Abstract
A mechanistic link between PPARγ and the renin-angiotensin system (RAS) has been previously proposed but clinical evidence supporting the relationship is incomplete. In the current issue of Arteriosclerosis Thrombosis Vascular Biology, Caron-Debarle et al. show that four patients with familial partial lipodystrophy associated with early-onset severe hypertension (FPLD3) carry mutations in PPARγ that impair its ability to act as a ligand-activated transcription factor. Cells isolated from these patients, and cells transfected with the same mutations in PPARγ exhibit activation of the cellular RAS, increased production of reactive oxygen species and markers of inflammation, all of which are dependent upon the angiotensin-II AT1 receptor. This translational study further supports a role for PPARγ as a regulator of blood pressure through its ability to modulate the RAS.
Peroxisome proliferator-activated receptor γ (PPARγ) is a ligand activated transcription factor and target of the thiazolidinedione TZD class of anti-diabetes medications. PPARγ is best recognized for its role in adipogenesis but is also a regulator of systemic metabolism as evidenced by the pleiotropic abnormalities (lipodystrophy, insulin-resistance, and metabolic syndrome) caused by PPARγ mutations.1-3 Clinical studies of TZD use in type 2 diabetes including the PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events) trial documented improved endothelial function and modest but significant reductions in blood pressure.4 Some of the same mutations which cause lipodystrophy and diabetes also cause severe hypertension and preeclampsia in human patients,3 and in knock-in mice.5,6 Evidence suggests that PPARγ activity in the vascular endothelium and smooth muscle are important regulators of endothelial function, smooth muscle contraction, and systemic blood pressure.7,8
Data suggesting a role for PPARγ in regulating blood pressure led many to search for downstream mediators. Early studies suggested that activation of PPARγ might antagonize the renin-angiotensin system (RAS) by inhibiting expression of the angiotensin-II (Ang-II) AT1 receptor (AT1R) in vascular smooth muscle cells (vSMC).9 PPARγ may also regulate expression of the renin and angiotensinogen (AGT) genes.10,11 TZD administration to Ang-II treated Sprague-Dawley rats blunts the development of hypertension, endothelial dysfunction, and the induction of proinflammatory mediators.12 Similarly, TZD treatment of hypertensive transgenic mice over-expressing the renin and AGT genes improved endothelial function and lowered arterial pressure.13 An association between PPARγ and the RAS was also suggested by Tsai et al. 5 (and reviewed in 14) who reported that mice carrying a mutant PPARγ allele equivalent to the mutation which causes hypertension in humans, exhibited increased blood pressure and elevated expression of AGT and AT1R in several adipose depots. That certain AT1R blockers (ARB) exhibit partial PPARγ agonist activity suggests an unexpected yet physiologically uncertain link between PPARγ and the RAS.15 What remained unclear is whether this association between PPARγ and the RAS is clinically relevant?
In the current issue of Arteriosclerosis Thrombosis Vascular Biology, Caron-Debarle et al.16 explore this question in 4 members of 2 unrelated families with familial partial lipodystrophy associated with early-onset severe hypertension (FPLD3). Blood pressure control in these patients required aggressive treatment with multiple antihypertensive agents (including ARBs) concurrent with treatment for hyperlipidemia, and in 3 of the 4 subjects, diabetes. They identified two previously unreported mutations in PPARγ. R165T occurs in a highly conserved residue in the DNA binding domain, whereas L339X truncates the protein to lack a portion of the ligand binding domain. All 4 patients were heterozygous for one of the mutations. In vitro studies of cultured fibroblasts and peripheral blood mononuclear cells (PBMC) derived from the patients, as well as human vSMCs transfected with the PPARγ mutants revealed that the mutant and wildtype alleles were equivalently expressed, but the mutants lacked transactivation capability. Unlike other mutations in PPARγ which cause hypertension, they do not act dominant negatively and most likely cause haploinsufficiency.3 TZD treatment improved glycemic control and eliminated the need for high dose insulin therapy in 2 subjects suggesting that the potential to activate the wildtype PPARγ allele was preserved. Although untested in the current study, it is possible that the activity of the wildtype PPARγ may have been impaired in these patients. Inflammation has been reported to impair PPARγ activity by CDK5-mediated phosphorylation, an effect prevented by TZDs.17 Indeed, hypertension and diabetes are commonly associated with inflammation and fibroblasts isolated from these patients exhibited increased NFκB activity, markers of inflammation, and increased reactive oxygen species (ROS). AT1R signaling is well known to cause inflammation and oxidative stress, and interestingly, expression of AT1R, renin, and AGT were all markedly increased in patient fibroblasts and PBMCs, cells we do not immediately associate with the RAS. The increase in AT1R expression occurred concomitantly with increased Ang-II-induced ERK phosphorylation, and AT1R silencing prevented the induction of ROS and inflammation suggesting that some of the pathological consequences of the mutations may be mediated by AT1R activation.
These data suggest a mechanism whereby impaired PPARγ activity induces AT1R expression and signaling which promotes oxidative stress and inflammation. That the silencing of AT1R in these cells also decreased expression of renin and AGT suggests their increase may be secondary to increased AT1R signaling. We could therefore hypothesize the existence (at least in the isolated cells from these patients) of a feed-forward mechanism whereby elevated AT1R action augments further Ang-II production which may then amplify the pathological response (see Figure). It is interesting to note that the induction of renin expression by AT1R in fibroblasts and PBMCs is contrary to Ang-II-induced inhibition of renin expression in kidney. Unfortunately, information regarding the status of the systemic RAS in these patients before treatment was not available, whereas under therapy, 2 patients had normal plasma renin activity (PRA), plasma and urinary aldosterone, and potassium. Although the clinical relevance of the RAS in fibroblasts and PBMCs remains uncertain, AT1R signaling in vSMC is of obvious importance in the regulation of vasomotor function. A feed-forward mechanism as described above could potentially induce endothelial dysfunction and smooth muscle contraction and exacerbate the hypertension.
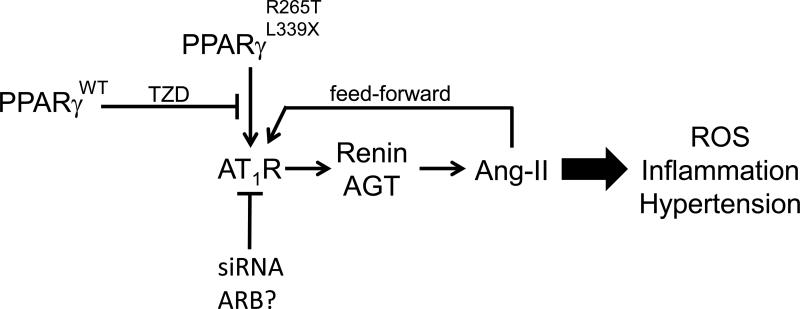
Figure. The PPARγ:RAS Relationship.
Schematic showing that PPARγ mutations cause an increase in expression of the AT1R which induces hypertension perhaps through ROS and inflammation. The increase in renin and AGT elevates production of Ang-II, which in cells from the effected patients, causes a feed-forward mechanism which may further increase AT1R signaling. TZD treatment activates the wildtype PPARγ allele and blunts the effects of the mutation. A similar effect is attained by blocking AT1R expression by an siRNA and presumably with an ARB.
Regardless of the many strengths of this translational study a number of important questions remain. First, did TZD treatment of the effected patients have an effect on arterial pressure; or in a more general sense, does PPARγ activation lower blood pressure in humans by antagonizing the RAS? We know that treatment of the patient fibroblasts with rosiglitazone, which presumably activated wildtype PPARγ decreased expression of the RAS genes, and blunted the increase in ROS, NFκB and IL-6 induced by the PPARγ mutations. Thus at the cellular level, a normal phenotype could be rescued by activation of wildtype PPARγ by TZD. Even with the declining clinical use of TZDs this may be important because new PPARγ activators, which do not act as full PPARγ agonists are in development. At least one of these new compounds prevents impairment of PPARγ activity by post-translational mechanisms induced by inflammation, and importantly, this compound may lack some of the detrimental side effects of TZDs.18 It's effect on the cardiovascular system has yet to be explored. Second, is the AT1R gene the primary PPARγ target gene or are their other PPARγ target genes in the relevant tissues which become dysregulated in response to mutant PPARγ? We recently reported that PPARγ induces expression of a target gene in the aorta which controls the activity of the Cullin-3 pathway, a regulator of RhoA/Rho kinase signaling and vasomotor function.19 We also recently identified a physiological connection between PPARγ and AT1R activity (but not AT1R expression) in mesenteric resistance vessels through Regulator of G protein signaling 5 (RGS5), a novel PPARγ target gene that functions as a small GTPase-activating protein to regulate AT1R signaling.20 Third, are all the cardiovascular effects in these patients mediated by PPARγ and the RAS? This may be important to consider because there are other inherited lipodystrophies which are not caused by mutations in PPARγ yet are associated with hypertension.21,22 A common feature of all these disorders is insulin resistance and a loss or redistribution of adipose tissue (e.g. loss of subcutaneous adipose with accumulation of abdominal adipose).23 The mechanistic contributions of these features to hypertension in these patients remains unclear. Interestingly, as these patients often display evidence of inflammation (e.g. increased plasma C-reactive peptide) a role for impaired PPARγ activity and thus increased RAS activity should be considered.
In closing, there are other FPLD3 subjects that carry different mutations in PPARγ and exhibit a much broader array of neurologic and hematologic symptoms in addition to severe metabolic syndrome.24 It is therefore likely that PPARγ has far reaching effects which may extend beyond the RAS. Studies of human patients and patient cells like Caron-Debarle et al.16 combined with studies employing animal models will likely uncover other mechanistic links between PPARγ, the RAS, and other important pathways that may lead to effective therapies for the spectrum of disorders which encompass the metabolic syndrome.
Acknowledgments
Funding:
NIH grants HL048058, HL061446, HL062984, HL084207 to CDS. The author also gratefully acknowledges the generous research support of the Roy J. Carver Trust.
This is a commentary on article Auclair M, Vigouroux C, Boccara F, Capel E, Vigeral C, Guerci B, Lascols O, Capeau J, Caron-Debarle M.Peroxisome proliferator-activated receptor-γ mutations responsible for lipodystrophy with severe hypertension activate the cellular renin-angiotensin system. Arterioscler Thromb Vasc Biol. 2013;33(4):829-38.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agostini M, Schoenmakers E, Mitchell C, et al. Non-DNA binding, dominant-negative, human PPARγ mutations cause lipodystrophic insulin resistance. Cell Metab. 2006;4:303–311. doi: 10.1016/j.cmet.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savage DB, Tan GD, Acerini CL, et al. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-gamma. Diabetes. 2003;52:910–917. doi: 10.2337/diabetes.52.4.910. [DOI] [PubMed] [Google Scholar]
- 3.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O'Rahilly S. Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 4.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 5.Tsai YS, Kim HJ, Takahashi N, Kim HS, Hagaman JR, Kim JK, Maeda N. Hypertension and abnormal fat distribution but not insulin resistance in mice with P465L PPARγ. J Clin Invest. 2004;114:240–249. doi: 10.1172/JCI20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyer AM, Baumbach GL, Halabi CM, Modrick ML, Lynch CM, Gerhold TD, Ghoneim SM, deLange WJ, Keen HL, Tsai Y-S, Maeda N, Sigmund CD, Faraci FM. Interference with PPARγ Signaling Causes Cerebral Vascular Dysfunction, Hypertrophy, and Remodeling. Hypertension. 2008;51:867–871. doi: 10.1161/HYPERTENSIONAHA.107.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beyer AM, de Lange WJ, Halabi CM, Modrick ML, Keen HL, Faraci FM, Sigmund CD. Endothelium-specific interference with peroxisome proliferator activated receptor gamma causes cerebral vascular dysfunction in response to a high-fat diet. Circ Res. 2008;103:654–661. doi: 10.1161/CIRCRESAHA.108.176339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM, Sigmund CD. Interference with PPARγ Function in Smooth Muscle Causes Vascular Dysfunction and Hypertension. Cell Metabolism. 2008;7:215–226. doi: 10.1016/j.cmet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeda K, Ichiki T, Tokunou T, Funakoshi Y, Iino N, Hirano K, Kanaide H, Takeshita A. Peroxisome proliferator-activated receptor gamma activators downregulate angiotensin II type 1 receptor in vascular smooth muscle cells. Circulation. 2000;102:1834–1839. doi: 10.1161/01.cir.102.15.1834. [DOI] [PubMed] [Google Scholar]
- 10.Vernochet C, Peres SB, Davis KE, McDonald ME, Qiang L, Wang H, Scherer PE, Farmer SR. C/EBPalpha and the corepressors CtBP1 and CtBP2 regulate repression of select visceral white adipose genes during induction of the brown phenotype in white adipocytes by peroxisome proliferator-activated receptor gamma agonists. Mol Cell Biol. 2009;29:4714–4728. doi: 10.1128/MCB.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desch M, Schreiber A, Schweda F, Madsen K, Friis UG, Weatherford ET, Sigmund CD, Sequeira Lopez ML, Gomez RA, Todorov VT. Increased renin production in mice with deletion of peroxisome proliferator-activated receptor-gamma in juxtaglomerular cells. Hypertension. 2010;55:660–666. doi: 10.1161/HYPERTENSIONAHA.109.138800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diep QN, El Mabrouk M, Cohn JS, Endemann D, Amiri F, Virdis A, Neves MF, Schiffrin EL. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: role of peroxisome proliferator-activated receptor-gamma. Circulation. 2002;105:2296–2302. doi: 10.1161/01.cir.0000016049.86468.23. [DOI] [PubMed] [Google Scholar]
- 13.Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. PPARγ agonist rosiglitazone improves vascular function and lowers blood pressure in hypertensive transgenic mice. Hypertension. 2004;43:661–666. doi: 10.1161/01.HYP.0000116303.71408.c2. [DOI] [PubMed] [Google Scholar]
- 14.Hegele RA, Leff T. Unbuckling lipodystrophy from insulin resistance and hypertension. J Clin Invest. 2004;114:163–165. doi: 10.1172/JCI22382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARγ-modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- 16.Caron-Debarle M, Auclair M, Vigouroux C, Boccara F, Capel E, Vigarel C, Guerci B, Lascols O, Capeau J. PPARG mutations responsible for lipodystrophy with severe hypertension activate the cellular renin-angiotensin system. Arterioscler Thromb Vasc Biol. 2013 doi: 10.1161/ATVBAHA.112.300962. in press. [DOI] [PubMed] [Google Scholar]
- 17.Choi JH, Banks AS, Estall JL, Kajimura S, Bostrom P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Bluher M, Griffin PR, Spiegelman BM. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARγ by Cdk5. Nature. 2010;466:451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi JH, Banks AS, Kamenecka TM, et al. Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation. Nature. 2011;477:477–481. doi: 10.1038/nature10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelham CJ, Ketsawatsomkron P, Groh S, Grobe JL, de Lange WJ, Ibeawuchi SR, Keen HL, Weatherford ET, Faraci FM, Sigmund CD. Cullin-3 Regulates Vascular Smooth Muscle Function and Arterial Blood Pressure via PPARγ and RhoA/Rho-Kinase. Cell Metab. 2012;16:462–472. doi: 10.1016/j.cmet.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ketsawatsomkron P, Lorca RA, Keen HL, Weatherford ET, Liu X, Pelham CJ, Grobe JL, Faraci FM, England SK, Sigmund CD. PPARγ Regulates Resistance Vessel Tone Through a Mechanism Involving RGS5-Mediated Control of PKC and BKCa Channel Activity. Circ Res. 2012;111:1446–1458. doi: 10.1161/CIRCRESAHA.112.271577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hegele RA, Anderson CM, Wang J, Jones DC, Cao H. Association between nuclear lamin A/C R482Q mutation and partial lipodystrophy with hyperinsulinemia, dyslipidemia, hypertension, and diabetes. Genome Res. 2000;10:652–658. doi: 10.1101/gr.10.5.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim CA, Delepine M, Boutet E, et al. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J Clin Endocrinol Metab. 2008;93:1129–1134. doi: 10.1210/jc.2007-1328. [DOI] [PubMed] [Google Scholar]
- 23.Hegele RA. Phenomics, lipodystrophy, and the metabolic syndrome. Trends Cardiovasc Med. 2004;14:133–137. doi: 10.1016/j.tcm.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Campeau PM, Astapova O, Martins R, Bergeron J, Couture P, Hegele RA, Leff T, Gagne C. Clinical and molecular characterization of a severe form of partial lipodystrophy expanding the phenotype of PPARγ deficiency. J Lipid Res. 2012;53:1968–1978. doi: 10.1194/jlr.P025437. [DOI] [PMC free article] [PubMed] [Google Scholar]