body weight; 4Differences and correlations were considered statistically significant when P value was < 0.05. BH: Body height; BMI: Body mass index; WC: Waist circumference; FM: Fat mass.
Physical parameters influencing the RMR: BP, HR, SpO2, and BT were considered (Table 4). No significant correlation was found for BP (r = 0.048/0.035, for systolic and diastolic BP, respectively) and SpO2 (r = 0.158) indicating a lack of influence of these parameters on the measured RMR. 29 (69%) of the total subjects were treated with anti- hypertensive agents, likely accounting for normal BP values. On the other hand, correlations were obtained with HR (r = 0.296, P = 0.050; Figure 1B: P < 0.05), and a significant association was found with BT (r = 0.409, P = 0.007; Figure 1A). As shown in Figure 1A (P < 0.05), BT was positively correlated with RMR.
Table 4.
Correlation of subjects’ physical characteristics with resting metabolic rate (n = 42)
| Variables | Correlation (r) | P1 |
| Blood pressure (mmHg) systolic/diastolic | 0.048/0.035 | 0.759/0.825 |
| Heart rate (b/min) | 0.296 | 0.050 |
| SpO2 (%) | 0.158 | 0.318 |
| Body temperature (°C) | 0.409 | 0.007 |
1Differences and correlations were considered statistically significant when P value was < 0.05.
Figure 1.
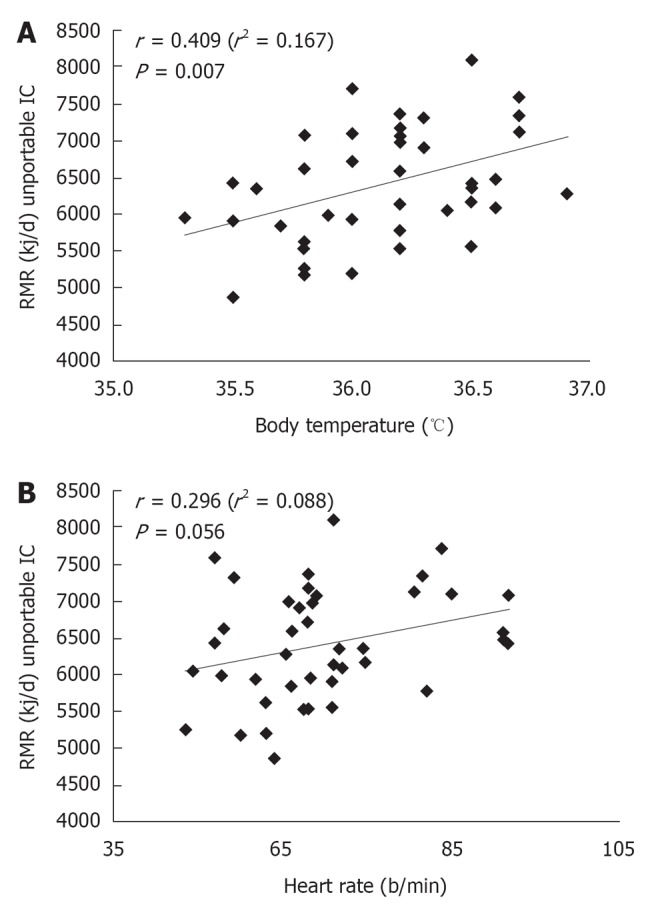
Correlation analysis between resting metabolic rate and body temperature (A) and heart rate (B). RMR: Resting metabolic rate.
A slight positive correlation between RMR and HR was described, showing a trend toward a higher estimated RMR consistently with an increase of HR, but without statistically significant results.
Blood parameters influencing the RMR: In Table 3 correlation analysis among RMR and serum TC, TG, LDL- cholesterol, HDL- cholesterol, glucose, insulin, TSH, T4, albumin and pre-albumin levels is shown. Just one significant positive association has been reported between RMR and serum T4 concentrations (r = 0.331, P = 0.036) (Figure 2: P < 0.05); as illustrated in Figure 2, a significant positive correlation has been found between RMR and T4 concentrations, even considering progressively increasing serum T4 levels within the normal range of values. Eighteen subjects had hypothyroidism and were on levothyroxine therapy (Figure 2).
Figure 2.
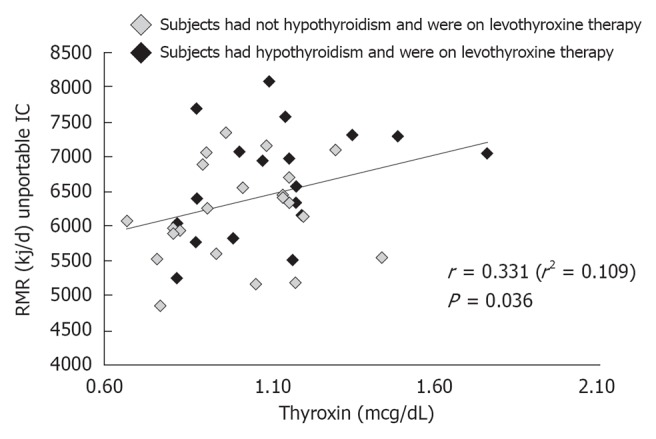
Correlation analysis between resting metabolic rate and thyroxin levels. RMR: Resting metabolic rate.
Other factors influencing the RMR: A correlation analysis among RMR and disorders, diseases, medications, and smoking was performed. A total of 13 parameters were included: type 2 diabetes mellitus (T2DM- including patients on metformin therapy), IGT, hyperinsulinism, thyroid function (hypothyroidism and dysthyroidism), dyslipidemia, hypertension (including subjects on hypertensive therapy), OSAS, depression, anxiety, menstrual cycle or postmenopausal state, antidepressant medications, metformin, levothyroxine, and smoking. A significant relationship was demonstrated between RMR and smoking (P = 0.031), OSAS (P = 0,023), IGT (P = 0.005), and impaired glycemic status- including IGT, T2DM, and hyperinsulinism (P = 0.003).
Environmental conditions influencing the RMR: Data provided by correlation analysis excluded any significant association among RMR and environmental variables (room temperature: r = 0.118, P = 0.456; room humidity: r = 0.076, P = 0.634, and atmospheric air pressure: r = 0.018, P = 0.910).
DISCUSSION
In the present study we selected and considered anthropometric and physical characteristics, blood parameters, as well as environmental conditions, to ascertain their impact on the RMR of obese female subjects.
As expected, BW and BH were significantly positively correlated with RMR, as well as BMI did, confirming the important role of BW and BH in modulating the basal metabolic rate, as these variables were already taken into account in early studies for the estimation of RMR.
WC, MAC and MAMC were found to be significantly positively associated with RMR. WC is a strong predictor for visceral adipose tissue, and it is currently and universally accepted as an independent cardiovascular risk marker for obese people[16]. Just few studies exploring and assessing the relationship between WC and RMR exist[17,18]. The correlation of MAC and MAMC with RMR could be explained by a higher accumulation of FM in the mid-upper arm in obese subjects, which is proportional to the accumulation of adipose tissue in other parts of the body.
BW as a significant predictor of increased RMR, and the higher percentage of FM and FFM in obese subjects, may lead also to a higher MAC and MAMC, hence to a higher RMR.
Several studies reported that RMR decreases paralleling aging, because of the loss of FFM and the increase in FM, since body mass and BC are the major predictors of EE[1]. In the present study no significant correlation was found among FM, FFM, BCM, MM, BCMI and RMR. FM was expected to contribute slightly to RMR, thus no significant correlation was obtained, and it is supported by previous evidence[19]. The influence of FM on RMR has not been univocally assessed, results being inconsistent across studies, even if it seems to play only a minor additional role.
FFM alone is well known to be related to RMR[20] though in the present study no correlation emerged, nor with values obtained by anthropometry neither by BIA, whereas a positive association was found between RMR and TBW. Special considerations should be highlighted when body composition of obese subjects is determined by BIA. In obese and overweight individuals the higher hydration of FFM is responsible for an overestimation of FFM and underestimation of FM. Additionally, the body build of obese subjects, especially abdominal obesity, concurs to the mentioned impairment of the results provided by the BIA[21]. Surprisingly in the present study TBW was significantly positively correlated with RMR, while FFM and FM were not. Even if BIA could not be completely reliable in our sample of obese participants, an increased total body water content mirroring a higher FFM may explain the positive association with RMR, that is notably higher as FFM increases.
Hence, the finding of a positive association between RMR and TBW, in absence of the same relation linking RMR and FFM, could be likely justified by the limited accuracy of BIA for FFM and FM assessment in such an altered hydration status that is obesity.
Otherwise, we evaluated FFM as a unique compartment, while growing evidence recognizes that FFM is energetically heterogeneous because it encompasses organs and tissues having a different metabolic rate[22].
Age did not show any correlation with RMR. Basal metabolism was shown to drop by 1%-2% per decade over the age of 20 to 75 years[23], and that aging process is accompanied by the apparent replacement of a certain proportion of MM by a gain in FM[24]. Actually, a potential reason for the inconsistency of our findings may be that mean age of our participants was lower than age of subjects in prior studies reporting an association between age and RMR[25].
Evaluating physical characteristics and the environmental conditions of the examination room, significant results were obtained only regarding BT. No significant results were obtained by the correlation analysis of RMR with BP, HR, SpO2, MET, room humidity and atmospheric air pressure.
No significant correlation with RMR was found for the selected blood parameters, save for serum T4 levels. The other blood parameters did not show any significant effect on the RMR, but it is likely that a detection of significantly abnormal values was difficult, since most subjects were on pharmacological therapy to maintain blood values in the normal range.
A wide spectrum of evidence supports the involvement of thyroid hormone in mechanisms responsible for thermogenesis, affecting EE and basal metabolic rate[26].
Resting energy expenditure (REE) has been shown to be very sensitive to modulation by thyroid hormone[27].
In the present study higher T4 concentrations were associated with higher RMR. Although thyroid hormone concentration was within the normal range, a significant effect of T4 concentration on RMR was however detectable. 19 subjects in the present study had hypothyroidism with no significant effect on metabolism, likely justified by levothyroxine chronic supplementation.
The effect of smoking status on EE has been at the centre of a number of studies attempting to assess the relationship linking the smoking attitude or its cessation to BW fluctuations[28,29]. The acute or short- term effect of cigarette smoking or nicotine leads to changes in metabolism that imply an increase in total EE or in RMR in few studies[30,31], despite this hypothesis remains to be confirmed. Moreover, smoking effect has been found to be different in obese smokers when compared to lean smokers[32]. In our study, smokers were all obese, and their RMR was increased by 10.4% than non-smokers’ RMR. Mechanisms other than the direct influence on energy and metabolism may account for these results, as nicotine can affect appetite and sympathetic nervous system action.
An aspect that strengthens our finding is that the positive correlation between smoking and RMR exists even after patients refrained from smoking at least 2 h before the examination, eliminating the very short-term nicotine effects.
Disorders and diseases as well as medicines did not show any significant correlation with RMR, save OSAS, IGT, and impaired glycaemic status (including IGT, T2DM, and hyperinsulinism), that were significantly correlated with RMR.
In agreement with prior studies, we found that RMR was increased in OSAS obese subjects when compared to obese women without OSAS[33,34].
OSAS patients are usually obese, and abnormalities in intrathoracic pressure lead to an increased work of breathing, as well as frequent arousals and increased sympathetic activity, that may account for the increased EE[33,34].
Additionally, we found that RMR was significantly different in obese female subjects with an impaired glycemic status, when compared to their normoglycemic counterparts.
We considered subgroups including patients with IGT, diabetes mellitus, and hyperinsulinism, respectively, and subgroup analysis showed that RMR was significantly increased in patients with IGT or in whom with hyperinsulinism, whereas only a trend toward higher values of RMR was observed in patients with diabetes mellitus; previous studies had demonstrated an increased RMR in diabetic patients, and several mechanisms were implicated to explain the increased EE, such as the activation of energy- consuming metabolic processes- gluconeogenesis and other substrate cycles- and an increased sympathetic nervous system activity. We have to stress that in the subgroup of type 2 diabetic patients, those who were on metformin therapy were not considered separately, and it may account for the slight increase in RMR in the whole subgroup. Moreover, no significant difference has emerged from the comparison of RMR between diabetic subjects treated with metformin and non-treated patients. Metformin is responsible for improved glycemic control, thus it may avoid overcoming metabolic adaptations of RMR that typically occur in T2DM[35]. Therefore, the significant association between increased RMR and IGT and the tendency toward a higher RMR in type 2 diabetic patients could be due to the common soil of insulin resistance underlying the different aspects of glucose abnormalities; on the other hand, just a little body of evidence is available at present investigating the relationship between insulin resistance and RMR[36].
Definitive conclusions may not be drawn. First, sample size was relatively small. Moreover, we studied a group of white female subjects exclusively, and influence of both gender and ethnicity on RMR has been widely recognized.
It is noteworthy that 33 of the 77 subjects, originally entering the study, failed to achieve the steady state criterion for IC measurements, hence they were subsequently excluded.
In stable, spontaneously breathing patients, like those evaluated in the present study, anxiety or hyperventilation have been frequently addressed to impact the achievement of the steady state[37]. The failure in satisfying the steady state criterion could be attributable to the assumption of a very stringent definition of steady state: less than 10% for changes in Vo2 and VCO2 over a period of 5 min. In existing literature less rigorous steady state criteria were applied, even if a weaker strength of correlation measurements with the 24-h REE was obtained when establishing a Vo2/Vco2 change by ≤ 15% or 20%. We adopted a more stringent definition of steady state in order to confer a higher accuracy to the results. We do not know if obesity may represent a limitation for the satisfaction of a very strict steady state criterion, and similar data are lacking for obese population at present.
In conclusion, the association among several anthropometric, physical and environmental factors, as well as disorders, diseases, medications, smoking habit and RMR was explored. Although significant results emerged from the correlation analysis, showing that RMR was positively related to BW, BH, BMI, WC, MAC, MAMC, TBW, BT, serum T4 levels, levothyroxine and smoking, the reason for the significance was not always thoroughly clear, and it could be related to coincidental association within and between parameters, considering that RMR can range widely within a group of people.
The precise identification of factors influencing the RMR is not only an experimental concern, but it represents a real clinical challenge. IC is an important method allowing an individualized obese patient care. Defining factors interfering with RMR will be useful to maximize the beneficial effects deriving from a tailored therapeutic approach. Moreover it would prompt further research in order to address and to improve obesity management.
COMMENTS
Background
Obesity is spreading worldwide in an epidemic fashion. Research should be prompted to increase knowledge about mechanisms underlying metabolism regulation in order to optimize tools and potential care interventions.
Research frontiers
Currently technology offers tools that could be useful in a personalized approach to the management of obesity. Indirect calorimetry (IC) could be a technologic resource to improve the tailored treatment of obese subjects.
Innovations and breakthroughs
Because of the larger life expectancy and the presence of comorbidities linked both to the ageing process and to obesity itself, a variety of factors potentially affecting the resting metabolic rate (RMR) should be taken into account when considering obese subjects than their lean and healthy counterparts.
Applications
By understanding how resting energy expenditure (REE) is modulated in obese subjects, this study may represent a future strategy addressed to a technologically supported treatment and a more precise dietary intervention in this subset of patients.
Terminology
IC is a common method to assess exactly the REE, based on respiratory gas exchange, allowing defining thoroughly the characteristics of nutritional interventions.
Peer review
The authors examined selected factors potentially influencing the RMR in obese women. REE was evaluated using IC. The results suggest that this experimental approach could represent an interesting method for an individualized obese patient care.
Footnotes
Peer reviewer: Moses Elisaf, Professor of Medicine, University of Ioannina, Medical School, Department of Internal Medicine, 451 10 Ioannina, Greece
S- Editor Li JY L- Editor A E- Editor Zheng XM
References
- 1.Leitzmann C. Nutrition ecology: origin and definition. Forum Nutr. 2003;56:220–221. [PubMed] [Google Scholar]
- 2.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 3.Pi-Sunyer FX. The obesity epidemic: pathophysiology and consequences of obesity. Obes Res. 2002;10 Suppl 2:97S–104S. doi: 10.1038/oby.2002.202. [DOI] [PubMed] [Google Scholar]
- 4.Reeves MM, Capra S. Predicting energy requirements in the clinical setting: are current methods evidence based. Nutr Rev. 2003;61:143–151. doi: 10.1301/nr.2003.apr.143-151. [DOI] [PubMed] [Google Scholar]
- 5.Haugen HA, Chan LN, Li F. Indirect calorimetry: a practical guide for clinicians. Nutr Clin Pract. 2007;22:377–388. doi: 10.1177/0115426507022004377. [DOI] [PubMed] [Google Scholar]
- 6.Livingston EH, Kohlstadt I. Simplified resting metabolic rate-predicting formulas for normal-sized and obese individuals. Obes Res. 2005;13:1255–1262. doi: 10.1038/oby.2005.149. [DOI] [PubMed] [Google Scholar]
- 7.Compher C, Frankenfield D, Keim N, Roth-Yousey L. Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc. 2006;106:881–903. doi: 10.1016/j.jada.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Levine JA. Measurement of energy expenditure. Public Health Nutr. 2005;8:1123–1132. doi: 10.1079/phn2005800. [DOI] [PubMed] [Google Scholar]
- 9.Horgan GW, Stubbs J. Predicting basal metabolic rate in the obese is difficult. Eur J Clin Nutr. 2003;57:335–340. doi: 10.1038/sj.ejcn.1601542. [DOI] [PubMed] [Google Scholar]
- 10.Solomon SJ, Kurzer MS, Calloway DH. Menstrual cycle and basal metabolic rate in women. Am J Clin Nutr. 1982;36:611–616. doi: 10.1093/ajcn/36.4.611. [DOI] [PubMed] [Google Scholar]
- 11.Nieman DC, Trone GA, Austin MD. A new handheld device for measuring resting metabolic rate and oxygen consumption. J Am Diet Assoc. 2003;103:588–592. doi: 10.1053/jada.2003.50116. [DOI] [PubMed] [Google Scholar]
- 12.Haugen HA, Melanson EL, Tran ZV, Kearney JT, Hill JO. Variability of measured resting metabolic rate. Am J Clin Nutr. 2003;78:1141–1145. doi: 10.1093/ajcn/78.6.1141. [DOI] [PubMed] [Google Scholar]
- 13.Severinghaus JW. Water vapor calibration errors in some capnometers: respiratory conventions misunderstood by manufacturers. Anesthesiology. 1989;70:996–998. doi: 10.1097/00000542-198906000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Chumlea WC, Guo SS. Bioelectrical impedance and body composition: present status and future directions. Nutr Rev. 1994;52:123–131. doi: 10.1111/j.1753-4887.1994.tb01404.x. [DOI] [PubMed] [Google Scholar]
- 15.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 16.Janssen I, Katzmarzyk PT, Ross R. Duration of overweight and metabolic health risk in American men and women. Ann Epidemiol. 2004;14:585–591. doi: 10.1016/j.annepidem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Armellini F, Robbi R, Zamboni M, Todesco T, Castelli S, Bosello O. Resting metabolic rate, body-fat distribution, and visceral fat in obese women. Am J Clin Nutr. 1992;56:981–987. doi: 10.1093/ajcn/56.6.981. [DOI] [PubMed] [Google Scholar]
- 18.den Besten C, Vansant G, Weststrate JA, Deurenberg P. Resting metabolic rate and diet-induced thermogenesis in abdominal and gluteal-femoral obese women before and after weight reduction. Am J Clin Nutr. 1988;47:840–847. doi: 10.1093/ajcn/47.5.840. [DOI] [PubMed] [Google Scholar]
- 19.Usui C, Takahashi E, Gando Y, Sanada K, Oka J, Miyachi M, Tabata I, Higuchi M. Relationship between blood adipocytokines and resting energy expenditure in young and elderly women. J Nutr Sci Vitaminol (Tokyo) 2007;53:529–535. doi: 10.3177/jnsv.53.529. [DOI] [PubMed] [Google Scholar]
- 20.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 21.Coppini LZ, Waitzberg DL, Campos AC. Limitations and validation of bioelectrical impedance analysis in morbidly obese patients. Curr Opin Clin Nutr Metab Care. 2005;8:329–332. doi: 10.1097/01.mco.0000165013.54696.64. [DOI] [PubMed] [Google Scholar]
- 22.Javed F, He Q, Davidson LE, Thornton JC, Albu J, Boxt L, Krasnow N, Elia M, Kang P, Heshka S, et al. Brain and high metabolic rate organ mass: contributions to resting energy expenditure beyond fat-free mass. Am J Clin Nutr. 2010;91:907–912. doi: 10.3945/ajcn.2009.28512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keys A, Taylor HL, Grande F. Basal metabolism and age of adult man. Metabolism. 1973;22:579–587. doi: 10.1016/0026-0495(73)90071-1. [DOI] [PubMed] [Google Scholar]
- 24.Lazzer S, Bedogni G, Lafortuna CL, Marazzi N, Busti C, Galli R, De Col A, Agosti F, Sartorio A. Relationship between basal metabolic rate, gender, age, and body composition in 8,780 white obese subjects. Obesity (Silver Spring) 2010;18:71–78. doi: 10.1038/oby.2009.162. [DOI] [PubMed] [Google Scholar]
- 25.Manini TM, Everhart JE, Anton SD, Schoeller DA, Cummings SR, Mackey DC, Delmonico MJ, Bauer DC, Simonsick EM, Colbert LH, et al. Activity energy expenditure and change in body composition in late life. Am J Clin Nutr. 2009;90:1336–1342. doi: 10.3945/ajcn.2009.27659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid. 2008;18:141–144. doi: 10.1089/thy.2007.0266. [DOI] [PubMed] [Google Scholar]
- 27.Tagliaferri M, Berselli ME, Calò G, Minocci A, Savia G, Petroni ML, Viberti GC, Liuzzi A. Subclinical hypothyroidism in obese patients: relation to resting energy expenditure, serum leptin, body composition, and lipid profile. Obes Res. 2001;9:196–201. doi: 10.1038/oby.2001.21. [DOI] [PubMed] [Google Scholar]
- 28.Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87:801–809. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 29.Bradley DP, Johnson LA, Zhang Z, Subar AF, Troiano RP, Schatzkin A, Schoeller DA. Effect of smoking status on total energy expenditure. Nutr Metab (Lond) 2010;7:81. doi: 10.1186/1743-7075-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perkins KA, Epstein LH, Stiller RL, Marks BL, Jacob RG. Acute effects of nicotine on resting metabolic rate in cigarette smokers. Am J Clin Nutr. 1989;50:545–550. doi: 10.1093/ajcn/50.3.545. [DOI] [PubMed] [Google Scholar]
- 31.Hofstetter A, Schutz Y, Jéquier E, Wahren J. Increased 24-hour energy expenditure in cigarette smokers. N Engl J Med. 1986;314:79–82. doi: 10.1056/NEJM198601093140204. [DOI] [PubMed] [Google Scholar]
- 32.Audrain JE, Klesges RC, Klesges LM. Relationship between obesity and the metabolic effects of smoking in women. Health Psychol. 1995;14:116–123. doi: 10.1037//0278-6133.14.2.116. [DOI] [PubMed] [Google Scholar]
- 33.Lin CC, Chang KC, Lee KS. Effects of treatment by laser-assisted uvuloplasty on sleep energy expenditure in obstructive sleep apnea patients. Metabolism. 2002;51:622–627. doi: 10.1053/meta.2002.31969. [DOI] [PubMed] [Google Scholar]
- 34.Ryan CF, Love LL, Buckley PA. Energy expenditure in obstructive sleep apnea. Sleep. 1995;18:180–187. doi: 10.1093/sleep/18.3.180. [DOI] [PubMed] [Google Scholar]
- 35.Nawata K, Sohmiya M, Kawaguchi M, Nishiki M, Kato Y. Increased resting metabolic rate in patients with type 2 diabetes mellitus accompanied by advanced diabetic nephropathy. Metabolism. 2004;53:1395–1398. doi: 10.1016/j.metabol.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 36.De Luis DA, Aller R, Izaola O. Resting energy expenditure and insulin resitance in obese patients, differences in women and men. Eur Rev Med Pharmacol Sci. 2006;10:285–289. [PubMed] [Google Scholar]
- 37.da Rocha EE, Alves VG, da Fonseca RB. Indirect calorimetry: methodology, instruments and clinical application. Curr Opin Clin Nutr Metab Care. 2006;9:247–256. doi: 10.1097/01.mco.0000222107.15548.f5. [DOI] [PubMed] [Google Scholar]


