Abstract
Infection with a variety of bacterial pathogens results in hematopoietic stem and progenitor cell (HSPC) mobilization. The mechanism and kinetics of HSPC mobilization during infection are largely unknown. Previously, we found altered HSPC activity in bone marrow (BM), spleen and blood during infection with Anaplasma phagocytophilum, the agent of granulocytic anaplasmosis. We hypothesized that altered CXCL12/CXCR4 signaling, a central pathway for HSPC homing to and retention within the BM, plays a role in infection-induced alterations in HSPC number and trafficking. Mice were infected with A. phagocytophilum. Lineage- cKit+ HSPCs were enumerated and proliferation determined. CXCL12 and CXCR4 mRNA were quantified along with CXCL12 protein. CXCR4 surface, intracellular and total protein expression in HSPCs was determined. Increased BM proliferation of HSPCs began at 2 days post-infection followed by HSPC mobilization and splenic homing. Proliferation of resident HSPCs contributed to increased splenic HSPC numbers. BM CXCL12 mRNA and protein levels were decreased at 4-8 days post-infection concurrent with HSPC mobilization. CXCR4 protein parameters were decreased in BM HSPCs throughout 2-6 days post-infection. Reduction of CXCL12/CXCR4 signaling occurs simultaneously with HSPC mobilization from BM. Findings suggest that deranged CXCL12/CXCR4 signaling plays a causal role in HSPC mobilization during acute A. phagocytophilum infection.
Keywords: Anaplasma, CXCL12, hematopoiesis, progenitor, rickettsia
Introduction
The CXCL12/CXCR4 signaling axis controls the homing and mobilization of hematopoietic stem and progenitor cells (HSPCs) and mature leukocytes.1-3 Previous work on this signaling pathway has focused primarily on its role in transplantation and stem cell biology, as HSPC mobilization can be initiated clinically using a selective antagonist to this ligand/receptor interaction.4,5 More recently, a role has emerged for CXCL12 signaling in neutrophil trafficking to and from bone marrow (BM), both in homeostasis and during inflammation.6-8 Chemokine signaling in leukocyte trafficking regulates the availability of immune effector cells at sites of inflammation and infection.
Alterations in CXCL12 signaling during bacterial infection may also be critical for controlling the trafficking of HSPCs and influencing infection outcome. Regulation of hematopoiesis via altered HSPC kinetics can increase production of multiple hemic cell lineages to replace and/or augment cell populations consumed in responding to infection. Any serious derangement of hematopoiesis during bacterial infection may reduce availability of immune effector cells and influence disease pathology. To date, there is no mechanistic, kinetic evaluation of how a bacterial infection regulates CXCL12/CXCR4 signaling in vivo.
A. phagocytophilum is a gram-negative, LPS-negative, obligate intracellular bacterium that resides primarily within circulating granulocytes.9,10 A. phagocytophilum is the etiologic agent of granulocytic anaplasmosis, the second most common human tick-borne disease in the United States.11 Infection with A. phagocytophilum typically results in multiple cytopenias (thrombocytopenia, leukopenia and anemia) in natural disease and in animal models of infection.12-17 Cytopenias and BM dysfunction contribute to serious clinical sequelae of infection including fatalities due to immunosuppression and secondary infections.12,18 Pathogen effects on host granulocytes have been well-studied.19,20 Experimentally, A. phagocytophilum infection results in altered neutrophil trafficking, increased neutrophil surface integrin expression and decreased neutrophil apoptosis with resultant effects on pathogen clearance.16,21 The impact of infection on other hemic cell lineages and hematopoiesis are poorly understood. A. phagocytophilum infection provides a useful model for investigating hematopoietic alterations arising in infections with diverse non-LPS and obligate intracellular pathogens.
BM CXCL12 mRNA expression is downregulated during acute A. phagocytophilum infection.22 This downregulation occurs in concert with altered HSPC colony-forming activity in the BM, spleen and peripheral blood along with shifts in lineage-committed cells. HSPCs express CXCR4, the primary CXCL12 receptor. HSPCs respond to directional cues created by a CXCL12 concentration gradient by mobilizing when BM CXCL12 levels decrease and homing toward an increasing chemokine gradient. 23 Downregulated CXCL12 signaling therefore may actuate some of the hemic cell alterations in A. phagocytophilum infection.
The objectives of this study were to quantify HSPC proliferation, mobilization and trafficking to spleen and blood during acute A. phagocytophilum infection and to fully characterize the regulation of CXCL12/CXCR4 signaling during infection. We hypothesized that acute infection would result in HSPC proliferation, disruption of BM CXCL12/CXCR4 interactions and mobilization of HSPCs from BM to the periphery. We found that A. phagocytophilum infection resulted in rapidly increased BM HSPC proliferative activity followed by mobilization of HSPCs into peripheral blood. Proliferative activity also increased in splenic HSPCs which contributed slightly to total mobilized HSPCs in blood. Our data implicate kinetic regulation of CXCL12/CXCR4 signaling as the mechanism of HSPC mobilization. Simultaneous downregulation of BM CXCL12 mRNA transcription and protein production occurred along with reduction in both surface and intracellular BM CXCR4 expression. Infection with this non-LPS pathogen altered CXCL12/CXCR4 signaling and increased HSPC proliferation and mobilization during the innate immune response to infection.
Methods
Mice and in vivo experimental infection
Mouse experiments were performed exactly as previously described.21 Female, 5- to 7-week-old, C3H/HeN (C3H) and C3H/HeN SCID (C3H-SCID) mice were purchased from Harlan Sprague-Dawley (Indianapolis, IN) and Taconic (Hudson, NY). For some experiments, C3H mice were splenectomized by Taconic according to their protocol and specifications. Experimental C3H mice were inoculated via i.p. injection with 200 μl of pooled whole blood from infected C3H-SCID mice.24 A. phagocytophilum strain NCH-1 was utilized for all infections. In some experiments, mice were injected i.p. two hours prior to euthanasia with 100 μL of 10 mg/mL BrdU solution (APC BrdU Flow Kit, BD Biosciences, San Jose, CA). Mice were maintained according to approved institutional animal use and care protocols. Mice were euthanized with CO2 on days 0 (control), 2, 4, 6 and 8 post infection.
Tissue collection and processing
Blood, spleen and BM were collected at necropsy. Blood was collected into EDTA by cardiocentesis. Whole blood was used for quantitative PCR (qPCR) to detect A. phagocytophilum p44 DNA, complete blood cell counts (CBCs), differential leukocyte counts and flow cytometry.16 CBCs were performed on an automated analyzer (Beckman/Coulter AcT Hematology Analyzer, Holbrook, NY) within 4 hours of blood collection. For flow cytometry, RBCs were lysed using ammonium chloride (Stem Cell Technologies, Vancouver, BC, Canada) and samples washed twice in PBS prior to labeling. Remaining whole blood was centrifuged at 1500xg for 5 minutes and plasma was banked for ELISA analysis. The spleen was removed and weighed. A section of spleen was placed in 10% formalin for immunohistochemistry. A single cell suspension was made from the remaining spleen for flow cytometic analysis, RT-PCR, cell sorting and/or culture for determination of secreted CXCL12. In brief, the spleen was gently crushed through a 70 μm nylon sterile strainer (BD Biosciences, San Jose, CA) and resuspended in Iscove’s Modified Dulbecco’s Media (IMDM; Invitrogen, Carlsbad, CA) + 2% fetal bovine serum (FBS). Splenic cells were also banked at −80°C for RT-PCR evaluation. The sternum was removed and placed in 10% formalin for immunohistochemistry. BM was sterilely harvested from femurs and tibias. In brief, intact bones with musculature removed were disinfected in 70% ethanol for 2 minutes and swashed with sterile PBS. BM was flushed from tibias and femurs with IMDM + 2% FBS + 5 mM EDTA and a single cell suspension was made. One aliquot of BM was counted (Beckman/Coulter AcT Hematology Analyzer) and the remaining BM was processed for flow cytometric analysis, cell sorting and/or culture for secreted CXCL12 measurement. BM cells were also banked at −80°C for RT-PCR evaluation.
DNA extraction and Quantitative PCR
To verify infection, DNA was extracted from 25 μl of whole blood from each mouse using the DNeasy Tissue Kit (QIAGEN, Valencia, CA) according to manufacturer’s instructions and exactly as previously described.21,25 DNA amplification, data acquisition, and data analysis were performed in an 7300 Real-Time system (Applied Biosystems, Foster City, CA) exactly as previously described.16,26
RNA extraction and Reverse Transcription PCR (RT-PCR)
Total RNA was isolated using the RNeasy mini kit (Qiagen), reverse transcribed to cDNA using the Quantitect Reverse Transcription kit (Qiagen) and amplified using Taqman Universal PCR Mastermix (Applied Biosystems). Inventoried primer/probe sets (Taqman Gene Expression Assays, Applied Biosystems) were used for mouse CXCL12, CXCR4 and GAPDH (housekeeping gene). Reactions were performed in duplicate or triplicate using 4 to 12 ng of template cDNA. Quantitative PCR was performed using a 7300 Real-Time System (Applied Biosystems). Ct values were analyzed using the 2(−ddCt) method.27
Flow cytometry and cell sorting
Single cell suspensions of BM, spleen and whole blood nucleated cells were brought to 1 × 106 cells/mL in staining buffer (PBS + 3% FBS). Cells were labeled with antibodies against CD117/c-kit (clone 2B8), CD45 (clone 30-F11), and a cocktail of lineage markers comprised of antibodies against Gr-1/Ly6C/G (clone RB6-8C5), TER119/Ly7/6 (clone TER119), CD3 (clone 17A2), and B220 (clone RA3-6B2). All antibodies were obtained from BD Pharmingen (San Jose, CA) except B220 (R&D Systems, Minneapolis, MN). FITC− and PE-conjugated isotype antibodies were used to prepare negative controls. Cells were incubated with antibody at room temperature for 20 minutes, washed and resuspended in staining buffer. For BrdU analysis, cells were labeled as above with the addition of anti-BrdU antibody (APC BrdU Flow Kit, BD Biosciences). Cells were then fixed and permeabilized according to kit directions prior to resuspension in staining buffer. Negative controls were generated using cells from mice not administered BrdU, utilizing the same labeling protocol as above. For CXCR4 analysis, samples were labeled as above with the addition of an antibody against CXCR4 (clone 247506, R&D Systems). Some cell aliquots were analyzed to detect surface expression of CXCR4; other aliquots were re-labeled with CXCR4 antibody after fixation and permeabilization to detect intracellular/total CXCR4 expression. Samples were analyzed the same day on a flow cytometer (Becton Dickinson FACScan, Franklin Lakes, NJ); 30,000 events were acquired per sample. Flow cytometry data was analyzed using FlowJo flow cytometry software (Treestar Inc, Ashland, OR). For cell sorting, single-cell suspensions harvested from BM or spleen were labeled according to the above protocol. Cells were sorted based on negative staining for lineage markers (TER119, CD3, B220 and Gr-1 above) and positive staining for c-kit using a Mo Flo cell sorter (Beckman Coulter).
CXCL12 immunohistochemistry
Formalin fixed tissues were embedded in paraffin then 4 μm sections were cut and mounted on glass slides. Slides were deparaffinized with xylene and rehydrated by serial incubations with decreasing concentrations of ethanol. For antigen retrieval, slides were placed in TBS + 0.05% Tween 20 at pH 9.0 and heated in a steamer. The sections were first blocked with PeroxAbolish (Biocare Medical, Concord, CA) followed by Background Sniper (Biocare Medical) then incubated with the primary antibody (mouse anti-human/mouse CXCL12 MAb (clone 79018, R&D Systems) for 45 minutes. MACH2 mouse HRP Polymer (Biocare Medical) was applied (30 minute incubation) followed by Betazoid DAB Chromagen (8 minute incubation, Biocare Medical) with a final counterstain with hematoxylin. For BM evaluation, all positive cells were counted in 3 entire sternebrae in sagittal section. For splenic evaluation, positive cells were enumerated in 5- 50X fields of white pulp and 5- 50X fields of red pulp; results were evaluated both separately (white pulp vs. red pulp) and together.
BM and spleen CXCL12 production
BM and spleen cells from infected and control mice were resuspended in Mesencult mouse basal medium (Stem Cell Technologies) + 10% FBS and plated in a 96-well plate at 4 × 106 cells/well. Cells were incubated at 37°C in 5% CO2 for 48 hours prior to harvesting of supernatants. Supernatants were concentrated using Amicon Ultra 3 kD centrifugal filter units (Millipore, Billerica, MA) per manufacturer instructions prior to measurement of CXCL12 protein.
CXCL12 ELISA
Banked plasma and supernatants from cultured BM and splenic cells were analyzed using CXCL12 Quantikine ELISA kit (R&D Systems) according to kit instructions.
Statistical analyses
Statistics were performed with a Student’s t-test (Microsoft Office Excel 2003, Microsoft Corporation, Bellevue, WA). All tests were performed comparing data from infected mice at each timepoint with data from control mice (time 0). Unless otherwise stated, data are included from 3 experiments, with 2 control and 4 infected mice per timepoint per experiment. A p value of < 0.05 was considered significant.
Results
HSPCs are increased in BM, spleen and peripheral blood during acute infection
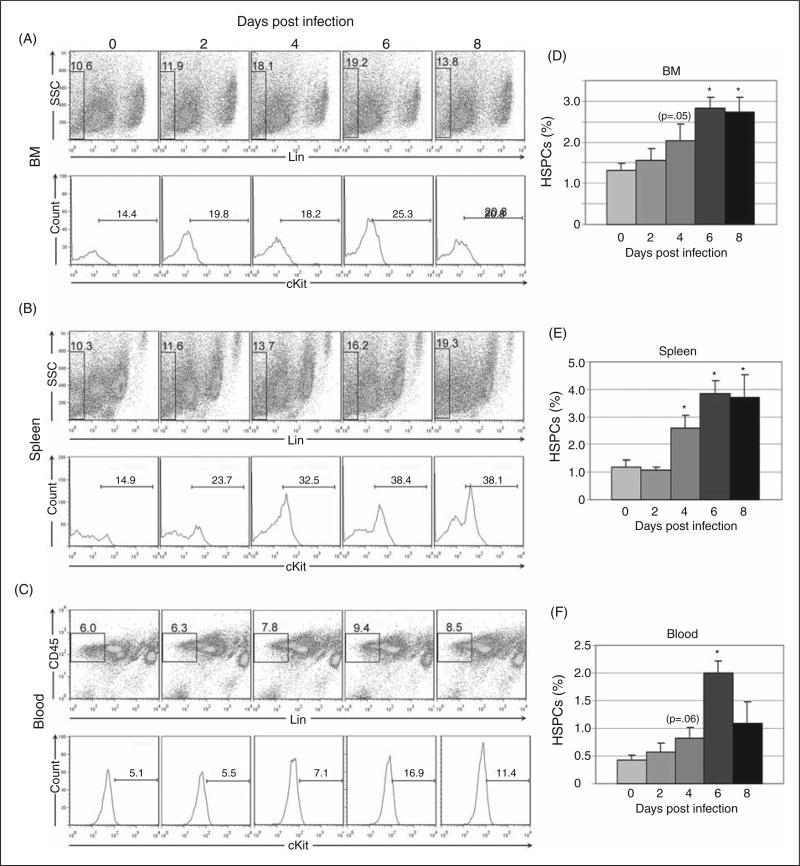
To define HSPC kinetics during acute infection, populations of lineage (lin)-negative, cKit-positive cells were identified using flow cytometry in BM, spleen and peripheral blood (Figure 1). Lineage negative cells were gated out using a cocktail of lineage markers; CD45 positivity was additionally used for gating in peripheral blood. Lin-negative cells were then evaluated for c-Kit positivity. Acute A. phagocytophilum infection resulted in increased numbers of HSPCs in BM, spleen and blood. The percentage of BM HSPCs increased by day 2 post-infection with significant increases at days 6 and 8 post-infection (Fig. 1a and 1d). The percentage of splenic HSPCs was significantly increased at days 4-8 post-infection (Fig. 1b and 1e) and the percentage of blood HSPCs increased at day 4 post-infection with a significant increase on day 6 post-infection (Fig. 1c and 1f).
Figure 1.
Flow cytometric quantification of hematopoietic stem and progenitor cells (HSPCs) in bone marrow (BM), spleen and peripheral blood during acute infection. Lineage-negative (and CD45-positive for peripheral blood) cells were evaluated for cKit positivity; lineage-negative and cKit-positive populations are considered equivalent to HSPCs. Representative plots are shown for days 0, 2, 4, 6 and 8 post infection (A-C). Mean values for HSPC percentage from three experiments were compared (D-F); statistically significant differences were found in BM, spleen and blood at multiple timepoints (asterisks = p<.05; bars = standard error).
Increased numbers of HSPCs in BM and spleen are due to HSPC proliferation during acute infection
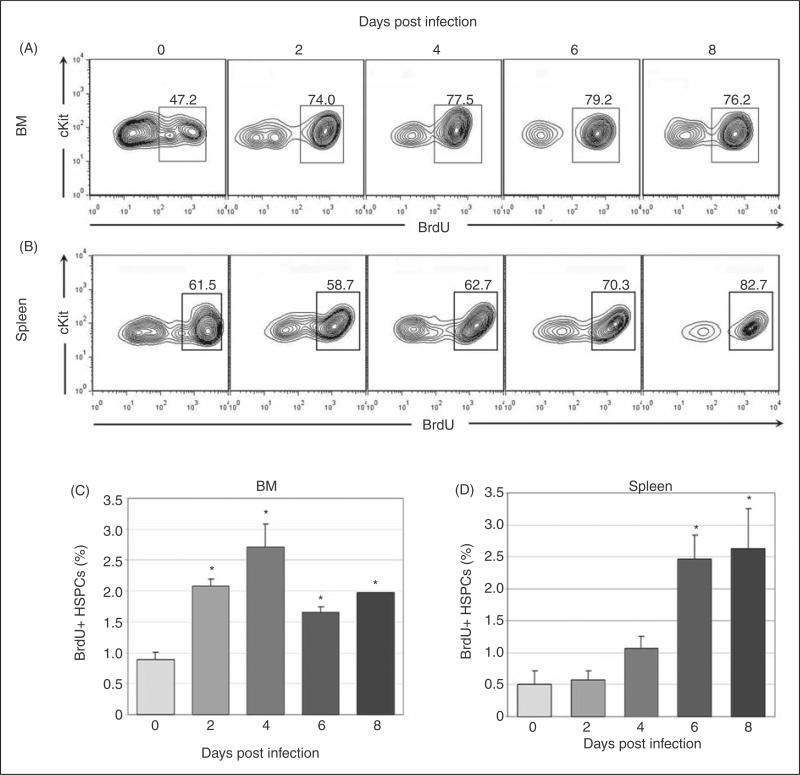
To verify that HSPC numbers increased due to proliferation, synthetic activity was evaluated with BrdU in gated HSPC populations. Significantly increased synthetic activity in BM HSPCs was found at days 2-8 post-infection (Fig. 2a and 2c) and in splenic HSPCs at days 6-8 post-infection (Fig. 2b and 2d). BM HSPC proliferation preceded the increase in BM HSPC number suggesting kinetic alignment of these processes. However, splenic HSPC proliferation began later than BM HSPC proliferation and increased splenic HSPC number preceded splenic HSPC proliferation. These data suggest that the initial increase in splenic HSPC number may have been due to an initial wave of HSPC mobilization from the BM and subsequent homing to the spleen, rather than independent splenic HSPC proliferation.
Figure 2.
Synthetic activity in BM and spleen HSPCs during acute infection. Cells were first gated as in figure 1 for lineage-negative, cKit-positive HSPC populations. BrdU positivity was then evaluated via flow cytometry. Representative plots are shown for days 0, 2, 4, 6 and 8 post infection (A-B). Mean values for BrdU positivity in BM (C) and spleen (D) HSPCs are charted; statistically significant differences were found at all infection timepoints in BM HSPCs and at days 6-8 post infection in splenic HSPCs (asterisks = p<.05; bars = standard error).
The spleen contributes to total mobilized blood HSPCs
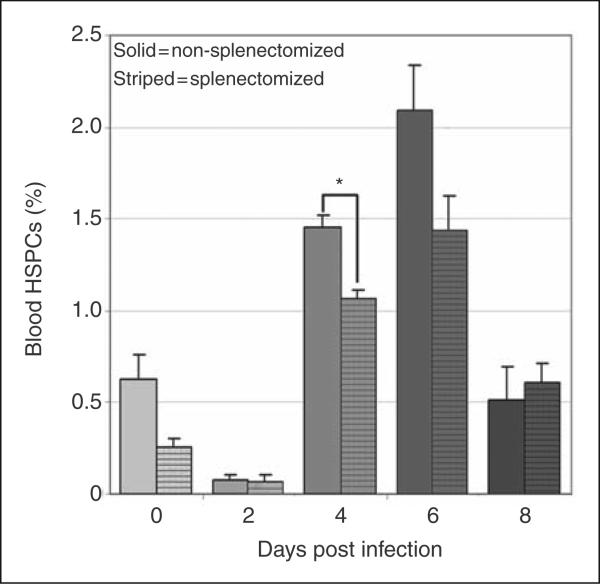
Peripheral blood HSPC numbers increased significantly at day 6 post-infection (Fig. 1e). Mobilized BM HSPCs are the likely source of these cells, but the spleen may also contribute to the peripheral blood pool especially during A. phagocytophilum infection, as infection results in extramedullary hematopoiesis and reactive hyperplasia.22,28 To determine the relative contribution of splenic HSPCs to the blood pool, blood HSPC numbers were compared in splenectomized and non-splenectomized mice. Splenectomized mice had significantly fewer blood HSPCs on day 4 post-infection compared to infected mice with a spleen (Fig. 3). Nonetheless, infected splenectomized mice still had a significant increase in blood HSPCs at days 4-6 post-infection, similar to the results in non-splenectomized mice shown in Figure 1. These results suggest that the spleen contributes to mobilized blood HSPCs at day 4 post-infection, but BM HSPCs are sufficient, even in the absence of the spleen, to mobilize significant numbers of HSPCs during infection.
Figure 3.
Contribution of mobilized splenic HSPCs to circulating HSPCs. Cells were gated as in figure 1 for lineage-negative, cKit-positive HSPC populations. Compared to non-splenectomized mice (solid bars), splenectomized mice (striped bars) had significantly lower mean values for circulating HSPCs at day 4 post infection (asterisk = p<.05, bars = standard error).
BM and plasma CXCL12 are decreased during acute infection with variable increases in splenic CXCL12
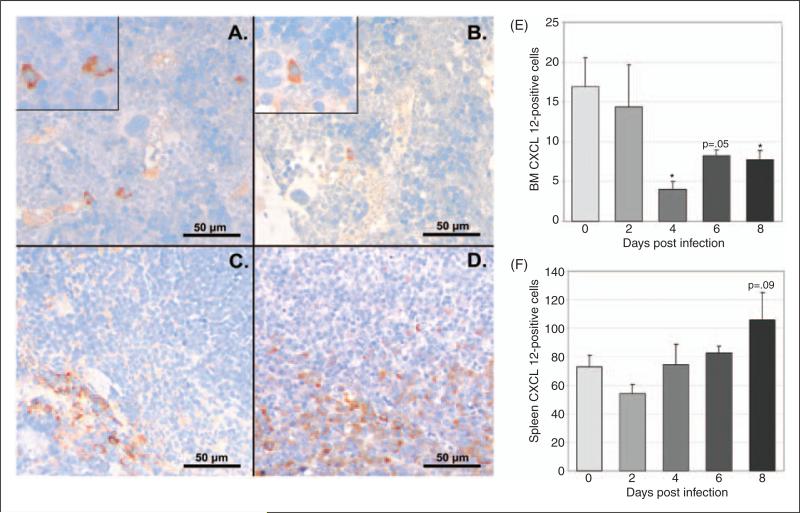
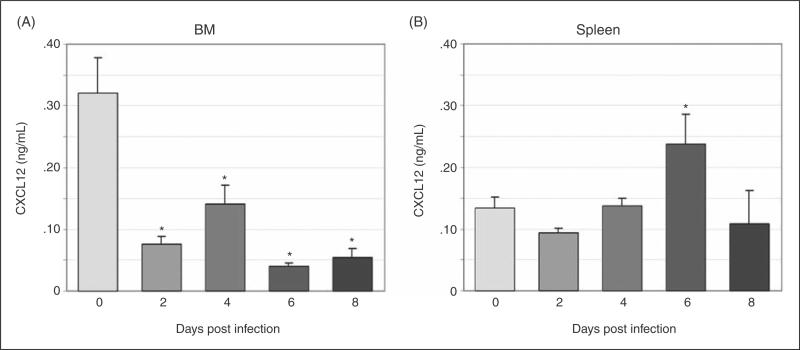
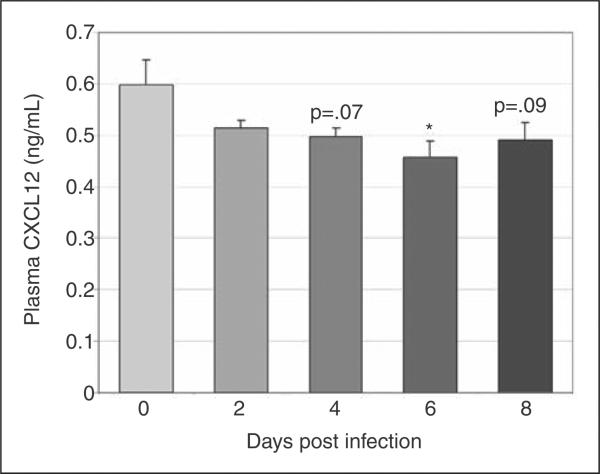
To define changes in CXCL12 protein during infection, we evaluated CXCL12 expression via immunohistochemistry in BM and spleen and secreted CXCL12 via ELISA of plasma and cultured BM and splenic cell supernatant. CXCL12-positive cells were significantly decreased in BM at days 4 and 8 post-infection, with a trend towards a decrease at day 6 post-infection (Fig. 4a and 4b, images at 40X; Fig. 4e). The percentage of splenic CXCL12-positive cells did not change with infection; however, a trend towards increased splenic CXCL12-positive cells was noted on day 8 post-infection (Fig. 4c and 4d, images at 40X; Fig. 4f). Similar to the immunohistochemical findings, the concentration of secreted BM CXCL12 was decreased on day 6 with significant decreases noted on days 4 and 8 post-infection and the concentration of secreted splenic CXCL12 was significantly increased on day 6 post-infection (data not shown). Plasma CXCL12 followed a similar pattern with a significant decrease in plasma CXCL12 concentration at day 6 post-infection and a trend towards decreased plasma CXCL12 noted at days 4 and 8 post-infection (Fig. 5).
Figure 4.
Immunohistochemical evaluation of CXCL12 protein in BM and spleen during acute infection. Tissues from a total of 8 control mice and 4 infected mice per timepoint were evaluated. Representative images of tissue sections from control and infected (day 8) mice are shown (A-D). Mean numbers of BM CXCL12-positive cells (E) were significantly decreased at days 4 and 8 post infection, with a trend toward decrease at day 6. No significant changes in mean numbers of CXCL12-positive cells were found in splenic sections, though a trend toward increase was seen at day 8 post infection (F) (asterisk = p<.05, bars = standard error).
Figure 5.
BM and splenic CXCL12 secretion. Unfractionated BM and splenic cells from a total of 12 control mice and 8 infected mice per timepoint were incubated in media for 48 hours and secreted CXCL12 was measured via ELISA. BM CXCL12 production (A) was significantly decreased at days 2-8 post-infection. Splenic CXCL12 production (B) was significantly increased at day 6 post-infection (asterisk = p<.05, bars = standard error).
CXCL12 mRNA was quantified in BM and spleen using RT-PCR. In BM, CXCL12 mRNA levels were significantly decreased at days 6-8 post-infection (data not shown), consistent with our previous results.22 In spleen, CXCL12 mRNA levels were variable and non-significant.
CXCR4 is decreased in BM HSPCs and variably increased in splenic HSPCs during acute infection
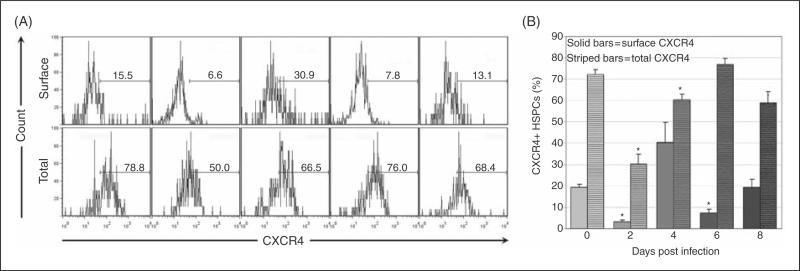
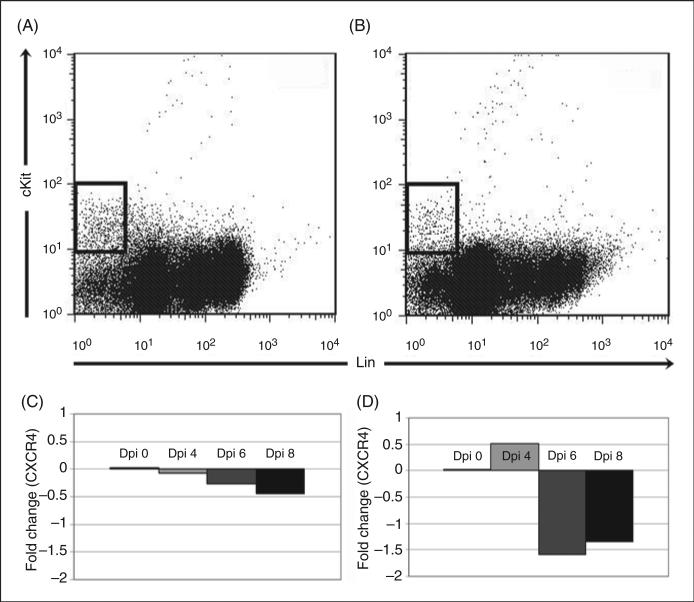
Surface and total CXCR4 expression was quantified on BM HSPCs throughout acute infection (Fig. 6a). Mean surface CXCR4 expression on BM HSPCs was significantly decreased on days 2 and 6 post-infection (Fig. 6b), while mean total BM HSPC CXCR4 expression (representing both surface and intracellular protein) was significantly decreased at days 2 and 4 post-infection (Fig. 6b). Surface CXCR4 expression on splenic HSPCs was significantly increased at day 6 post-infection and mean total CXCR4 expression was significantly increased at day 8 post-infection (data not shown). CXCR4 mRNA was quantified from sorted BM (Fig. 7a) and spleen (Fig. 7b) HSPCs on days 4, 6 and 8 post-infection. CXCR4 mRNA did not significantly change in HSPCs from BM (Fig. 7c) or spleen (Fig. 7d), indicating that CXCR4 regulation in HSPCs primarily occurs at a post-transcriptional level. Reciprocal changes in BM and splenic HSPC receptor/ligand regulation are compatible with a model of infection-induced changes in CXCL12-CXCR4 signaling resulting in BM HSPC mobilization and homing to the spleen.
Figure 6.
Plasma CXCL12 levels during acute infection. CXCL12 was evaluated in plasma via ELISA. Mean values for CXCL12 from three experiments were compared; a significant decrease was found at day 6 post infection with trends toward decrease at days 4 and 8 post infection (asterisk = p<.05, bars = standard error).
Figure 7.
Flow cytometric evaluation of CXCR4 expression on BM HSPCs during acute infection. BM from a total of 8 control mice and 4 infected mice per timepoint was assessed. Cells were first gated as in Figure 1 for lineage-negative, cKit-positive HSPC populations. CXCR4 positivity was then evaluated via flow cytometry. Representative plots are shown for days 0, 2, 4, 6 and 8 post infection for surface and total CXCR4 expression (A). Mean values for percentage of CXCR4+ BM HSPCs were compared (B); significant decreases were found in surface CXCR4 (solid bars) on days 2 and 6 post infection and in total CXCR4 (striped bars) on days 2-4 post infection (asterisk = p<.05, bars = standard error).
Discussion
Downregulation of CXCL12/CXCR4 signaling occurs in a murine model of infection with A. phagocytophilum, an obligate intracellular, LPS-negative and peptidoglycan layer-negative pathogen. CXCL12 is a CXC chemokine found in BM stromal cells including CXCL12-abundant reticular (CAR) cells, immature osteoblasts and endothelial cells.29-31 CXCR4 is expressed on HSPCs as well as other hemic cells.32 CXCL12/CXCR4 signaling can be modulated by inflammatory mediators. Cytokines such as tumor necrosis factor-α (TNFα) and granulocyte colony-stimulating factor (G-CSF) may suppress CXCL12 expression.33,34 Degradation of CXCL12 protein by granulocyte-derived proteases also downregulates signaling.3,35-37 Potential mediators of decreased BM CXCL12 in our infection model include TNFα and G-CSF.3,22,31 TNFα concentration is increased in BM supernatants during infection; G-CSF alterations in A. phagocytophilum infection have not been evaluated.22 Neutrophil proteases may also play a role in diminishing BM CXCL12 protein levels.
Regulation of CXCR4 primarily occurs at the level of protein expression, as the half-life of CXCR4 mRNA is limited; however, transcriptional control does occur. Factors implicated in downregulation of CXCR4 transcription include IFN-γ, TNFα and IL-1β.38 The brief half-life of CXCR4 mRNA may partly explain the mild and inconsistent changes in CXCR4 via RT-PCR in sorted HSPCs noted in this study. Alternately, regulation may not occur via transcriptional control. Post-translational regulation of CXCR4 in hemic cells includes increased internalization after IFN-γ or TNFα stimulation.39 Taken together, our data suggest that BM or systemic cytokine alterations may serve as upstream mediators of diminished CXCL12/CXCR4 signaling during acute A. phagocytophilum infection. Plasma CXCL12 levels, representing total body levels, were diminished in the later stages of acute infection reflecting a decline in CXCL12 BM production. HSPCs mobilize in response to a CXCL12 chemokine gradient, so BM CXCL12 reduction likely shifts the gradient toward the periphery, resulting in HSPC mobilization.23
Kinetic changes in CXCL12/CXCR4 in our in vivo infection model fit as a plausible mechanism of hemic cell mobilization. The role of inflammatory cytokines in recruitment of hemic cells to a focus of infection has been well-studied. However, pathogen-induced perturbations of CXCL12/CXCR4 signaling in the BM are essentially uncharacterized in vivo during bacterial infections. Viral perturbations of this pathway are better described, in part due to the fact that CXCR4 is a co-receptor for HIV.40 Our work as well as work with our collaborators on a related monocyte-tropic pathogen (Ehrlichia muris) demonstrates that infection with an obligate intracellular bacterial pathogen can have a similar viral-like impact on hematopoiesis.22,41 A. phagocytophilum and viral pathogens both have an intracellular life cycle and a trend towards disturbing hematopoiesis, resulting in BM dysfunction and cytopenias, rather than stimulating effective hematopoiesis. Although acute A. phagocytophilum infection clearly results in HSPC proliferation and mobilization, other effects on hematopoiesis, including the induction of myelosuppressive chemokines, decreased BM CFU activity, and a clinical presentation of bi- or pancytopenia, are more indicative of BM dysfunction.42
Acute infection with A. phagocytophilum triggers alterations in proliferation and trafficking of HSPCs. Specifically, acute infection triggers increased BM HSPC synthetic activity that is first detected at day 2 post-infection and peaks at days 6-8. This burst of proliferative activity correlates with increased numbers of HSPCs in BM as well as in spleen and peripheral blood. Our previous work found substantial increases in splenic colony-forming unit (CFU) activity during acute A. phagocytophilum infection, corresponding with increased splenic HSPC numbers noted in this study.22 Both mobilization from BM and proliferation within the spleen appear to play a role in expansion of splenic HSPC populations. Interestingly, CFU activity was decreased in BM throughout infection in contrast to increased BM HSPC numbers. Several potential factors may contribute to this disparity. Colony-forming potential does not necessarily correlate with in vivo proliferation, as demonstrated via IFN-γ effects on BM; colony formation was suppressed in the face of LSK population expansion.43 Additionally, the prior findings occurred during infection via tick-borne injection versus intraperitoneal inoculation, raising the possibility of differing immune responses affecting hematopoiesis as tick saliva is immunomodulatory.44
The relationship between downregulated CXCL12 signaling, HSPC proliferation, HSPC mobilization and the development of cytopenias during A. phagocytophilum infection is unclear. Cytopenias are detected as early as 2-3 days post-infection.17 HSPC-related changes in the BM are therefore not a likely immediate cause of acute cytopenias, but altered trafficking of mature blood cells due to downregulated CXCL12 signaling remains a potential cause. Our prior studies found substantially increased numbers of mature neutrophils in the spleen during acute A. phagocytophilum infection; increased splenic leukocyte homing and sequestration due to shifts in CXCL12 gradients may therefore play a role in development of leukopenia.22 Work with a related pathogen , E. muris, has demonstrated that the functional alterations in BM are accompanied by a decrease in classically defined megakaryocytic-erythroid progenitors (MEPs) and common myeloid progenitors (CMPs) with an increase in functionally and phenotypically IFN-γ “activated” (Sca-1 positive) cells that correlate with decreased BM CFUs.45 Cytopenias may also be due to delayed hematopoietic suppression during A. phagocytophilum infection. Infection with similar tick-borne agents, particularly Ehrlichia canis, can result in chronic severe myelosuppression leading to sustained pancytopenia, and prolonged disruption of the stem/progenitor cell compartment is a plausible mechanism.46,47 Other infections with both LPS-positive and LPS-negative pathogens disrupt HSPC proliferation and mobilization kinetics, but the net effect is generally to augment innate immune cell production during infection.48-51 A causal link between the hematopoietic disturbances reported here and development of cytopenias during infection with A. phagocytophilum and similar pathogens remains to be shown.
HSPC proliferation and mobilization in response to gram positive,50 gram negative,51,52 fungal,49,53 and protist48 pathogens has been demonstrated in vitro and in models of systemic infection. However, how these pathogens specifically impact hematopoietic signaling has not been established. In this paper we specifically implicate regulation of CXCL12/CXCR4 signaling as one mechanism by which this LPS-negative pathogen impacts hematopoiesis and hemic cell mobilization. Upstream effectors of signaling disruption remain to be elucidated. Further investigation of the mechanisms involved in these and other infection-induced hematopoietic disturbances may lead to improved therapeutic responses in the clinical setting.
Figure 8.
RT-PCR for CXCR4 mRNA in sorted BM and spleen HSPCs. Cells were first sorted in a similar fashion as in figure 1 for lineage-negative, cKit-positive HSPC populations in BM (A) and spleen (B). CXCR4 mRNA was then quantified via RT-PCR using the comparative Ct approach. No significant change in CXCR4 mRNA was found in BM HSPCs (C). A mild but significant increase in fold expression of CXCR4 mRNA was found in splenic HSPCs at day 4 post infection (D).
Acknowledgements
This investigation was supported by the National Institutes of Health, Ruth L. Kirschstein National Research Service Award T32 RR207038 from the National Center for Research Resources (JLJ). We thank Carol Oxford at the University of California Davis Optical Biology Core Facility for flow cytometric sorting of HSPCs, and Michael Lamé and Naomi Walker at the University of California Davis School of Veterinary Medicine for technical assistance.
Footnotes
Conflict of Interest Disclosure The Author(s) declare(s) that there is no conflict of interest.
References
- 1.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201(8):1307–18. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma Q, Jones D, Borghesani PR, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4× and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95(16):9448–53. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ueda Y, Yang K, Foster SJ, et al. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J Exp Med. 2004;199(1):47–57. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levesque JP, Hendy J, Takamatsu Y, et al. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003 Jan;111(2):187–96. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pusic I, DiPersio JF. Update on clinical experience with AMD3100, an SDF-1/CXCL12-CXCR4 inhibitor, in mobilization of hematopoietic stem and progenitor cells. Curr Opin Hematol. 2010 Jul;17(4):319–26. doi: 10.1097/MOH.0b013e328338b7d5. [DOI] [PubMed] [Google Scholar]
- 6.Eash KJ, Greenbaum AM, Gopalan PK, et al. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010 Jul 1;120(7):2423–31. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furze RC, Rankin SM. Neutrophil mobilization and clearance in the bone marrow. Immunology. 2008 Nov;125(3):281–8. doi: 10.1111/j.1365-2567.2008.02950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wengner AM, Pitchford SC, Furze RC, et al. The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation. Blood. 2008 Jan 1;111(1):42–9. doi: 10.1182/blood-2007-07-099648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen SM, Dumler JS, Bakken JS, et al. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32(3):589–95. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumler JS, Barbet AF, Bekker CPJ, et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. International Journal of Systemic and Evolutionary Microbiology. 2001;51:2145–65. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- 11.Sukumaran B, Narasimhan S, Anderson JF, et al. An Ixodes scapularis protein required for survival of Anaplasma phagocytophilum in tick salivary glands. J Exp Med. 2006 Jun 12;203(6):1507–17. doi: 10.1084/jem.20060208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakken JS, Krueth J, Wilson-Nordskog C, et al. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996 Jan 17;275(3):199–205. [PubMed] [Google Scholar]
- 13.Batungbacal MR, Scott GR, Burrells C. The lymphocytopaenia in tick-borne fever. J Comp Pathol. 1982 Jul;92(3):403–7. doi: 10.1016/0021-9975(82)90026-3. [DOI] [PubMed] [Google Scholar]
- 14.Madigan JE, Gribble D. Equine ehrlichiosis in northern California: 49 cases (1968-1981) J Am Vet Med Assoc. 1987 Feb 15;190(4):445–8. [PubMed] [Google Scholar]
- 15.Pusterla N, Pusterla JB, Braun U, et al. Experimental cross-infections with Ehrlichia phagocytophila and human granulocytic ehrlichia-like agent in cows and horses. Vet Rec. 1999;145(11):311–4. doi: 10.1136/vr.145.11.311. [DOI] [PubMed] [Google Scholar]
- 16.Borjesson DL, Simon SI, Hodzic E, et al. Kinetics of CD11b/CD18 up-regulation during infection with the agent of human granulocytic ehrlichiosis in mice. Lab Invest. 2002;82(3):303–11. doi: 10.1038/labinvest.3780424. [DOI] [PubMed] [Google Scholar]
- 17.Borjesson DL, Simon SI, Tablin F, et al. Thrombocytopenia in a Mouse Model of Human Granulocytic Ehrlichiosis. J Infect Dis. 2001;184:1475–9. doi: 10.1086/324518. [DOI] [PubMed] [Google Scholar]
- 18.Dumler JS, Madigan JE, Pusterla N, et al. Ehrlichioses in Humans: Epidemiology, Clinical Presentation, Diagnosis, and Treatment. Clinical Infectious Diseases. 2007;45(Suppl 1):45–51. doi: 10.1086/518146. [DOI] [PubMed] [Google Scholar]
- 19.Rikihisa Y. Anaplasma phagocytophilum and Ehrlichia chaffeensis: subversive manipulators of host cells. Nat Rev Microbiol. 2010 May;8(5):328–39. doi: 10.1038/nrmicro2318. [DOI] [PubMed] [Google Scholar]
- 20.Lee HC, Goodman JL. Anaplasma phagocytophilum causes global induction of antiapoptosis in human neutrophils. Genomics. 2006 Oct;88(4):496–503. doi: 10.1016/j.ygeno.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Borjesson DL, Simon SI, Hodzic E, et al. Roles of neutrophil beta 2 integrins in kinetics of bacteremia, extravasation, and tick acquisition of Anaplasma phagocytophila in mice. Blood. 2003;101(8):3257–64. doi: 10.1182/blood-2002-04-1019. [DOI] [PubMed] [Google Scholar]
- 22.Johns JL, MacNamara KC, Walker NJ, et al. Infection with Anaplasma phagocytophilum Induces Dynamic Alterations in Hematopoietic Subsets. Infect Immun. 2009;77(9):4070–80. doi: 10.1128/IAI.00570-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hattori K, Heissig B, Tashiro K, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001 Jun 1;97(11):3354–60. doi: 10.1182/blood.v97.11.3354. [DOI] [PubMed] [Google Scholar]
- 24.Hodzic E, Ijdo JW, Feng S, et al. Granulocytic ehrlichiosis in the laboratory mouse. J Infect Dis. 1998;177(3):737–45. doi: 10.1086/514236. [DOI] [PubMed] [Google Scholar]
- 25.Borjesson DL, Barthold SW. The Mouse as a Model for Investigation of Human Granulocytic Ehrlichiosis: Current Knowledge and Future Directions. Comparative Medicine. 2002;52(5):403–13. [PubMed] [Google Scholar]
- 26.Hodzic E, Feng S, Fish D, et al. Infection of mice with the agent of human granulocytic ehrlichiosis after different routes of inoculation. J Infect Dis. 2001;183(12):1781–6. doi: 10.1086/320735. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Blas-Machado U, Fuente J, Blouin EF, et al. Experimental infection of C3H/HeJ mice with the NY18 isolate of Anaplasma phagocytophilum. Vet Pathol. 2007 Jan;44(1):64–73. doi: 10.1354/vp.44-1-64. [DOI] [PubMed] [Google Scholar]
- 29.Sugiyama T, Kohara H, Noda M, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immun. 2006;25:977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Bleul CC, Fuhlbrigge RC, Casasnovas JM, et al. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996 Sep 1;184(3):1101–9. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semerad CL, Christopher MJ, Liu F, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005 Nov 1;106(9):3020–7. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broxmeyer HE. Chemokines in hematopoiesis. Curr Opin Hematol. 2008 Jan;15(1):49–58. doi: 10.1097/MOH.0b013e3282f29012. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Q, Guo R, Schwarz EM, et al. TNF inhibits production of stromal cell-derived factor 1 by bone stromal cells and increases osteoclast precursor mobilization from bone marrow to peripheral blood. Arthritis Res Ther. 2008;10(2):R37. doi: 10.1186/ar2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Semerad CL, Christopher MJ, Liu F, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106(9):3020–7. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002 Jul;3(7):687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 36.Delgado MB, Clark-Lewis I, Loetscher P, et al. Rapid inactivation of stromal cell-derived factor-1 by cathepsin G associated with lymphocytes. Eur J Immunol. 2001 Mar;31(3):699–707. doi: 10.1002/1521-4141(200103)31:3<699::aid-immu699>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 37.McQuibban GA, Butler GS, Gong JH, et al. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001 Nov 23;276(47):43503–8. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- 38.Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta. 2007 Apr;1768(4):952–63. doi: 10.1016/j.bbamem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brühl H, Cohen CD, Linder S, et al. Post-translational and cell type-specific regulation of CXCR4 expression by cytokines. Eur J Immunol. 2003;33(11):3028–37. doi: 10.1002/eji.200324163. [DOI] [PubMed] [Google Scholar]
- 40.Oberlin E, Amara A, Bachelerie F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382(6594):833–5. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 41.MacNamara KC, Racine R, Chatterjee M, et al. Diminished hematopoietic activity associated with alterations in innate and adaptive immunity in a mouse model of human monocytic ehrlichiosis. Infect Immun. 2009 Sep;77(9):4061–9. doi: 10.1128/IAI.01550-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein MB, Hu S, Chao CC, et al. The agent of human granulocytic ehrlichiosis induces the production of myelosuppressing chemokines without induction of proinflammatory cytokines. J Infect Dis. 2000;182(1):200–5. doi: 10.1086/315641. [DOI] [PubMed] [Google Scholar]
- 43.Zhao X, Ren G, Liang L, et al. Brief report: interferon-gamma induces expansion of Lin(−)Sca-1(+)C-Kit(+) Cells. Stem Cells. 2010 Jan;28(1):122–6. doi: 10.1002/stem.252. [DOI] [PubMed] [Google Scholar]
- 44.Gillespie RD, Mbow ML, Titus RG. The immunomodulatory factors of blood-feeding arthropod saliva. Parasite Immunol. 2000;22(7):319–21. doi: 10.1046/j.1365-3024.2000.00309.x. [DOI] [PubMed] [Google Scholar]
- 45.Macnamara KC, Oduro K, Martin O, et al. Infection-induced myelopoiesis during intracellular bacterial infection is critically dependent upon IFN-{gamma} signaling. J Immunol. 2011 Jan 15;186(2):1032–43. doi: 10.4049/jimmunol.1001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearce CJ, Conrad ME, Nolan PE, et al. Ehrlichiosis: a cause of bone marrow hypoplasia in humans. Am J Hematol. 1988 May;28(1):53–5. doi: 10.1002/ajh.2830280111. [DOI] [PubMed] [Google Scholar]
- 47.Mylonakis ME, Koutinas AF, Breitschwerdt EB, et al. Chronic canine ehrlichiosis (Ehrlichia canis): a retrospective study of 19 natural cases. J Am Anim Hosp Assoc. 2004 May-Jun;40(3):174–84. doi: 10.5326/0400174. [DOI] [PubMed] [Google Scholar]
- 48.Belyaev NN, Brown DE, Diaz AI, et al. Induction of an IL7-R(+)c-Kit(hi) myelolymphoid progenitor critically dependent on IFN-gamma signaling during acute malaria. Nat Immunol. 2010 Jun;11(6):477–85. doi: 10.1038/ni.1869. [DOI] [PubMed] [Google Scholar]
- 49.Yáñez A, Murciano C, O’Connor JE, et al. Candida albicans triggers proliferation and differentiation of hematopoietic stem and progenitor cells by a MyD88-dependent signaling. Microbes Infect. 2009 Apr;11(4):531–5. doi: 10.1016/j.micinf.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Scumpia PO, Kelly-Scumpia KM, Delano MJ, et al. Cutting edge: bacterial infection induces hematopoietic stem and progenitor cell expansion in the absence of TLR signaling. J Immunol. 2010;184(5):2247–51. doi: 10.4049/jimmunol.0903652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang P, Nelson S, Bagby GJ, et al. Lineage-c-kit+Sca-1+ cell response to Escherichia coli bacteremia in Balb/c mice. Stem Cells. 2008 doi: 10.1634/stemcells.2007-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shahbazian LM, Quinton LJ, Bagby GJ, et al. Escherichia coli pneumonia enhances granulopoiesis and the mobilization of myeloid progenitor cells into the systemic circulation. Crit Care Med. 2004;32(8):1740–6. doi: 10.1097/01.ccm.0000132900.84627.90. [DOI] [PubMed] [Google Scholar]
- 53.Yáñez A, Flores A, Murciano C, et al. Signalling through TLR2/MyD88 induces differentiation of murine bone marrow stem and progenitor cells to functional phagocytes in response to Candida albicans. Cell Microbiol. 2010 Jan;12(1):114–28. doi: 10.1111/j.1462-5822.2009.01382.x. [DOI] [PubMed] [Google Scholar]