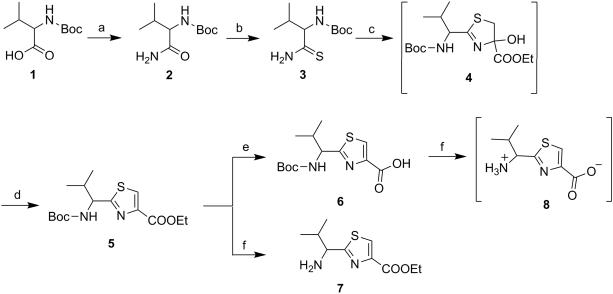
Scheme 1.
Reagents and conditions: (a) (i) Isobutyl chloroformate, N-Methyl morpholine, THF, −20°C, 4 h; (ii) 30% NH4OH in excess, −20°C to rt, 4 h; (b) Lawesson's reagent, THF, 50°C, 16 h; (c) Ethyl bromopyruvate, KHCO3, DME, −15°C to rt, 12 h; (d) (i) TFAA, 2,6-Lutidine, DME, −15°C to rt, 15 h; (ii) NaOEt, Ethanol, −15°C to rt, 6 h; (e) NaOH, THF:Methanol:H2O (10:2:3), rt, 4 h; (f) TFA, CH2Cl2, rt, 12 h