Abstract
Immune checkpoint inhibitors such as ipilimumab and targeted BRAF inhibitors have dramatically altered the landscape of melanoma therapeutics over the past few years. Agents targeting the programmed cell death-1/ligand (PD-1/PD-L1) axis are now being developed and appear to be highly active clinically with favorable toxicity profiles. We report two patients with BRAF V600E mutant melanoma who were treated with anti-PD-1 agents as first-line therapy without significant toxicity, followed by vemurafenib at disease progression. Both patients developed severe hypersensitivity drug eruptions with multi-organ injury early in their BRAF inhibitor treatment course. One patient subsequently developed acute inflammatory demyelinating polyneuropathy (AIDP) and the other developed anaphylaxis upon low-dose vemurafenib rechallenge. Further investigation of the immune response during combination or sequences of melanoma therapeutics is warranted. Furthermore, clinicians should maintain a high index of suspicion for these toxicities when vemurafenib is administered following an anti-PD-1 agent.
Keywords: Melanoma, vemurafenib, anti-PD-1, immunotherapy
Background
Metastatic melanoma is historically associated with limited treatment options and poor outcomes. In 2011, two agents were approved for the treatment of advanced melanoma. Vemurafenib, a selective BRAF inhibitor, improved overall survival compared to cytotoxic chemotherapy in patients with BRAF V600E mutant melanoma (1, 2). Ipilimumab, an immune modulator, also demonstrated an overall survival advantage with a minority of patients experiencing durable remissions (3). Additional immune-based therapies are being developed, notably agents targeting the PD-1/PD-L1 axis (Programmed Cell Death-1/Ligand), which also unleash suppressed tumor-specific immune responses by blocking a key immune regulatory checkpoint. In early trials, objective response rates ranged from 30-50%, many of which appear durable (4, 5). These newer agents are well-tolerated although immune-related adverse events including pneumonitis occur infrequently.
Approximately 50% of metastatic melanomas harbor BRAF V600E mutations (6, 7). First-line therapy options for these patients include BRAF inhibitors or immune-based therapies although the optimal sequence has not been defined. As these treatments are now more widely used, defining efficacy and toxicity profiles for various sequences and even combinations of immune-based and targeted therapies has become essential (8-10). We report two patients treated with anti-PD-1 agents on clinical trials, who at disease progression were rapidly switched to commercially available vemurafenib and subsequently developed severe systemic toxicities (including cutaneous, neurologic, and allergic) during vemurafenib therapy.
Case 1
A 62 year old woman was diagnosed with AJCC stage IIIB melanoma on the abdomen in March 2012 (4.65mm Breslow depth with ulceration; two axillary lymph nodes harbored micro-metastases). Molecular testing revealed a BRAF V600E mutation. In July 2012, she developed in-transit melanoma on her breast and was briefly treated with imiquimod and “debulking” surgery. Further disease progression ensued and in November 2012 she initiated anti-PD-1 (nivolumab, NCT00730639) treatment. Complications consisted of a self-limited pruritic rash and hypothyroidism. Subsequent to her final dose, she developed pulmonary and hepatic metastases and enlarging subcutaneous lesions. See Table 1 for timing of therapies.
Table 1.
| Patient 1 | Patient 2 | |||
|---|---|---|---|---|
| Anti-PD-1 therapy | Nivolumab 3mg/kg | Lambrolizumab 2mg/kg | ||
| Anti-PD-1 treatment dates | 11/5/12 – 12/17/12 | 10/12/12 – 12/14/12 | ||
| Vemurafenib (Vem) start date | 1/08/13 | 1/22/13 | ||
| Hospitalization dates | 1/21/13 – 1/29/13 | 2/3/13 – 2/7/13 | ||
| Laboratory values (reference) | Pre-vem | Admission | Pre-vem | Admission |
| Hemoglobin (11.8–16.0 g/dL) | 12.2 | 9.5 (after IV fluids) | 12.6 | 10.8 (after IV fluids) |
| Platelets (135,000–371,000/mm3) | 412 | 43 (after IV fluids) | 240 | 93 (after IV fluids) |
| Creatinine (0.7-1.5 g/dL) | 0.7 | 3.70 | 0.5 | 2.09 |
| Aspartate aminotransferase (4–40U/L) | 20 | 200 | 27 | 56 |
| Date of subsequent admission | 2/4/13 | 3/5/13 | ||
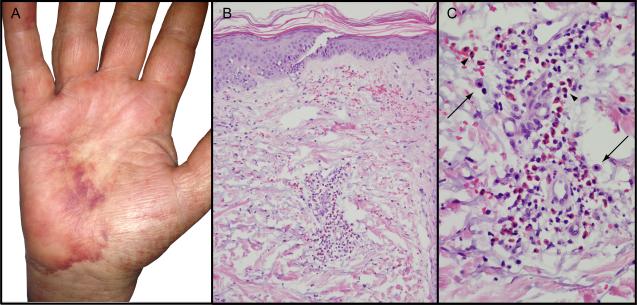
In January 2013 she initiated vemurafenib treatment. After seven days, she developed a tender erythematous macular eruption on her back that spread to her chest, extremities, and face; methylprednisolone (40mg/day) and diphenhydramine were prescribed. The rash worsened over the next week, predominantly on the palms, soles, and face; she developed fever to 101°F, tachycardia, and hypotension. Her trunk, cheeks, and extremities had warm, erythematous, blanching macules coalescing to patches without epidermal involvement. On her palms and feet were tender, violaceous, nonblanching patches with pedal and acral edema (Figure 1A). She had hemorrhagic crusting on the lips and mild conjunctival injection, but no mucosal involvement, skin fragility or bullae. Laboratory testing showed anemia, thrombocytopenia, and acute kidney and liver injury (Table 1); no eosinophilia or evidence of hemolysis was present. Skin biopsy demonstrated a dense superficial perivascular lymphocytic infiltrate with numerous eosinophils, occasional mast cells, and no evidence of epidermal necrosis, consistent with a dermal hypersensitivity reaction (Figure 1B and C). Due to somnolence and fever, cerebrospinal fluid (CSF) analysis was obtained and revealed elevated protein, normal glucose, and 39 nucleated cells (89% lymphocytes). CSF cytology, cultures, and viral and rickettsial serologies were negative. Prednisone 60 mg daily and broad spectrum antibiotics were administered. The patient was felt to have a severe hypersensitivity reaction with multiorgan involvement from vemurafenib. Her symptoms and laboratory abnormalities rapidly improved on prednisone and she was discharged with a prolonged steroid taper; vemurafenib was not restarted.
Figure 1.
Left hand biopsy sections show dense superficial perivascular lymphocytic infiltrate with numerous eosinophils (A, B), occasional mast cells (C, arrows) and no evidence of epidermal necrosis. (B H&E, 20 × orig. obj. mag., C H&E 100× orig. obj. mag.)
During the next week, the patient developed progressive and severe bilateral lower extremity weakness. On exam, she had decreased lower extremity strength (2-3/5 strength with hip and knee flexion bilaterally), absent patellar and ankle reflexes and decreased vibratory sensation; arm strength was preserved. MRI of the thoracic and lumbar spine did not demonstrate spinal cord compression or enhancement. CSF evaluation re-demonstrated elevated protein and lymphocytic pleocytosis. Electromyography showed partial conduction block consistent with acute focal nerve injury and possible demyelination in multiple nerves. She was presumed to have acute inflammatory demyelinating polyneuropathy (AIDP) due to vemurafenib. She completed a five day course of intravenous immunoglobulin (IVIG), and continued a prolonged prednisone taper. Her strength slowly improved although her melanoma continued to worsen with subsequent initiation of temozolomide.
Case 2
A 41 year old woman was diagnosed with AJCC stage IIIA melanoma in her left index finger in April 2011 (Breslow depth 3.1mm, non-ulcerated, one axillary lymph node involved). In December 2011 she developed in-transit lesions on her upper arm and was treated with imiquimod; molecular profiling revealed a BRAF V600E mutation. She then developed axillary lymphadenopathy and underwent resection which was followed by further regional progression. She subsequently began anti-PD-1 (MK-3475, NCT01295827) and tolerated four cycles of therapy without side effects but developed new splenic and hepatic metastases.
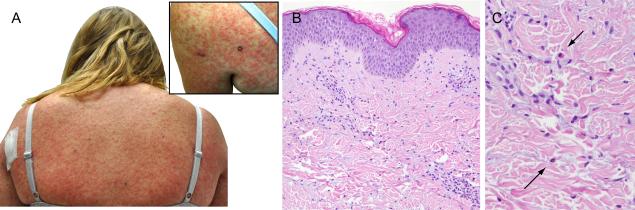
She initiated vemurafenib in January 2013. Nine days later, she developed a diffuse, pruritic eruption, composed of blanching erythematous macules and papules on the trunk and extremities and was started on prednisone 60 mg daily. Despite steroid administration, her rash became more confluent on the trunk with development of dark brown papules, plaques and few pustules (Figure 2A). She developed fever to 104.9°F, tachycardia, and hypotension; acute kidney injury, lactic acidosis, and mild transaminitis were noted (Table 1). Broad spectrum antibiotics and aggressive intravenous fluid resuscitation were administered; vemurafenib was discontinued. Skin biopsy of the upper back demonstrated superficial perivascular dermatitis with occasional eosinophils consistent with a dermal hypersensitivity reaction (Figure 2B and C). Her prednisone was increased to 80 mg daily. Blood and urine cultures remained negative; her symptoms and metabolic abnormalities improved dramatically over several days.
Figure 2.
Diffuse erythematous papules covering the back and upper extremities (A and inset) with histologic evidence of superficial perivascular dermatitis with occasional eosinophils (B H&E, 20 × orig. obj. mag., C H&E 100× orig. obj. mag.)
During the next month her rash completely resolved although her cutaneous melanoma lesions enlarged. She restarted a low dose of vemurafenib (240 mg) while continuing prednisone 20mg concurrently. Less than 6 hours following her first dose, she acutely developed a diffuse erythematous rash, severe shortness of breath, stridor, and vomiting. She was treated with epinephrine, corticosteroids, and anti-histamines with rapid symptom resolution. Vemurafenib therapy was abandoned and the patient is currently enrolled in a clinical trial evaluating a MEK inhibitor and PI3K/AKT inhibitor.
Discussion
Both of these patients experienced severe cutaneous and systemic toxicities while being treated with vemurafenib preceded by a course of anti-PD-1 antibodies. Their initial presentations were consistent with a severe drug hypersensitivity reaction with widespread morbilliform eruption, fever, hypotension, and multiorgan dysfunction. Initial differential diagnosis included DRESS (drug rash with eosinophilia and systemic symptoms) but diagnostic criteria, including peripheral eosinophilia, were not met (11). Severe neurologic and systemic toxicities subsequently ensued with presumed AIDP in one patient and anaphylaxis upon vemurafenib rechallenge in the other.
Vemurafenib has a well-defined toxicity profile of arthralgias, fatigue, and cutaneous manifestations, including hand-foot syndrome, photosensitivity, hyperkeratotic rash, and cutaneous squamous cell carcinomas (cuSCCs) (1, 12, 13). Additionally, up to 50% of patients treated with vemurafenib on clinical trials are described to develop rashes which are not classified more specifically, some of which may represent hypersensitivity reactions (1, 2). These cutaneous effects are usually treatable with antihistamines, topical corticosteroids, or excision of cuSCCs; dose modification or systemic steroids are required occasionally (14). Rare side effects reported include facial nerve palsy, hepatotoxicity, pancreatitis, and Stevens-Johnson syndrome, although life-threatening toxicities are uncommon (2, 15-17). To our knowledge, the toxicities observed in our patients have not been described with vemurafenib in the medical literature, leading to the concern that the preceding anti-PD-1 may have played a role.
There has been significant interest in combining BRAF inhibitors with immune-based therapies, tempered by a concern that combination or sequential therapy may augment toxicities. BRAF inhibitors likely mediate their anti-melanoma effects in part through immune-based mechanisms. In pre-clinical and clinical studies, vemurafenib caused marked tumor infiltration of CD8+ cytotoxic T lymphocytes as well as upregulation of the immune checkpoint PD-L1 (18, 19). Furthermore, the presence of functional host cytotoxic T cells appears to play an essential role in effecting responses to BRAF inhibitor therapy (20). This observed effector T cell activation may be explained by paradoxical activation of MAPK signaling in BRAF wild-type cells through enhanced CRAF dimerization (21).
In both of our patients, anti-PD-1 therapy had been well-tolerated prior to vemurafenib. However, these agents may have “primed” the immune system, predisposing these patients to severe BRAF inhibitor toxicities. This phenomenon appeared to occur in a small series of patients with unusually high rates of cutaneous toxicity and hypersensitivity reactions when treated with vemurafenib preceded by ipilimumab, although no systemic toxicities were reported (22). Additionally, the combination of ipilimumab and vemurafenib administered simultaneously led to an unacceptable frequency of severe hepatotoxicity (23). With generally more tumor-specific immune responses with anti-PD-1 therapy, it could be predicted that these agents would be better tolerated in combination therapy.
Earlier in their treatment courses, both patients received imiquimod for in-transit metastases. Imiquimod is a Toll-like receptor 7 agonist which induces a local inflammatory response through recruitment of cytotoxic T cells and cytokine production (24). Although imiquimod is immune-activating, more than 3 months in one case and 6 months in the other had elapsed since its cessation. Furthermore, generalized hypersensitivity reactions have not been described during therapy (25). The role of imiquimod is not clear in these cases. Notably, from our two institutions, this report represents two of only three patients treated with anti-PD-1 followed by vemurafenib. The additional patient received nivolumab without an objective response and then was treated with commercially available vemurafenib at an outside institution. Subsequent clinic notes describe that the patient developed a confluent generalized eruption but was able to continue therapy after dose interruption.
Anti-PD-1 agents are being evaluated in clinical trials currently. With frequent and durable responses observed and a favorable toxicity profile (rarely including immune-related pneumonitis), these novel agents have been attractive treatment options in clinical trials (4, 5). If patients with BRAF mutant melanoma have disease progression on anti-PD-1 therapy, their treating clinicians should maintain a high index of suspicion for dangerous toxicities if vemurafenib is selected as a subsequent treatment option. Furthermore, trials to explore the combination or sequence of anti-PD-1 with BRAF targeted treatment will need to be performed with great caution.
Acknowledgments
Research Support: Douglas Johnson: K12 CA 0906525
Footnotes
This study has not been presented or published elsewhere.
Conflicts of interest: Dr. Joseph has a paid consulting role for Merck. Dr. Sosman has a paid consulting role for Genentech and Bristol Myers Squibb.
References
- 1.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–14. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and Tumor Responses with Lambrolizumab (Anti-PD-1) in Melanoma. N Engl J Med. 2013 doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lovly CM, Dahlman KB, Fohn LE, Su Z, Dias-Santagata D, Hicks DJ, et al. Routine multiplex mutational profiling of melanomas enables enrollment in genotype-driven therapeutic trials. PLoS One. 2012;7:e35309. doi: 10.1371/journal.pone.0035309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 8.Jang S, Atkins MB. Which drug, and when, for patients with BRAF-mutant melanoma? Lancet Oncol. 2013;14:e60–9. doi: 10.1016/S1470-2045(12)70539-9. [DOI] [PubMed] [Google Scholar]
- 9.Ascierto PA, Simeone E, Giannarelli D, Grimaldi AM, Romano A, Mozzillo N. Sequencing of BRAF inhibitors and ipilimumab in patients with metastatic melanoma: a possible algorithm for clinical use. J Transl Med. 2012;10:107. doi: 10.1186/1479-5876-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N Engl J Med. 2013 doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camous X, Calbo S, Picard D, Musette P. Drug Reaction with Eosinophilia and Systemic Symptoms: an update on pathogenesis. Curr Opin Immunol. 2012;24:730–5. doi: 10.1016/j.coi.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Su F, Viros A, Milagre C, Trunzer K, Bollag G, Spleiss O, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207–15. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boussemart L, Routier E, Mateus C, Opletalova K, Sebille G, Kamsu-Kom N, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013 doi: 10.1093/annonc/mdt015. [DOI] [PubMed] [Google Scholar]
- 14.Anforth R, Fernandez-Penas P, Long GV. Cutaneous toxicities of RAF inhibitors. Lancet Oncol. 2013;14:e11–8. doi: 10.1016/S1470-2045(12)70413-8. [DOI] [PubMed] [Google Scholar]
- 15.Klein O, Ribas A, Chmielowski B, Walker G, Clements A, Long GV, et al. Facial Palsy As a Side Effect of Vemurafenib Treatment in Patients With Metastatic Melanoma. J Clin Oncol. 2013 doi: 10.1200/JCO.2012.45.7028. [DOI] [PubMed] [Google Scholar]
- 16.Muluneh B, Buie LW, Collichio F. Vemurafenib-Associated Pancreatitis: Case Report. Pharmacotherapy. 2013 doi: 10.1002/phar.1208. [DOI] [PubMed] [Google Scholar]
- 17.Minor DR, Rodvien R, Kashani-Sabet M. Successful desensitization in a case of Stevens- Johnson syndrome due to vemurafenib. Melanoma Res. 2012;22:410–1. doi: 10.1097/CMR.0b013e3283573437. [DOI] [PubMed] [Google Scholar]
- 18.Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res. 2012;18:1386–94. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- 19.Tompers Frederick D, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knight DA, Ngiow SF, Li M, Parmenter T, Mok S, Cass A, et al. Host immunity contributes to the anti-melanoma activity of BRAF inhibitors. J Clin Invest. 2013;123:1371–81. doi: 10.1172/JCI66236. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–30. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harding JJ, Pulitzer M, Chapman PB. Vemurafenib sensitivity skin reaction after ipilimumab. N Engl J Med. 2012;366:866–8. doi: 10.1056/NEJMc1114329. [DOI] [PubMed] [Google Scholar]
- 23.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368:1365–6. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]
- 24.Wolf IH, Kodama K, Cerroni L, Kerl H. Nature of inflammatory infiltrate in superficial cutaneous malignancies during topical imiquimod treatment. Am J Dermatopathol. 2007;29:237–41. doi: 10.1097/01.dad.0000211531.33670.94. [DOI] [PubMed] [Google Scholar]
- 25.Narayan R, Nguyen H, Bentow JJ, Moy L, Lee DK, Greger S, et al. Immunomodulation by imiquimod in patients with high-risk primary melanoma. J Invest Dermatol. 2012;132:163–9. doi: 10.1038/jid.2011.247. [DOI] [PMC free article] [PubMed] [Google Scholar]