Abstract
AIM: To investigate the effects of exercise on healthy individuals of both genders.
METHODS: This study lasted 6 years and involved about 800 healthy people. Individuals were divided into females and males and further sub-divided into two groups; in the first group individuals run (or skied in the winter time) and then rested for 3 h, whereas individuals in the second group intensely cycled for 5 min. The status of health was determined by measuring the sedimentation rate and the intensity of exercises by measuring the heart rate. Blood samples were collected before and after exercise.
RESULTS: We observed that in the first group a significant increase of the total white blood cells, segmented neutrophils, band neutrophils, eosinophils and to a lesser extent lymphocytes but not monocytes in the blood circulation. However, all cell types were increased in the circulation after 5 min intense exercise. No differences in the pattern of cell increase were observed among the genders. Activated partial thromboplastin time (APTT) and D-dimer were also measured in the blood of individuals who cycled intensely for 5 min to determine the coagulation and fibrinolytic activities in the blood. APTT is reduced and D-dimer values significantly increased after intense exercise. However, APTT was statistically lower in males than females, whereas no differences in the D-dimer values were observed among the genders.
CONCLUSION: Our results indicate that exercise whether leisure or strenuous affects leukocytosis and hemostasis in both genders. A major advantage of this study is the high numbers of individuals involved and the inclusion of both females and males values.
Keywords: Exercise, Leukocytosis, Activated partial thromboplastin time, D-dimer
INTRODUCTION
Recreational exercise is now part of everyday life style because it is important for maintaining cardiovascular fitness. Emphasis is on improving cardiac input and activity as well as manipulating the immune system for a better and prolonged life. Hence, several studies were dedicated to examine the blood hemodynamic and hemostasis after exercise whether being leisure, intense or strenuous. The total number of white blood cells (WBCs) was increased during and immediately after exercise[1], and both leukocytosis and thrombocytosis occurred in the first 10 min of high-intensity exercise[2]. In 16 male volunteers (average age 30 years), all leukocytes except basophils and eosinophils were increased very early after resistance exercise but declined after 15 and 30 min with the exception of neutrophils[3]. About 5 h after exercise, there was a marked increase in the numbers of blood granulocytes and monocytes along with several inflammatory cytokines and chemokines for both well-trained and untrained runners[4]. Another study examined leukocytosis in 10 female soccer players and observed neutrocytosis but not lymphocytosis after one bout of intense exercise “at 75% maximal heart rate”[5]. Upon comparing the immunological parameters in age and gender different groups it was observed that exercise induced significant increases in total leukocytes and lymphocytes in 11 girls as compared to 13 boys studied[6].
Neutrocytosis may depend on the duration rather than the intensity of the exercise, which also depends on the release of adrenocorticotropic hormone[7]. Intensive short-term exercise resulted in increased leukocytosis which included lymphocytes, granulocytes and monocytes concomitant with alterations in plasma catecholamine levels[8]. A 30 min exercise in 8 male volunteers resulted in increased catecholamine and leukocytosis, whereas another round of exercise 3 h later also resulted in enhanced leukocytosis “neutrophils and lymphocytes” and increased cortisol levels[9]. It was proposed that catecholamine increases the number of circulating leukocytes, whereas cortisol which has a later effect maintains this increase in the vascular compartments[10]. Similarly, a short period of recreational vigorous exercise induced significant leukocytosis, which could be due to the release of adrenalin[11]. In contrast to these results it was reported that neither the total increase nor the subsequent decline in plasma cortisol concentration after exercise in 7 healthy male volunteers is important for leukocytosis[12].
Physical exercise also affects blood hemostasis since blood coagulation cascade was activated as demonstrated by shortening the activated partial thromboplastin time “activated partial thromboplastin time (APTT)”[13]. In 10 healthy adolescents, APTT is shortened by about 15% immediately after exercise which returned to normal value after 1 h concomitant with a high increase of fibrinolytic activity[14]. Similarly, activation of the coagulation cascade is detectable after acute physical exercise in 10 healthy subjects, which led to increased thrombin generation[15]. It was also reported that in 11 healthy male subjects irrespective of the type of exercise they performed, alterations in markers of thrombin and fibrin formation were pronounced after 1 h exercise. In this study of high impact, it was suggested that prolonged exercise is necessary for exercise-induced activation of coagulation resulting in thrombin and fibrin formation[16].
In 15 healthy individuals who performed strenuous exercise for 15, 45 and 90 s, it was observed that this exercise did not induce blood coagulation, whereas fibrinolysis, e.g., generation of tissue-plasminogen activator “t-PA” was increased after 15 s and remained high through the duration of exercise. The release of t-PA might be due to increased catecholamine concentrations and blood sheer-stress, whereas no increase in D-dimer generation was observed[17]. In contrast, Gunga et al[18] observed that PT and APTT were both increased in 15 healthy individuals after 30 s exercise, and that t-PA and D-dimer levels were also elevated after the same period suggesting that short-time intensive exercise shifts the homeostasis system into a higher equilibrium. The influence of moderate exercise was also studied and it was observed that after 30 min exercise there was an increase in t-PA[19]. On the other hand, the levels of APTT or D-dimer were not significantly increased during 15 min short-term extreme exercise, but APTT was decreased and D-dimer increased after termination of the exercise[20].
Although these studies are informative, drawbacks include the small numbers of samples included in each study (not more than 30 individuals at best). Therefore, it was difficult to draw a reasonable conclusion from these studies regarding the effects of exercise. In the current study we combined monitoring blood leukocytosis and hemodynamic in about 800 healthy individuals, a study that lasted about 6 years and included comparison of both males and females.
MATERIALS AND METHODS
Physical exercise protocols
Healthy volunteers (age 19-44 years, average 23 years) were divided into two groups with different experimental designs. Individuals in the first group were instructed to run or go cross-country skiing for at least 1 h without any pauses. Blood samples were collected before exercise and 3 h after exercise, and all samples were analyzed immediately after blood withdrawal. Individuals in the other group were instructed to use an exercise bike for 5 min at high resistance and with as high rpm as they could manage. Blood samples were collected before exercise and immediately after exercise. An overview of the numbers of individuals involved and the sort of exercise they performed are shown in Table 1.
Table 1.
Overview of individuals involved in the current study
| Blood examinations | Subjects | Exercise | Gender |
| SR | 795 | - | F |
| 506 | - | M | |
| WBCs | 241 | Running | F = 121/M = 120 |
| 273 | Cycling | F = 133/M = 140 | |
| Differential counting | 241 | Running | F = 121/M = 120 |
| 273 | Cycling | F = 133/M = 140 | |
| PCV | 241 | Running | F = 121/M = 120 |
| 273 | Cycling | F = 133/M = 140 | |
| APTT | 291 | Cycling | F = 132/M = 159 |
| D-dimer | 279 | Cycling | F = 125/M = 154 |
SR: Sedimentation rate; PCV: Packed cell volume; APTT: Activated partial thromboplastin time; WBCs: White blood cells; F: Female; M: Male.
Hematological tests
Duplicates of total white blood cell counts before and after exercise were performed using coulter counter Z1 from Beckman Coulter (Miami, FL, United states). Full blood was diluted with an isotonic salt solution and mixed with Zap-Oglobin II (Beckman Coulter) to lyse red blood cells. Blood smears were made from each EDTA-tube and stained using color rapid set from Lucerna-Chem (Lucerne, Switzerland). Differential counts of at least 100 WBCs were done on these smears. Triplicates of hematocrit were measured before and after exercise to determine the changes in packed cell volume (PCV). Determination of hematocrit was done by centrifuging the hematocrit capillary tubes in hematocrit centrifuges and then measuring on a circular micro capillary reader (Damon IEC division).
Coagulation tests
Vacuette sodium citrate tubes from before and immediately after 5 min intensive exercise were centrifuged for 15 min at 2000 × g to obtain platelet free plasma. Plasma samples were then tested for APTT and D-dimer values. APTT was measured using DG-APTT kit and a Thrombotrack coagulometer (Axis-Shield PoC AS Oslo, Norway). D-dimer test was done using NycoCard D-dimer test kit and NycoCard READER II (Axis Shield PoC AS). The purpose of this test was to determine the levels of D-dimer as an indirect measurement of plasma t-PA. Citrated plasma (500 μL) from before and after exercise was added to 0.1 U/mL thrombin (Sigma-Aldrich, St. Louis, MO, United states) and incubated for 5 min. A positive test was done from 500 μL plasma mixed with 0.01 mg/mL actilyse “recombinant t-PA” from Boehringer Ingelheim (Ingelheim am Rhein, Germany) and 0.1 U/mL thrombin. D-dimer concentration was measured using NycoCard READER. In these tests, the coagulation was performed on an independent set of individuals performing only intense exercise.
Heart rate and sedimentation rate
Heart rate was measured before and after exercise at the same time blood was collected for the two different groups. For sedimentation rate (SR), blood was drawn in BD Vacutainer glass seditainer tubes, and SR was measured in a seditainer manual ESR stand (BD Diagnostics, Plymouth, United Kingdom) from all individuals involved in these experiments.
Statistical analysis
Data was collected over a period of 6 years from anonymous individuals reporting only gender and physical condition. All statistical analyses were determined utilizing Graphpad Prism program (San Diego, CA, United States), and significant values were determined using the two-tailed Student’s t test. A P value < 0.05 was considered to be statistically significant.
RESULTS
Hematological evaluations
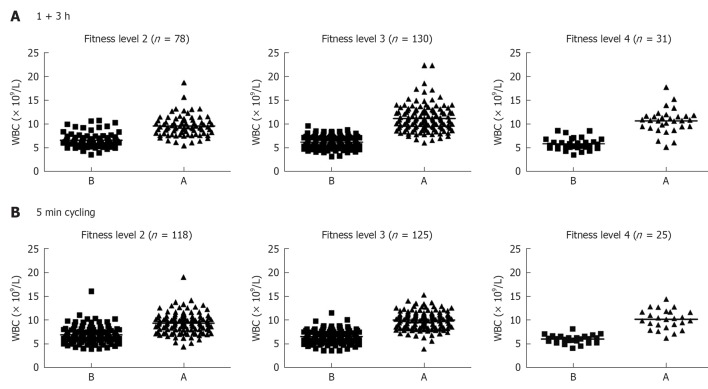
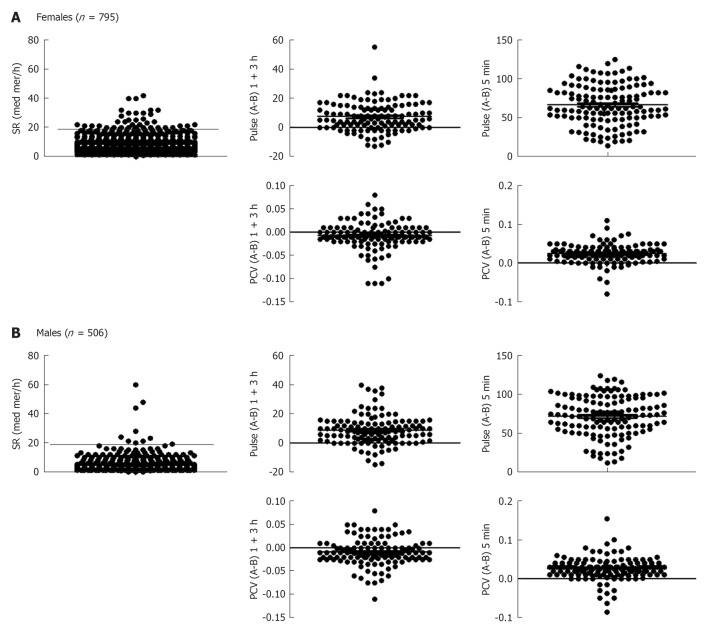
Individuals were divided into females and males and each were further sub-divided into two groups; individuals in one group run or skied for 1 h and then rested for 3 h, whereas those in the other group cycled at a maximum intensity for 5 min. Fitness levels ranking from 1-4 were investigated, where 1 indicates lack of training and 4 indicates athletic “training sports at a national level” (Figure 1). Only 4 subjects in total reported fitness level 1, and that group was not further analyzed. Blood was withdrawn before and after each exercise. A total number of 795 females were examined for SR, and those who showed 20 mm/h or more were excluded from the evaluation (Figure 2A). Heart rate measurement was important to determine whether individuals optimally followed the instructions, particularly with the intense exercise (5 min cycling). As can be seen pulse (heart rate) was higher after 1 + 3 h exercise and was more pronounced after the 5 min intense exercise (Figure 2A). There was no effect of 1 + 3 h exercise on PCV of females, but an increase was observed after the 5 min intense exercise. Exactly similar pattern was observed with males; those who had SR 15 mm/h or more were excluded from the evaluation. In addition heart rate difference between before (B) and after (A) exercise was more pronounced with the 5 min intense cycling. Similar to females, PCV was not increased after the 1 + 3 h exercise but increased after the 5 min intense exercise (Figure 2B).
Figure 1.
Fitness levels of individuals involved in this study. The numbers of white blood cells (WBCs) were counted according to the fitness levels of individuals (levels 1-4 from low to high intensity) in each group (those that run for 1 h and then rested for 3 h, and those that cycled for 5 min). The numbers of individuals in each category are shown. Also shown the numbers of WBCs before and after exercise. B: Before; A: After.
Figure 2.
Evaluation of blood and hearts. A: Sedimentation rate (SR) was measured in 795 female students (age 19-44 years, average 23 years old). Females with SR more than 20 mm/h were excluded from the evaluation (above the thick line). Packed cell volume (PCV) and heart rate (pulse) changes were calculated after exercise minus before exercise in 120 females who run or skied for 1 h and then rested for 3 h (1+3 h). PCV and pulse were also measured in about 135 females who exercised for 5 min at a maximum intensity; B: This is similar to Panel A except that 506 males were examined for SR; those that have more than 15 mm/h were excluded (above the thick line). Lines in the other panels show the PCV or pulse after minus before exercise. Each dot represents one individual. B: Before; A: After.
Leukocytosis examination after 1 + 3 h exercise
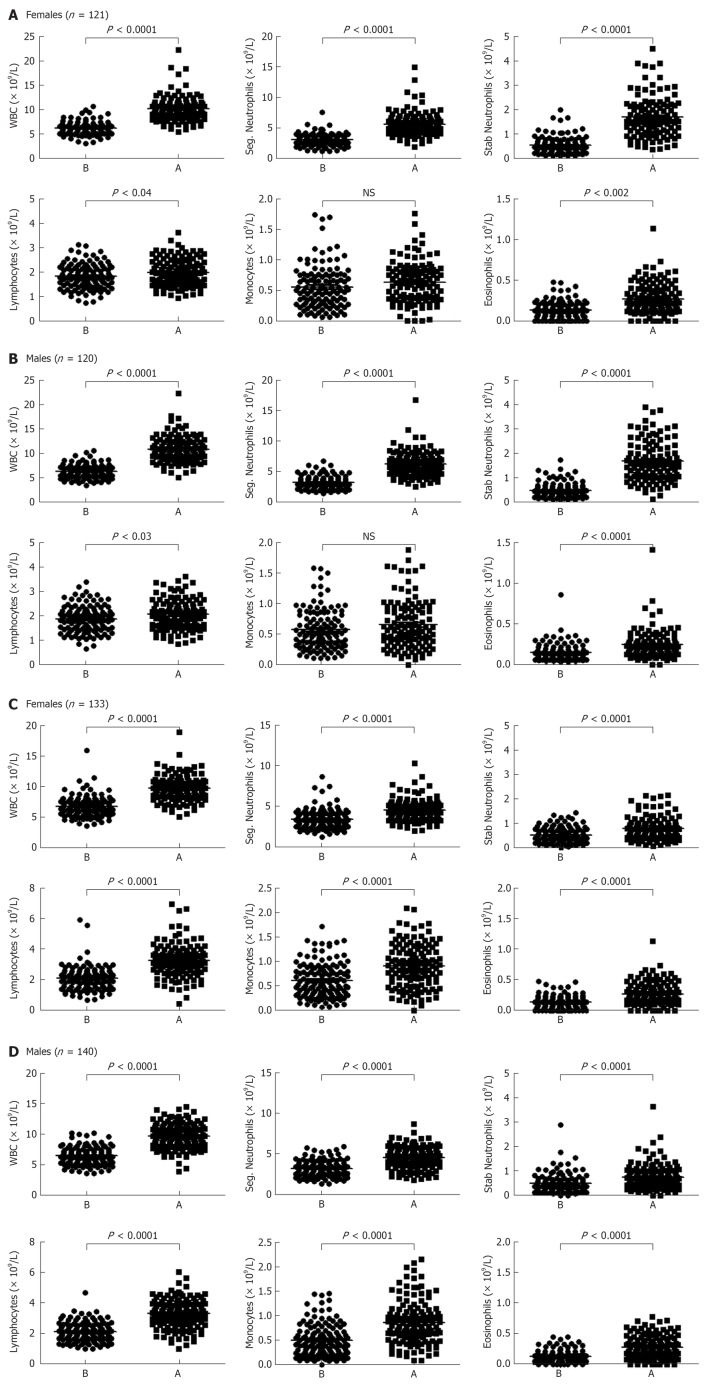
A significant increase in the number of WBCs was observed in the blood of females who run (or skied) for 1 h and then rested for 3 h (P < 0.0001 when compared to the number of WBCs before exercise, Figure 3A). Both segmented and stab (band) neutrophils numbers were also significantly increased after this mode of exercise (P < 0.0001 for both cell types, Figure 3A). There was a significant increase in the number of lymphocytes (P < 0.04), albeit it was much lower than neutrophils. Monocytes number was not increased, whereas the number of eosinophils in the blood circulation was significantly increased after exercise (P < 0.002). Similar findings were observed with males blood where the numbers of total WBCs, segmented neutrophils, stab neutrophils, lymphocytes and eosinophils, but not monocytes were increased as compared to the resting state (P < 0.0001, P < 0.0001, P < 0.0001, P < 0.03, P < 0.0001, and not significant, respectively; Figure 3B).
Figure 3.
Evaluation of leukocytosis before and after leisure exercise. A: Total numbers of white blood cells, segmented (seg.) neutrophils, stab (band) neutrophils, total lymphocytes, monocytes and eosinophils were calculated from 121 healthy females before and after 1 h run (or ski) and 3 h rest. Lines indicate the mean values. P values compared the differences in the numbers between after and before exercise; B: This is similar to panel A, except that 120 healthy males were examined. Each dot represents one individual; C: Total numbers of white blood cells, segmented neutrophils, stab (band) neutrophils, total lymphocytes, monocytes and eosinophils were calculated from 133 healthy females before and after 5 min of intense exercise. Lines indicate the mean values. P values compared the differences in the numbers between after and before exercise; D: This is similar to panel A, except that 140 healthy males were examined. Each dot represents one individual. B: Before; A: After; WBC: White blood cell.
Leukocytosis examination after 5 min intense exercise
WBCs numbers significantly increased in the blood circulation of females 5 min after intense exercise (P < 0.0001; Figure 3C). In this category, the numbers of granulocytes, lymphocytes, monocytes and eosinophils were also significantly increased after intense exercise (P < 0.0001 for all cell types). Similar results were observed with males blood where the numbers of total WBCs, segmented granulocytes, stab neutrophils, lymphocytes, monocytes and eosinophils were increased when compared to the numbers of these cells in the blood of individuals at resting state (P < 0.0001 for all cell types; Figure 3D). Fold increases in the numbers of WBCs after both bouts of exercise are shown in Figure 4.
Figure 4.
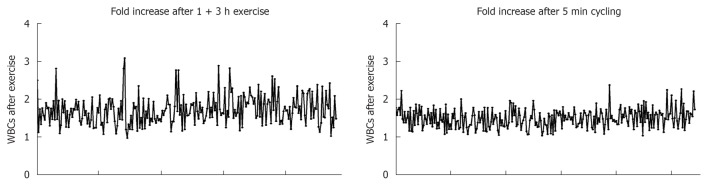
Fold increase in the numbers of white blood cells after exercise. Each dot represents one Individual. The increase in the numbers of leukocytes after exercise varies between 1-3 times. WBC: White blood cell.
It should also be mentioned that data based on fitness levels showed no significant differences among the various groups on leukocytosis either after running or after cycling (Table 2). The only significant difference observed was the lower number of WBCs circulating at resting situation between members of group 4 as compared to those in group 2 (mean 5.8 × 109/L for group 4 and 6.6 × 109/L for group 2) and also a larger change “after-before” numbers of leukocytosis for members in group 4 (Table 2). For those who cycled significantly higher changes in heart rate was observed after exercise for those in groups 3 and 4 as compared to those in group 2. No significant differences were found when APTT and D-dimer were analyzed based on the fitness levels (Table 2). Further analysis showed that there was a significant (P < 0.0001) relationship between the number of WBC’s and heart rate, i.e., increased heart rates are correlated with increased leukocytosis (Figure 5).
Table 2.
Comparison of the exercise intensity levels among the various groups (mean ± SD)
| Fitness level | Exercise form (n) | WBC increase (× 109/L) | Change in heart rate right after 5 min cycling | APTT (after-before)(s) | D-dimer (after-before) (mg/L) |
| 2 | Running (78) | 2.97 ± 0.24 | |||
| 2 | Cycling (130) | 2.44 ± 0.20 | 59.82 ± 2.35 | -2.11 ± 0.22 | 0.459 ± 0.22 |
| 3 | Running (118) | 4.93 ± 0.41 | |||
| 3 | Cycling (125) | 3.54 ± 0.17 | 78.67 ± 2.34 | -2.16 ± 0.19 | 0.631 ± 0.16 |
| 4 | Running (31) | 4.84 ± 0.27 | |||
| 4 | Cycling (25) | 3.80 ± 0.41 | 86.80 ± 5.30 | -2.05 ± 0.31 | 0.959 ± 0.67 |
Individuals were divided into groups according to physical activities ranging from 1 (no activity) to 4 (high intense activity). The numbers of increased WBCs after 1 h run and 3 h rest (Running group), and after 5 min intense cycling (Cycling group) are shown. Shown are changes in the heart rates among the various groups. Also shown are the differences in activated partial thromboplastin time (APTT) and D-dimer measurements. WBCs: White blood cells.
Figure 5.
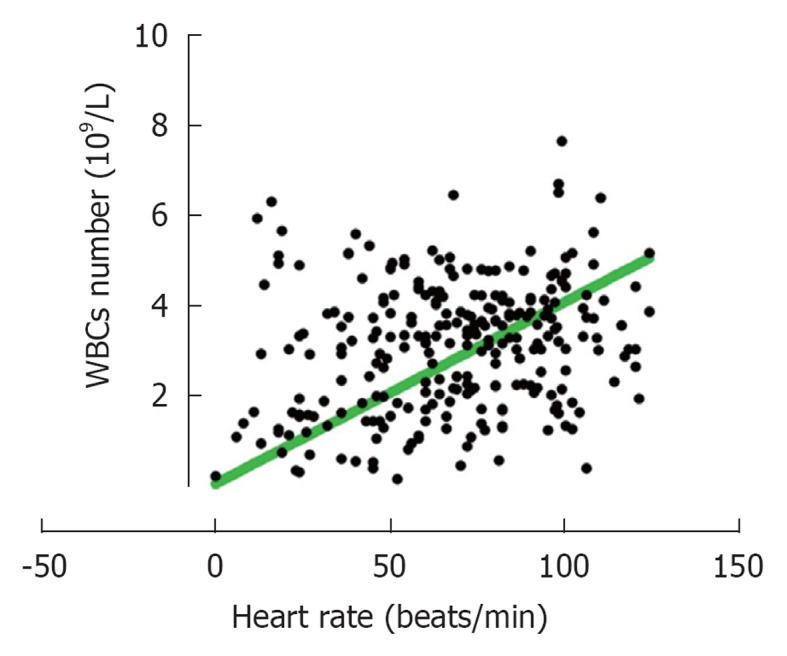
Correlations between the numbers of white blood cells and heart rate after intense exercise. The increase in the numbers of white blood cells accumulating in the blood after 5 min intense cycling is correlated with increased heart rate. WBCs: White blood cells.
Hemostasis shifts into a higher equilibrium after 5 min intense exercise
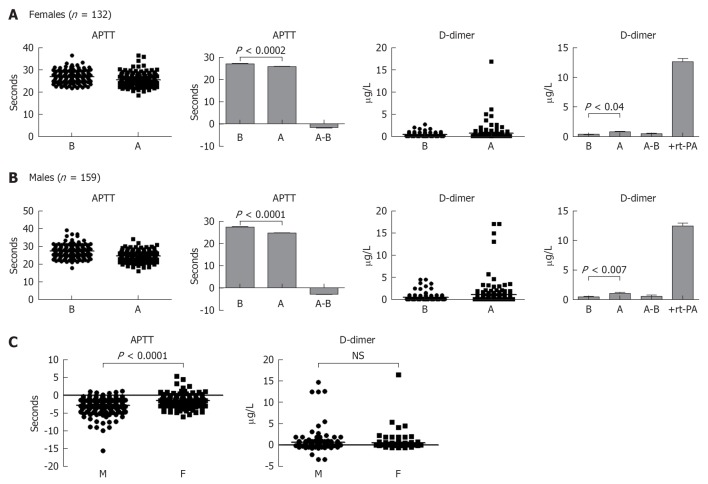
Next, we investigated the influence of short-term exercise on the hemodynamic of blood coagulation system. Both female and male healthy individuals cycled for 5 min at high intensity. Blood samples were withdrawn before and after exercise and APTT as well as D-dimer values were measured. Results shown in Figure 6A demonstrate that APTT was decreased in females, i.e., shortened time after this type of exercise. APTT is decreased after exercise and was significantly lower than before exercise (P < 0.0001). From the same blood, plasma levels of D-dimer showed increased values after exercise (P < 0.04). A high D-dimer level was also observed when the plasma from females was supplemented with thrombin and recombinant t-PA. Similar pattern was observed with the blood of healthy males, where APTT was significantly shortened (P < 0.0001), and D-dimer significantly increased (P < 0.007) after 5 min intense exercise (Figure 6B). Curiously, the decrease in APTT found in males was significantly higher than those found in females (Figure 6C), whereas no significant difference was observed when D-dimer was compared among the genders (Figure 6C).
Figure 6.
Evaluation of blood hemostasis. A: Activated partial thromboplastin time (APTT) and D-dimer were measured in 132 healthy females before and after 5 min intense exercise. P values show the difference between after and before exercise. In the last panel actilyse “recombinant tissue-PA = rt-PA” was added to the assay in the presence thrombin and plasma samples collected from these individual and represent positive control. Dot and column graphs are shown to indicate the differences between after and before exercise; B: This is similar to panel A except that plasma samples of 159 healthy males were examined; C: Comparison among males (M) and females (F) for differences in APTT or D-dimer values (before minus after exercise). B: Before; A: After; NS: Not significant.
DISCUSSION
The numbers of granulocytes, lymphocytes and monocytes have been reported to be increased after exercise[1-12], but the numbers of samples in these studies were too small to come up with a consensus. In addition, effects of exercise on gender differences are surprisingly unclear. Avloniti showed that there is a significant increase in total WBCs of elite female national-team soccer players as well as increased their heart rates after exercise[5]. Also, a great weight loss occurred after running in both males and females[21]. However, no differences in the post-exercise systolic blood pressure or heart rates were detected among healthy young males and females after exercise[22]. These results were supported by the study of Fernandez-Fernandez et al[23], showing no differences among females and males heart rates during badminton match play. Here, we examined a large number of medical students whom we taught the blood course in the department of Physiology at the University of Oslo medical school between January 2007 and June 2012. The students were divided into two groups (males and females) and were further sub-divided into a group where individuals run (or skied during winter time) for 1 h and then rested for three h, whereas those in the other group cycled at a maximum intensity for 5 min. The intensity of the exercise was monitored by measuring the heart pulse after exercise and compared it to before exercise. PCV showed no difference in the group that run for 1 h and then rested for 3 h, but an increase in PCV occurred after 5 min exercise. This could be due to the pressure exerted on RBCs during intense exercise due to blood sheer stress and outward filtration of plasma from vasculature into tissue space, among other factors.
In the 1 + 3 h group, significant increases in the numbers of segmented neutrophils, band neutrophils and eosinophils were observed after this bout of exercise. Lymphocyte numbers were also increased, although to a much lower extent than the other cells, whereas no increase in the numbers of monocytes was observed. These results could be due to the recruitment of cells from the bone marrow. It was previously observed that CXCL1/interleukin (IL)-8 is increased in the blood circulation of female soccer athletes performing exercise[6]. This chemokine is also increased in the blood of 16 male volunteers 5 h after exercise[3]. Increased CXCL8/IL-8 concentration could explain the higher numbers of neutrophils observed after this bout of exercise. However, the numbers of eosinophils could not be explained by increased CXCL8/IL-8 levels, since these cells do not express CXCR1 or CXCR2, receptors that bind CXCL8/IL-8. Instead, the results may be due to increased cortisol levels which peaks after 3 h of exercise and which maintains cells in the vascular compartments, as shown by others[10]. Alternatively, the levels of eotaxin/CCL11 may also be increased which could be responsible for the recruitment of eosinophils into the circulation since these cells express CCR3 that binds this chemokine[24-26]. One can also exclude the possibility that MCP-1/CCL4 is released at this stage, since it is the major chemokine recruiting monocytes which express CCR2 that binds this chemokine[24-26]. Lastly, no differences were demonstrated in the patterns of cell accumulation in the blood circulation after 1+3 h exercise between females and males.
However, different patterns of cell accumulation in the blood circulation were observed after 5 min intense exercise. Blood samples collected from individuals that exercised intensely showed increased numbers of all cell types including segmented and stab (band) neutrophils, lymphocytes, monocytes, and eosinophils. This increase could be due to potentiating the sheer blood flow that occurs during this sort of exercise as previously described[17]. It could also be due to the release of catecholamine which was reported to be increased in plasma after intensive short-term exercise[8,11]. We also noted a similar pattern of cell accumulation in the blood circulation of males and females performing similar activity, suggesting that there are no gender differences in relation to neutrocytosis, lymphocytosis or monocytosis after this type of exercise. A correlation between increased heart rate and enhanced leukocytosis was also observed.
Our work also combined leukocytosis with hemostasis. We observed that in both males and females a shift towards higher homeostasis occurred after 5 min intense exercise. Hence, both higher coagulation (shortened APTT) and D-dimer activation were observed. These results suggest that during exercise, the increase in coagulation is offset by enhanced fibrin degradation. To this end, we observed that increased coagulation was significantly higher in males than females, albeit no difference was observed in their D-dimer activity. This may suggest that males might be more prone to higher coagulation after intense short-term exercise than females. The impact of these findings on the exercise ability among genders has not yet been publicized but it might be important to distinguish the coagulation patterns among genders when one is planning exercise regiments. Collectively, our results support those of Gunga et al[18] where both coagulation and fibrinolytic cascades are increased in healthy individuals after short-term intense exercise.
D-dimer is increased in patients with deep venous thrombosis and pulmonary embolism[27]. In this regard, elevated plasma concentrations of von Willebrand factor, t-PA and PAI-1 could be predictors of myocardial infarction and stroke incidence in older or in cardiac patients[28]. However, fibrinolysis is increased during 2 h triathlon where it neutralizes the increase in thrombin generation[29], and increased D-dimer activity in healthy individuals is a marker of hydrolyzing the cross-linked fibrin by plasminogen which is generated from plasmin by t-PA.
The advantages of our study as compared to other published data are: (1) large numbers of samples were examined; (2) comparison was done among male and female healthy individuals performing two different kinds of exercise; and (3) both leukocytosis and hemostasis observations were included. Taken together, we observed that WBCs are accumulated in the blood circulation after 5 min intense exercise or after 1 h run plus 3 h rest and that the mechanism of accumulation is different among the two types of exercise. The results also suggest that in both types of exercise, changes in the hemodynamic of blood cells in the circulation took place, and the effects may not dampen the immune system but instead may potentiate it due to increased numbers of immune cells in the circulation. Hence, although exercise may resemble acute inflammation regarding recruitment of leukocytes, the effect of exercise could be beneficial since most cells are recruited into the blood circulation rather than into inflamed tissues such as what happens during infections. Regarding hemostasis, our results continue to support others demonstrating a shift of the hemostasis system into a higher equilibrium after exercise. It was previously suggested that changes in hemostasis during exercise may induced coronary ischemia and possibly sudden death[30]. However, based on the numbers of individuals examined in our study and their gender status, we support the consensus that short-term intense exercise or leisure exercise that may last up to 1 h is beneficial and should be continued particularly for healthy individuals.
COMMENTS
Background
Exercise is part of normal life. However, the effects of exercise in health and diseases are not clearly defined, basically due to the low numbers of individuals examined in most studies published in the literature.
Research frontiers
This is a large study that involves about 800 individuals. Consequently, the results can be regarded as standards for the effects of both intense and leisure exercises on the numbers of blood cells as well as blood coagulation and fibrinolytic components. In contrast to other reports which examined similar effects, this study included large sample size and showed comparison among healthy females and males. Further, this report combined leukocytosis and hemostasis in one study.
Applications
Although not all aspects of blood components were examined, the results may form a reference guide for any future study examining the effects of exercise on blood hemodynamic.
Terminology
Activated partial thromboplastin time and D-dimer are tests which measure blood hemostasis; one measures the time of blood coagulation and the other the degradation of fibrin. Hence, these tests are widely used to examine blood hemodynamic.
Peer review
The data from this paper strongly support the notion that exercise (independent of the intensity) affects leukocytosis and blood hemostasis in both genders. The present results are promising and very important to the research in this area of knowledge once this study includes a high number of individuals (both females and males). In general the paper is well written and the results are consistent, thus supporting the conclusions.
Footnotes
Supported by The Faculty of Medicine at the University of Oslo
P-Reviewers Fortunato M, Robson Luiz P S- Editor Jiang L L- Editor A E- Editor Zheng XM
References
- 1.Rowbottom DG, Green KJ. Acute exercise effects on the immune system. Med Sci Sports Exerc. 2000;32:S396–S405. doi: 10.1097/00005768-200007001-00004. [DOI] [PubMed] [Google Scholar]
- 2.McKenzie MA, Greenleaf JE, Looft-Wilson R, Barnes PR. Leucocytosis, thrombocytosis, and plasma osmolality during rest and exercise: an hypothesis. J Physiol Pharmacol. 1999;50:259–273. [PubMed] [Google Scholar]
- 3.Simonson SR, Jackson CG. Leukocytosis occurs in response to resistance exercise in men. J Strength Cond Res. 2004;18:266–271. doi: 10.1519/R-12572.1. [DOI] [PubMed] [Google Scholar]
- 4.Risøy BA, Raastad T, Hallén J, Lappegård KT, Baeverfjord K, Kravdal A, Siebke EM, Benestad HB. Delayed leukocytosis after hard strength and endurance exercise: aspects of regulatory mechanisms. BMC Physiol. 2003;3:14. doi: 10.1186/1472-6793-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avloniti AA, Douda HT, Tokmakidis SP, Kortsaris AH, Papadopoulou EG, Spanoudakis EG. Acute effects of soccer training on white blood cell count in elite female players. Int J Sports Physiol Perform. 2007;2:239–249. doi: 10.1123/ijspp.2.3.239. [DOI] [PubMed] [Google Scholar]
- 6.Timmons BW, Tarnopolsky MA, Snider DP, Bar-Or O. Immunological changes in response to exercise: influence of age, puberty, and gender. Med Sci Sports Exerc. 2006;38:293–304. doi: 10.1249/01.mss.0000183479.90501.a0. [DOI] [PubMed] [Google Scholar]
- 7.Gabriel H, Kindermann W. The acute immune response to exercise: what does it mean. Int J Sports Med. 1997;18 Suppl 1:S28–S45. doi: 10.1055/s-2007-972698. [DOI] [PubMed] [Google Scholar]
- 8.Gray AB, Telford RD, Collins M, Weidemann MJ. The response of leukocyte subsets and plasma hormones to interval exercise. Med Sci Sports Exerc. 1993;25:1252–1258. [PubMed] [Google Scholar]
- 9.McCarthy DA, Macdonald I, Grant M, Marbut M, Watling M, Nicholson S, Deeks JJ, Wade AJ, Perry JD. Studies on the immediate and delayed leucocytosis elicited by brief (30-min) strenuous exercise. Eur J Appl Physiol Occup Physiol. 1992;64:513–517. doi: 10.1007/BF00843760. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy DA, Dale MM. The leucocytosis of exercise. A review and model. Sports Med. 1988;6:333–363. doi: 10.2165/00007256-198806060-00002. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy DA, Perry JD, Melsom RD, Dale MM. Leucocytosis induced by exercise. Br Med J (Clin Res Ed) 1987;295:636. doi: 10.1136/bmj.295.6599.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen JB, Wilsgård L, Osterud B. Biphasic changes in leukocytes induced by strenuous exercise. Eur J Appl Physiol Occup Physiol. 1991;62:157–161. doi: 10.1007/BF00643735. [DOI] [PubMed] [Google Scholar]
- 13.Iatridis SG, Ferguson JH. Effect of physical exercise on blood clotting and fibrinolysis. J Appl Physiol. 1963;18:337–344. doi: 10.1152/jappl.1963.18.2.337. [DOI] [PubMed] [Google Scholar]
- 14.Ribeiro J, Almeida-Dias A, Ascensão A, Magalhães J, Oliveira AR, Carlson J, Mota J, Appell HJ, Duarte J. Hemostatic response to acute physical exercise in healthy adolescents. J Sci Med Sport. 2007;10:164–169. doi: 10.1016/j.jsams.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Prisco D, Paniccia R, Guarnaccia V, Olivo G, Taddei T, Boddi M, Gensini GF. Thrombin generation after physical exercise. Thromb Res. 1993;69:159–164. doi: 10.1016/0049-3848(93)90013-e. [DOI] [PubMed] [Google Scholar]
- 16.Weiss C, Welsch B, Albert M, Friedmann B, Strobel G, Jost J, Nawroth P, Bärtsch P. Coagulation and thrombomodulin in response to exercise of different type and duration. Med Sci Sports Exerc. 1998;30:1205–1210. doi: 10.1097/00005768-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Hilberg T, Prasa D, Stürzebecher J, Gläser D, Schneider K, Gabriel HH. Blood coagulation and fibrinolysis after extreme short-term exercise. Thromb Res. 2003;109:271–277. doi: 10.1016/s0049-3848(03)00283-4. [DOI] [PubMed] [Google Scholar]
- 18.Gunga HC, Kirsch K, Beneke R, Böning D, Hopfenmüller W, Leithäuser R, Hütler M, Röcker L. Markers of coagulation, fibrinolysis and angiogenesis after strenuous short-term exercise (Wingate-test) in male subjects of varying fitness levels. Int J Sports Med. 2002;23:495–499. doi: 10.1055/s-2002-35070. [DOI] [PubMed] [Google Scholar]
- 19.Röcker L, Möckel M, Westpfahl KP, Gunga HC. Influence of maximal ergometric exercise on endothelin concentrations in relation to molecular markers of the hemostatic system. Thromb Haemost. 1996;75:612–616. [PubMed] [Google Scholar]
- 20.Kahraman S, Bediz CS, Pişkin O, Aksu I, Topçu A, Yüksel F, Demirkan F. The effect of the acute submaximal exercise on thrombin activatable fibrinolysis inhibitor levels in young sedentary males. Clin Appl Thromb Hemost. 2011;17:414–420. doi: 10.1177/1076029610385672. [DOI] [PubMed] [Google Scholar]
- 21.Williams PT. Greater Weight Loss from Running than Walking during 6.2-yr Prospective Follow-up. Med Sci Sports Exerc. 2012:Epub ahead of print. doi: 10.1249/MSS.0b013e31827b0d0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maruf FA, Ogochukwu UN, Dim PA, Alada AR. Absence of sex differences in systolic blood pressure and heart rate responses to exercise in healthy young adults. Niger J Physiol Sci. 2012;27:95–100. [PubMed] [Google Scholar]
- 23.Fernandez-Fernandez J, Gonzalez de la Aleja Tellez J, Moya-Ramon M, Cabello-Manrique D, Mendez-Villanueva A. Gender differences in game responses during badminton match play. J Strength Cond Res. 2012:Epub ahead of print. doi: 10.1519/JSC.0b013e31827fcc6a. [DOI] [PubMed] [Google Scholar]
- 24.Luster AD. The role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol. 2002;14:129–135. doi: 10.1016/s0952-7915(01)00308-9. [DOI] [PubMed] [Google Scholar]
- 25.Maghazachi AA. G protein-coupled receptors in natural killer cells. J Leukoc Biol. 2003;74:16–24. doi: 10.1189/jlb.0103019. [DOI] [PubMed] [Google Scholar]
- 26.Raman D, Sobolik-Delmaire T, Richmond A. Chemokines in health and disease. Exp Cell Res. 2011;317:575–589. doi: 10.1016/j.yexcr.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rectenwald JE, Myers DD, Hawley AE, Longo C, Henke PK, Guire KE, Schmaier AH, Wakefield TW. D-dimer, P-selectin, and microparticles: novel markers to predict deep venous thrombosis. A pilot study. Thromb Haemost. 2005;94:1312–1317. doi: 10.1160/TH05-06-0426. [DOI] [PubMed] [Google Scholar]
- 28.Gallistl S, Sudi KM, Borkenstein M, Troebinger M, Weinhandl G, Muntean W. Determinants of haemostatic risk factors for coronary heart disease in obese children and adolescents. Int J Obes Relat Metab Disord. 2000;24:1459–1464. doi: 10.1038/sj.ijo.0801427. [DOI] [PubMed] [Google Scholar]
- 29.Bärtsch P, Welsch B, Albert M, Friedmann B, Levi M, Kruithof EK. Balanced activation of coagulation and fibrinolysis after a 2-h triathlon. Med Sci Sports Exerc. 1995;27:1465–1470. [PubMed] [Google Scholar]
- 30.Thompson PD, Funk EJ, Carleton RA, Sturner WQ. Incidence of death during jogging in Rhode Island from 1975 through 1980. JAMA. 1982;247:2535–2538. [PubMed] [Google Scholar]