Abstract
Covalent conjugation of TLR agonists to protein antigens often facilitates the generation of a CD8+ T cell response. However, mechanisms underlying the efficacy of the conjugate over its unconjugated counterpart have been largely uninvestigated. In this study, we show that conjugation of a TLR7 agonist enhances CD8+ T cell responses without affecting antigen persistence and with minimal impact on cellular uptake of the antigen in vivo. Instead, the conjugated form induced a robust accumulation of DCs in regional lymph nodes. Perhaps more importantly, cross-presentation in DCs was detected only when the antigen was delivered in the conjugated form with the TLR7 agonist. Collectively these data represent the first demonstration that a TLR agonist-antigen conjuagte elicits CD8+ T cell responses based not on its capacity to induce DC maturation or antigen persistence and uptake, but on the engagement of DC cross-presentation pathways.
INTRODUCTION
TLR7 is an intracellular receptor that recognizes single-stranded RNA molecules and enables the host to sense RNA viruses such as the Influenza virus (1). However, synthetic compounds agonistic for TLR7 had already been discovered prior to the identification of RNA as the natural ligand (2). Collectively known as imidazoquinolines, these compounds structurally resemble RNA molecules, and similarly to the physiologic ligand, they induce innate immune activation via TLR7 (2–5). Imidazoquinolines up-regulate co-stimulatory molecules such as CD80, CD86, and MHC class II molecules on APCs and induce the localization of DCs to the T cell areas of secondary lymphoid tissues (5). In addition, rapid induction of inflammatory mediators, such as TNFα, IL-12, Type I IFNs, and MIP1α were reported in mice treated with TLR7 agonists (2–5). Given the presence of activated APCs and inflammation, which are necessary conditions for the priming of T cells, targeting TLRs represents a rational approach to designing vaccines that can effectively induce cross-priming of CD8+ T cells.
However, despite their ability to activate the innate immune system, TLR agonists, in general, were demonstrated to be relatively poor inducers of cellular immunity (6). Immunizations using protein antigens mixed with agonists targeting TLRs ranging from TLR2 to TLR9 failed to induce detectable CD8+ T cell responses (7), suggesting that simply the induction of APC activation and inflammatory cytokines are not sufficient to bridge the innate and adaptive immune systems. We previously explored the idea of covalently linking a TLR agonist to antigen as a means to mimic the natural recognition of pathogens (6). Using a TLR7 agonist covalently conjugated to the HIV-1 gag protein, we demonstrated that CD4+ and CD8+ T cell responses can indeed be generated in both mouse and non-human primates (6, 8). Other studies have shown a similar enhancement of the adaptive response when using antigens conjugated to ligands targeting TLR2, TLR4 or TLR9 (9–16). Thus, the use of chemical conjugation in a vaccine formulation appears to generally enhance the generation of cellular immune responses.
However, mechanisms underlying the efficacy of conjugation remain poorly defined. Several possibilities exist by which conjugation increases the immunogenicity of the antigen and TLR7 agonist. Limited comparisons between free TLR2 and TLR9 agonist vaccinations versus their respective conjugates suggested that mechanisms underlying the efficacy of the conjugate may be related to increased DC targeting and/or increased antigen uptake (12, 14). While this may be a general mechanism by which all TLR agonist-antigen conjugates can elicit more potent cellular immunity, it is difficult to imagine how agonists targeting TLRs expressed in intracellular compartments may mediate this process. Indeed, a TLR9 agonist-antigen conjugate was reported to be taken up by TLR9 deficient APCs with similar efficiencies as TLR9 sufficient APCs (17), indicating that any increased efficacy of antigen uptake is not related to the presence of the targeted TLR. Another possibility is that the conjugation of antigen and adjuvant insures their co-delivery into a common endosomal compartment, facilitating the efficiency with which the antigen is presented to responding T cells (16, 18). While these and other factors may be necessary, it remains unclear which of these factors truly dictates the success or failure of the vaccination. Comparing these various parameters between conjugated and free TLR agonist-based immunizations provides the opportunity to conclusively determine which of these mechanisms are responsible for facilitating vaccine-induced cellular immunity.
In this study, we examined the possibility that conjugation enhances the stability of the antigen resulting in prolonged persistence of the antigen. In addition, we also considered that conjugation could result in aggregation of the antigen and hypothesized that it would consequently affect the efficiency of antigen uptake. Using a TLR7 agonist conjugated to fluorescently labeled ovalbumin protein (OVA), we found that conjugation did not elongate the half-life of the antigen, and had a limited impact on efficiency of antigen uptake. Instead, conjugation resulted in a dramatic increase in the frequency of DCs accumulating in locally draining LNs. Perhaps more importantly, cross-presentation was detected in DCs only when the antigen was delivered as a conjugate to the TLR7 agonist. These results not only clarify the mechanism of action of this particular adjuvant, but more broadly suggest that the timely recruitment of DCs and facilitation of cross-presentation may be the key determinants by which any vaccine adjuvant successfully induces cellular immunity.
MATERIALS AND METHODS
Mice and immunizations
C57BL/6 and OTI mice were obtained from The Jackson Laboratory and bred at National Jewish Health. Mice were immunized subcutaneously in the lower/upper flanks or in the footpad with whole ovalbumin protein (OVA) (5–500 μg), or with the TLR7 agonist, 3M012 either in the unconjugated form or as a covalently linked conjugate. OVA was purchased from Sigma-Aldrich and any contaminating endotoxins were eliminated using Triton X-114 and SM-2 Bio-Beads (Bio-Rad Labs) as previously described (19). The TLR7 agonist, 3M012, was obtained through material transfer agreements with 3M Pharmaceuticals. OVA was covalently conjugated to 3M012 as previously described (6, 8). All components injected into mice were tested for LPS contamination using the Limulus Amebocyte Lysate assay (Lonza) and confirmed to be free of any detectable levels of LPS.
Primary and secondary CD8+ T cell responses
OVA-specific CD8+ T cell responses were measured from PBLs of mice either 7 days (primary) or 5 days (boost) following injections. Boost injections were administered 30–60 days following the primary injection. Cells were then stained with PE-conjugated H-2Kb tetramers loaded with the OVA peptide, SIINFEKL, for 90 minutes at 37°C. OVA-specific CD8+ T cells were identified by gating on CD8+ CD4− B220− events, then gating on tetramer staining events that were positive for the activation marker CD44. To monitor cytokine function of effector CD8+ T cells, PBLs and spleens of mice were harvested 5–6 days post boost injection, pulsed with the SIINFEKL peptide at 1 μg/mL in media containing brefeldin A and incubated at 37°C for 4 hours. Cells were fixed and stained for intracellular IFNγ and IL-2 production.
Protection Assay
Mice were immunized with 5 μg of the OVA-3M012 conjugate in the footpad as described above. Immunized mice were given the same dose of the conjugate as a boost immunization 30–60 days following the primary injection. Approximately 30–60 days following the boost immunization, mice were challenged intravenously with 2 × 105 c.f.u. Listeria monocytogenes expressing ovalbumin (Lm-OVA). Liver and spleen were removed 3 days after challenge and the harvested tissues were mechanically sheared while resuspended in PBS buffer containing NP-40 (Sigma-Aldrich). They were then cultured on BHI plates and incubated overnight at 37° C. The c.f.u. was calculated the following day. Lm-OVA was kindly provided by Michael Bevan (University of Washington, Seattle, WA).
Kinetics of antigen uptake, migration, and activation
Mice were immunized in the footpad with 5 μg of the Alexa-488-labeled OVA-3M012 conjugate. At the indicated time points following immunization, DCs were isolated from draining LNs using Collagenase D and DNase as previously described (5). The uptake of antigen by DCs was determined by examining the frequency of CD11chigh events (pDCa-1− and B220−) that were positive for Alexa-488 OVA. The magnitude of uptake on a per cell basis was determined by measuring the mean fluorescence intensity of Alexa-488 in CD11chigh OVA+ events.
Cross-presentation assay
DCs were isolated using either a magnetic bead enrichment method with anti-CD11c (N418) PE mAb and anti-PE microbeads (Miltenyi Biotec) or the MoFlow cell sorter. Purity of the sorted fractions was assessed by flow cytometry and confirmed to be approximately 80–90%. Sorted DCs were incubated at the indicated cell numbers with 0.5 × 106 OTI effector T cells purified from a 5-day stimulation culture. The magnitude of IFNγ production in the effector OTI T cells was used as a measure of the level of antigen cross-presentation by DCs. This was quantified as percent maximum IFNγ response, which was calculated based on the percent of the maximal OTI IFNγ response (achieved by SIINFEKL-pulsed DCs) that is induced by the sorted DCs.
Statistical analyses
Statistical analyses were performed in Prism (GraphPad Software, Inc.) using Student’s two-tailed t tests. Asterisk symbols (*) were used to mark the statistical significance of a given difference in sample means and summarize the P value in the following manner: one asterisk marker (*) represent P < 0.05, two asterisk markers (**) represent P < 0.005, and three asterisk markers (***) represent P < 0.0005. Unless otherwise indicated, shown values of bar graphs are the mean ± standard error of mean (SEM).
RESULTS
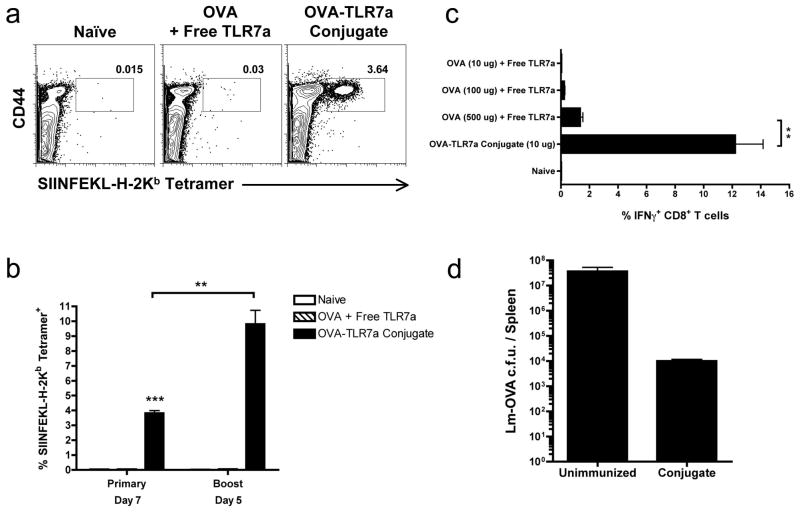
To understand the underlying mechanism by which conjugation of a TLR agonist to antigen serves as a protective vaccine formulation, we conjugated an imidazoquinoline TLR7 agonist (TLR7a) to ovalbumin (OVA) as previously described (6, 8), and first examined its ability to establish protective immune responses. We found that the OVA-TLR7a conjugate elicited a substantial OVA-specific CD8+ T cell response (Fig. 1a), similar to previously published results with the HIV-1 gag protein-TLR7a conjugates (6). Moreover, a robust production of IFNγ by CD8+ T cells was detected upon a boost injection using the same dose as the primary injection. These data suggest the OVA-TLR7a conjugate induces functional CD8+ effector T cells and generates memory CD8+ T cells (Fig. 1b,c). In contrast, using a 50 fold higher dose of the unconjugated mixture of the TLR7 agonist and OVA failed to induce any appreciable primary or secondary response (Fig. 1a,c), which together highlight the efficiency of conjugation in enhancing cross-priming of CD8+ T cells using minimal amounts of whole protein. Most importantly, mice that received only a single prime and boost injection of the OVA-TLR7a conjugate showed substantial protection against challenge with Lm-OVA. As shown in Figure 3.3, immunization with the OVA-TLR7a conjugate resulted in approximately a 1000 fold reduction in the frequency of bacteria in inoculated mice, demonstrating a striking ability of the response to control the infection and lower bacterial burden (Fig. 1d). Given the relatively low frequency of OVA-specific CD8+ T cells generated in the secondary response to the OVA-TLR7a conjugate (10–15% of CD8+ T cells) when compared to that generated in response to an actual infection such as vaccinia or LCMV (20–60% of CD8+ T cells) (20, 21), our results demonstrate the potency by which conjugation of the TLR7 agonist to protein antigens can establish protective immunity.
Figure 1. Conjugation of TLR7 agonist to OVA elicits protective CD8+ T cell responses.
(a) B6 mice were immunized with either a 50 μg mixture of OVA and unconjugated TLR7 agonist (TLR7a) or 5 μg of the OVA-TLR7a conjugate in the footpad and (b) given an equal dose of antigen for a boost injection 30–60 days following the primary immunization. Immunized mice were bled from the tail vein (a) 7 days following primary or (b) 5 days following boost immunization. Cells were stained with the SIINFEKL-loaded H-2Kb tetramers to measure the frequency of OVA-specific CD8+ T cells. (c,d) Mice were given the indicated doses of OVA and 10 μg of the unconjugated TLR7 agonist or the OVA-TLR7a conjugate. Thirty days later, immunized mice were injected again with the same dose used for the corresponding primary immunization. (c) Spleens from the immunized mice were harvested 6 days later and cell suspensions were prepared and pulsed with the OVA peptide, SIINFEKL, for 4 hours. Cells were then stained for intracellular IFNg. Frequency of IFNg+ cells shown were gated on live, B220− CD8+ cells. (d) Thirty days following the boost injection, mice were challenged intravenously with 2 × 105 c.f.u. of Lm-OVA per mouse. On Day 3, spleens from mice were harvested and cultured on BHI plates to assess the c.f.u. of Lm-OVA in the spleen as a measure of protection. Dot plots shown (a) are representative of three mice from each sample group. (b,c) Graphed values of the frequency of OVA-specific CD8+ T cells are expressed as the mean ± SEM. Data shown in (a,b,c) are representative of four independent experiments, and data shown in (d) are representative of two independent experiments. Statistical analyses (*) were performed as described in Materials and Methods.
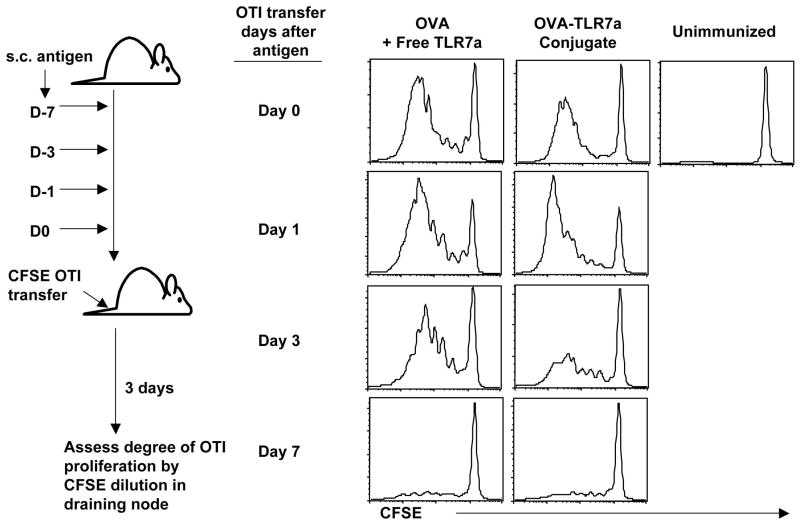
To determine the mechanism by which conjugation enhances the efficacy of the TLR7 agonist as a vaccine adjuvant, we examined the possibility that conjugation increased the half-life of the antigen in vivo resulting in a depot effect of the antigen. We addressed this hypothesis by utilizing the adoptive transfer of OTI TCR transgenic (Tg) T cells, which express a high affinity Tg TCR specific for the OVA peptide, SIINFEKL. OTI T cells can respond to concentrations as low as pico- or femtomolar ranges (22–24), allowing them to serve as a functional readout for a level of antigen presentation at which the endogenous OVA-specific T cells may not be responsive. To determine how long antigen persisted in the host in a functionally relevant manner, CFSE-labeled OTI T cells were adoptively transferred into mice at 0, 1, 3 or 7 days after antigenic challenge. The loss of CFSE fluorescence was used as an indicator of OTI T cells encountering antigen, and thus an indication that the antigen injected at a particular time prior to the OTI T cell transfer had persisted in vivo. Interestingly, the OVA-TLR7a conjugate showed a pattern of OTI stimulation similar to the unconjugated TLR7 agonist (Fig. 2). Evidence of cellular division was detected up to but not beyond 3 days prior to OTI T cell transfer, suggesting that the conjugated TLR7 agonist-antigen does not persist in vivo to any greater degree than antigen in the presence of the unconjugated TLR7 agonist. Therefore, the enhanced ability of the OVA-TLR7a conjugate to generate CD8+ T cell responses is not due to the establishment of an antigen depot in vivo.
Figure 2. Conjugation of the TLR7 agonist to antigen does not increase antigen persistence in vivo.
Mice were injected subcuntaneously with 10 μg OVA alone, OVA mixed with the unconjugated TLR7 agonist or the OVA-TLR7a conjugate at 0, 1, 3, or 7 days prior to the intravenous transfer of CFSE-labeled TCR transgenic OTI T cells. Locally draining LNs were harvested 3 days following OTI T cell transfer, and the dilution of CFSE was assessed by gating on OTI T cells using SIINFEKL-H-2Kb Tetramer+ events. The data shown are representative of two independent experiments.
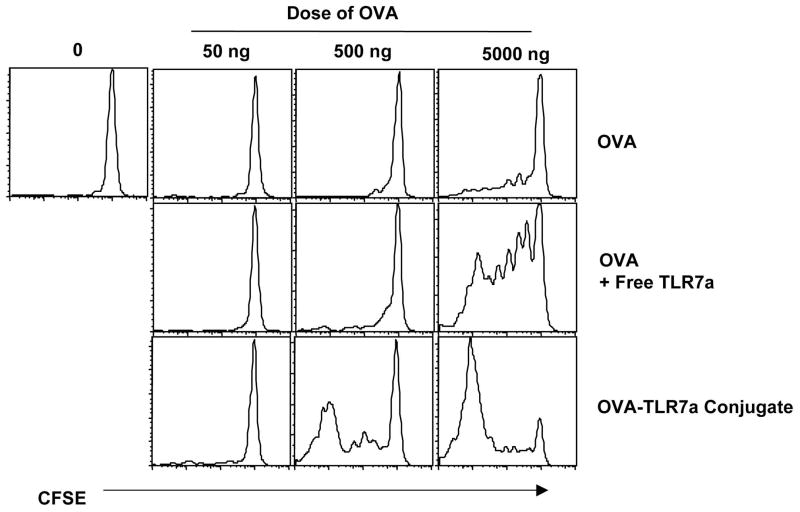
In related experiments, however, it was clear that conjugation increased the sensitivity of the responding T cells to limiting amounts of antigen. OTI T cells were adoptively transferred into naïve recipients followed by injection of limiting doses of the antigen either in combination with, or conjugated to, the TLR7 agonist. The starting dose of OVA (5 μg) elicited very little response from the transferred cells (Fig. 3). While the addition of the TLR7 agonist simply increased the response of the transferred cells to only the highest dose of OVA, the transferred cells responded vigorously to a 10 fold lower dose of the conjugate (Fig. 3). Thus, the differences in the effective dose of the antigen required to stimulate the OTI T cells were exponentially lower for the conjugated than the unconjugated antigen. Collectively, these data indicate that the conjugate facilitates an increased sensitivity to antigen by the responding T cells (Fig. 3) without the formation of an antigen depot (Fig. 2).
Figure 3. Conjugation increases sensitivity of responding T cells to limiting amounts of antigen.
Mice injected with CFSE-labeled OTI T cells were challenged with the indicated amounts of the various forms of antigen as shown. Three days after transfer of OTI T cells, cells from the dLN were isolated and the OTI T cells assessed for the loss of CFSE as an indication of the presence of antigen in the host. The histograms shown were gated on live, CD8+, SIINFEKL-Kb tetramer+ events. The data shown are representative of three independent experiments.
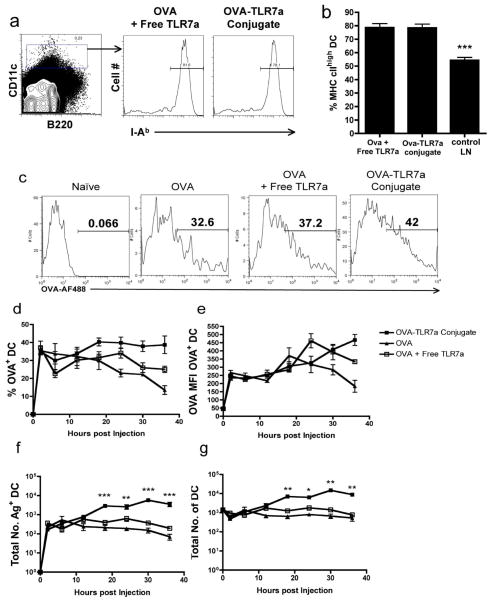
We (5, 7, 25) and others (4) have demonstrated the potency with which administration of free TLR7 agonist mediates the activation of DCs in vivo. However, it was still possible that the conjugation of the TLR7 agonist enhanced its immuno-stimulatory properties on the DCs. To test this hypothesis, we compared the level of DC activation induced by the unconjugated and conjugated forms of the TLR7 agonist. Using the level of MHC class II expression as a measure of DC activation, we found that both forms activated DCs equally well (Fig. 4a,b). Nearly 80% of the DCs had significantly up-regulated surface expression of I-Ab by 18 hours following immunization with either the unconjugated or conjugated forms of the TLR7 agonist, a dramatic difference from the phenotype observed in non-draining LNs. Similar results were obtained for the expression of costimulatory markers such as CD86 (data not shown and (5, 7, 25)). Therefore, the data suggest that conjugation status of the TLR7 agonist does not influence the maturation state of the DCs in a fashion that is consistent with the binary outcomes in cross-priming of CD8+ T cells (Fig. 1a).
Figure 4. Conjugation does not affect antigen uptake but enhances DC accumulation in dLNs.
(a–g) Mice were immunized in the footpad with 5 μg of Alexa-Fluor488-labeled OVA with the unconjugated TLR7 agonist or with the Alexa-Fluor488-OVA-TLR7a conjugate. At (a,b) 18 hours or (c–g) the indicated time points following immunization, popliteal LN draining the foot were harvested, minced, and digested with collagenase/DNase. (a) The histograms shown were generated by gating on CD11c+ cells and are representative of four lymph nodes from each treatment group. (b) Graphed values of the frequency of DCs expressing high levels of the MHC class II molecule, I-Ab, are expressed as the mean ± SEM. The frequency of antigen-bearing DCs shown in (c) representative histograms and (d) the percent of DCs harboring the antigen were calculated based on CD11c+ expression profiles. (e) The mean fluorescence intensity values of OVA-AF488 was derived from CD11c+ Alexa-Fluor 488+ gated events. The total numbers of (f) antigen-bearing DCs and (g) the entire DC population were calculated based on percent of CD11c+ or Alexa-Fluor 488+ gated events. Statistical analyses were performed as described in Materials and Methods. The summary of P values, as indicated by (*), denote statistical significance of differences between the means of the conjugate and OVA + TLR7 agonist treatments. The data shown are representative of three independent experiments.
Although the immuno-stimulatory activities between the two forms are comparable, a possible explanation for the success of the conjugate over the free TLR7 agonist may be related to differences in their capacity to influence overall uptake of the antigen by APCs. To address this, we labeled OVA with the fluorochrome, Alexa-Fluor488, with or without further conjugation to the TLR7 agonist, and tracked its uptake by DCs in immunized mice. Using the labeled OVA, we observed a rapid uptake by DCs, regardless of whether the TLR7 agonist was conjugated, unconjugated, or absent (Fig. 4c,d). OVA was taken up by approximately 30–40% of the DCs present in the draining lymph node (dLN). While this percentage was enhanced to some degree in mice injected with the OVA-TLR7a conjugate, the higher level of uptake was observed only at later time points (Fig. 4d). Furthermore, the overall mean fluorescence intensity (MFI) values for the fluorochrome-labeled OVA in the conjugated and unconjugated forms did not differ in antigen-bearing DCs at all time points examined (Fig. 4e), suggesting that the amount of antigen on a per cell basis is not influenced by the conjugation status of the TLR7 agonist. Interestingly, we found this to be true not only for the bulk population of DCs, but also for the multiple subsets of DCs present in skin-dLNs (Supplemental Fig. 1). Collectively, the data suggest that conjugation had a relatively minimal impact on either the proportion of DCs that take up the antigen or the duration of antigen uptake by the DCs when compared to the unconjugated form of the antigen. However, we noticed that conjugation had a more significant impact on the frequency of DCs in the dLN (Fig. 4f). As much as a 10 fold increase in the number of DCs was observed in mice immunized with the OVA-TLR7a conjugate when compared to either OVA alone or with the unconjugated TLR7 agonist (Fig. 4f). Thus, the overall antigen load appears to be higher in OVA-TLR7a conjugate-immunized mice, likely due more to the sizable increase in the number of DCs than to the relatively minor increase in the proportion of DCs acquiring antigen (Fig. 4f,g).
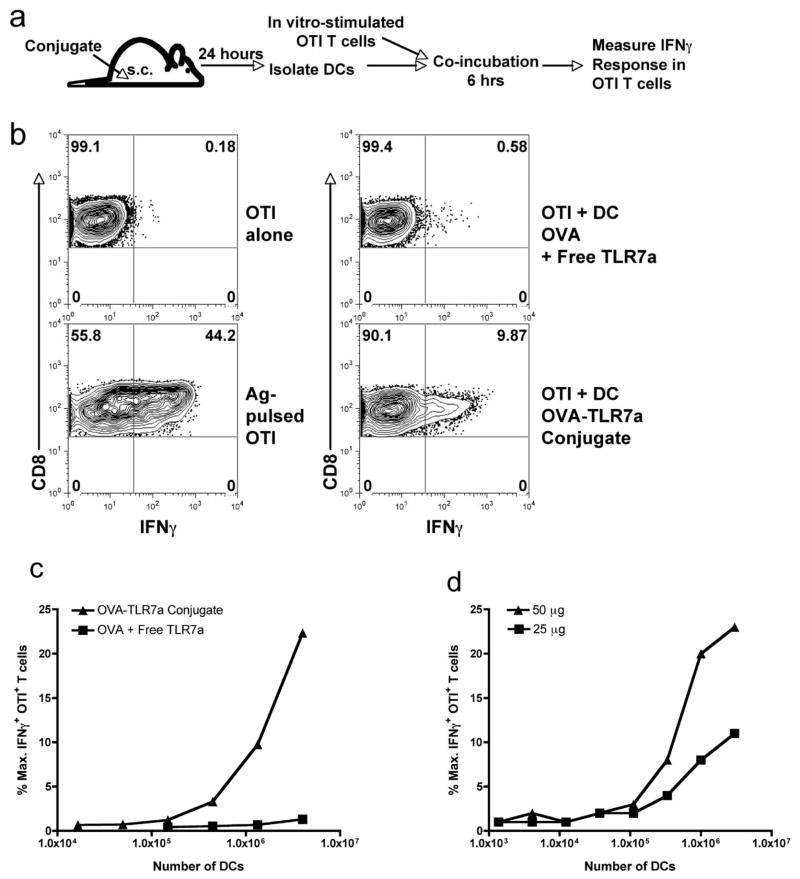
Taken together, the data show that while the conjugate has at best a two-fold enhancement of antigen uptake over time, it enhances both DC accumulation (Fig. 4) and sensitivity of T cells to antigen (Fig. 3) by at least ten fold. Thus it was plausible that the increased sensitivity to antigen was simply due to the increased numbers of antigen-bearing DCs. Alternatively, the increased sensitivity to antigen may be due to the capacity of the conjugate to enhance DC antigen processing and cross-presentation on a per cell basis. To test this possibility, we developed an assay to quantitate cross-presentation in vivo following immunization with the conjugate and non-conjugate forms of antigen. Mice were immunized subcutaneously using OVA either combined with or conjugated to the TLR7 agonist. The DCs from the dLNs were isolated 24 hours following immunization, mixed with in vitro stimulated effector OTI cells, and the magnitude of IFNγ production by the T cells was measured (Fig. 5a). Because we used effector T cells in this assay, the presentation of antigen by MHC class I is, alone, sufficient for the induction of IFNγ production by the T cells (Fig. 5b). As such, the response of the T cells can be used as a functional readout of the amount of peptide-MHC complexes on the DC cell surface.
Figure 5. Conjugation enhances efficiency of cross-presentation by DCs.
(a) A schematic outline of the method used to assess the level of antigen cross-presentation by DCs ex vivo. Mice were immunized in the footpad with 50 μg of the OVA-TLR7a conjugate or the unconjugated mixture of OVA and TLR7 agonist. Twenty-four hours following immunization, popliteal LNs were harvested, the cell suspensions from each mouse group pooled, and the DCs purified using anti-CD11c magnetic beads. In some experiments, DCs were purified using a cell sorter (MoFlow). Enriched DCs were incubated (b) at 4.0 × 106 cells per well or (c) at the indicated titration of cell numbers with 0.5 × 106 effector OTI T cells. The cells were co-incubated for 4 hours in the presence of brefeldin A and then stained for intracellular IFNγ. Production of IFNγ by OTI T cells was assessed by gating on expression of the congenic marker CD45.1. The level of IFNγ response by the OTI T cells was expressed as a percent of the maximal production of IFNγ induced by DCs pulsed with the SIINFEKL peptide. (d) Cross-presentation by DCs from mice immunized with either 50 μg or 25 μg of OVA- TLR7a conjugate was assessed as in (a–c). The data shown are representative of four independent experiments.
As expected, DCs purified from mice immunized with the OVA-TLR7a conjugate exhibited a high level of antigen cross-presentation (Fig. 5b,c). In contrast, the unconjugated mixture of TLR7 agonist and OVA, despite its level of antigen-uptake into the DCs (Fig. 4), did not result in cross-presentation. OTI T cells were incubated with a titration of increasing cell numbers of DCs (Fig. 5c), and even the highest titration of DCs used (4 × 106 cells) did not stimulate any OTI IFNγ production (Fig. 5b,c). This is significant since we found, at the 24 hour time point, relatively similar levels of antigen uptake on a per cell basis between the unconjugated and conjugated forms of the antigen (Fig. 4d,e). In addition, when we lowered the amount of conjugate injected to more closely match the amount of antigen uptake observed using the unconjugated form of the antigen, the DCs still cross-presented antigen well above background in response to the conjugate immunization (Fig. 5e).
We make two conclusions from these results. First, the observed failure of DCs to cross-present antigen delivered with the unconjugated TLR7 agonist is not due to the antigen uptake falling below some critical threshold. Second, the DCs from the conjugate immunized host have an increased capacity to process and present antigen on a per-cell basis as compared to the DCs from hosts immunized with the unconjugated form of the antigen. The ability to assess DC function on a per-cell basis allows the conclusion that, while the conjugate also augments the accumulation of antigen-bearing DCs in dLNs, the critical parameter underlying the efficacy of the conjugated TLR7 agonist is its enabling of cross-presentation. Thus, the phenotype of DC cross-presentation highly mirrors the phenotype of the T cell responses generated by the two forms of vaccination; both functional outcomes (cross presentation and T cell expansion) display an all-or-none type of a response. The conjugated TLR7 agonist elicited a robust functional response in both assays, while the unconjugated form did not elicit any detectable responses.
DISCUSSION
Mechanisms by which vaccine adjuvants enhance the immunogenicity of antigens have been examined for several decades. However, a full understanding of the key processes underlying their efficacy remains unclear and under debate. Early studies have implicated the formation of antigen depots as the underlying cause (reviewed in (26)). These findings have led to the proposal that adjuvants enhance the immune response through prolonging the persistence of antigen in vivo. Additionally, antigen depots are thought to form through aggregation, which may affect the mode and efficiency of uptake by APCs (27, 28). However, more recent data suggest that the activation of inflammation via various innate cells and factors is the component dictating the success of an adjuvant (29). To this end, experimental approaches used to identify alternative vaccine adjuvants focus now largely on characterizing innate immune receptor signaling pathways and what corresponding impact candidate adjuvants targeting these pathways have on the downstream adaptive immune system.
Targeting TLRs through the use of either natural or synthetic agonists have been widely explored as a potential alternative vaccination strategy to induce cellular immunity. This approach has drawn much attention on the basis that TLR stimulation results in robust activation of innate immune cells and the production of pro-inflammatory cytokines (2–5). However, the assumption that simply inducing these innate immune responses results in cellular adaptive immunity appears to be misplaced. This is particularly evidenced by the failure of TLR agonists to elicit CD8+ and CD4+ T cell responses when administered as a mixture with protein antigens into hosts (6, 7). Therefore, activation of the innate immune system by these agonists alone is insufficient to effectively induce cellular adaptive immune responses.
Previous studies by us (6, 8) and others (9–16) have shown that any demonstration of these agonists to elicit CD8+ T cell responses requires some kind of modification to the way in which the antigen and the TLR agonist are delivered. These include coupling the two components together through chemical conjugation, adsorption by synthetic microparticles, formation of aggregates or antigen depots, or the combinatorial use of anti-CD40 monoclonal antibodies. However, the current assessment of how these methods elicit cellular immune responses is gravely limited and fails to explain the inability of administering the TLR agonist alone to induce such responses. In the present study, we demonstrated the efficacy of co-delivering a synthetic TLR7 agonist with a soluble protein antigen through chemical conjugation as a means to establish cellular immunity. In the process of directly comparing this approach to the unconjugated mixture of the TLR7 agonist, we identified recruitment of DCs and activation of cross-presentation by DCs as two key determinants governing the success and failure of the conjugated and unconjugated forms of the TLR agonist, respectively. Our data suggest that although efficient uptake of antigen by APCs and inflammation are necessary, recruiting sufficient number of DCs and enabling antigen-bearing DCs to cross-present the acquired antigen are paramount to induce CD8+ T cell responses.
DC recruitment and cross-presentation are undoubtedly necessary components mediating CD8+ T cell responses to exogenous antigens. Of all parameters critical for vaccine-mediated immunity, the induction of cross-presentation, in particular, has long been known to hold some level of importance. However, our data elevate this parameter to a previously unappreciated degree of significance. Namely, when all other factors are made equal (antigen duration, antigen uptake, DC phenotype, DC numbers), the simple capacity of a given vaccination method to facilitate cross-presentation is the dominant factor dictating its success or failure. These results therefore provide a new focus for future vaccine adjuvant discovery and development.
Supplementary Material
References
- 1.Diebold S, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 2.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 3.Ito T, Amakawa R, Kaisho T, Hemmi H, Tajima K, Uehira K, Ozaki Y, Tomizawa H, Akira S, Fukuhara S. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med. 2002;195:1507–1512. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, Akira S, Reis e Sousa C. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol. 2003;33:827–833. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 5.Doxsee C, Riter T, Reiter M, Gibson S, Vasilakos J, Kedl R. The immune response modifier and toll-like receptor 7 agonist S-27609 selectively induces IL-12 and TNF-a production in CD11c+ CD11b+ CD8− dendritic cells. Journal of Immunology. 2003;171:1156–1163. doi: 10.4049/jimmunol.171.3.1156. [DOI] [PubMed] [Google Scholar]
- 6.Wille-Reece U, Wu C, Flynn B, Kedl R, Seder R. Immunization with HIV-1 Gag Protein Conjugated to a TLR7/8 Agonist Results in the Generation of HIV-1 Gag-Specific Th1 and CD8 T Cell Responses. Journal of Immunology. 2005:7676–7683. doi: 10.4049/jimmunol.174.12.7676. [DOI] [PubMed] [Google Scholar]
- 7.Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, Vasilakos JP, Noelle RJ, Kedl RM. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199:775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci U S A. 2005;102:15190–15194. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tighe H, Takabayashi K, Schwartz D, Marsden R, Beck L, Corbeil J, Richman DD, Eiden JJ, Jr, Spiegelberg HL, Raz E. Conjugation of protein to immunostimulatory DNA results in a rapid, long-lasting and potent induction of cell-mediated and humoral immunity. Eur J Immunol. 2000;30:1939–1947. doi: 10.1002/1521-4141(200007)30:7<1939::AID-IMMU1939>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Shirota H, Sano K, Hirasawa N, Terui T, Ohuchi K, Hattori T, Shirato K, Tamura G. Novel roles of CpG oligodeoxynucleotides as a leader for the sampling and presentation of CpG-tagged antigen by dendritic cells. J Immunol. 2001;167:66–74. doi: 10.4049/jimmunol.167.1.66. [DOI] [PubMed] [Google Scholar]
- 11.Jackson DC, Lau YF, Le T, Suhrbier A, Deliyannis G, Cheers C, Smith C, Zeng W, Brown LE. A totally synthetic vaccine of generic structure that targets Toll-like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc Natl Acad Sci U S A. 2004;101:15440–15445. doi: 10.1073/pnas.0406740101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan S, Bijker MS, Weterings JJ, Tanke HJ, Adema GJ, van Hall T, Drijfhout JW, Melief CJ, Overkleeft HS, van der Marel GA, Filippov DV, van der Burg SH, Ossendorp F. Distinct uptake mechanisms but similar intracellular processing of two different toll-like receptor ligand-peptide conjugates in dendritic cells. J Biol Chem. 2007;282:21145–21159. doi: 10.1074/jbc.M701705200. [DOI] [PubMed] [Google Scholar]
- 13.Tighe H, Takabayashi K, Schwartz D, Van Nest G, Tuck S, Eiden JJ, Kagey-Sobotka A, Creticos PS, Lichtenstein LM, Spiegelberg HL, Raz E. Conjugation of immunostimulatory DNA to the short ragweed allergen amb a 1 enhances its immunogenicity and reduces its allergenicity. J Allergy Clin Immunol. 2000;106:124–134. doi: 10.1067/mai.2000.107927. [DOI] [PubMed] [Google Scholar]
- 14.Maurer T, Heit A, Hochrein H, Ampenberger F, O’Keeffe M, Bauer S, Lipford GB, Vabulas RM, Wagner H. CpG-DNA aided cross-presentation of soluble antigens by dendritic cells. Eur J Immunol. 2002;32:2356–2364. doi: 10.1002/1521-4141(200208)32:8<2356::AID-IMMU2356>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 15.Heit A, Schmitz F, O’Keeffe M, Staib C, Busch DH, Wagner H, Huster KM. Protective CD8 T cell immunity triggered by CpG-protein conjugates competes with the efficacy of live vaccines. J Immunol. 2005;174:4373–4380. doi: 10.4049/jimmunol.174.7.4373. [DOI] [PubMed] [Google Scholar]
- 16.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 17.Heit A, Maurer T, Hochrein H, Bauer S, Huster KM, Busch DH, Wagner H. Cutting edge: Toll-like receptor 9 expression is not required for CpG DNA-aided cross-presentation of DNA-conjugated antigens but essential for cross-priming of CD8 T cells. J Immunol. 2003;170:2802–2805. doi: 10.4049/jimmunol.170.6.2802. [DOI] [PubMed] [Google Scholar]
- 18.Burgdorf S, Scholz C, Kautz A, Tampe R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 2008;9:558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 19.Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods. 1990;132:191–195. doi: 10.1016/0022-1759(90)90029-u. [DOI] [PubMed] [Google Scholar]
- 20.Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrington LE, Most R, Whitton RvJL, Ahmed R. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J Virol. 2002;76:3329–3337. doi: 10.1128/JVI.76.7.3329-3337.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zehn D, Bevan MJ. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity. 2006;25:261–270. doi: 10.1016/j.immuni.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potter TA, Grebe K, Freiberg B, Kupfer A. Formation of supramolecular activation clusters on fresh ex vivo CD8+ T cells after engagement of the T cell antigen receptor and CD8 by antigen-presenting cells. Proc Natl Acad Sci U S A. 2001;98:12624–12629. doi: 10.1073/pnas.221458898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez PJ, McWilliams JA, Haluszczak C, Yagita H, Kedl RM. Combined TLR/CD40 stimulation mediates potent cellular immunity by regulating dendritic cell expression of CD70 in vivo. J Immunol. 2007;178:1564–1572. doi: 10.4049/jimmunol.178.3.1564. [DOI] [PubMed] [Google Scholar]
- 26.Schijns VE, Tangeras A. Vaccine adjuvant technology: from theoretical mechanisms to practical approaches. Dev Biol (Basel) 2005;121:127–134. [PubMed] [Google Scholar]
- 27.Shen L, Rock KL. Priming of T cells by exogenous antigen cross-presented on MHC class I molecules. Curr Opin Immunol. 2006;18:85–91. doi: 10.1016/j.coi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Rock KL. Exiting the outside world for cross-presentation. Immunity. 2006;25:523–525. doi: 10.1016/j.immuni.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Stills HF., Jr Adjuvants and antibody production: dispelling the myths associated with Freund’s complete and other adjuvants. ILAR J. 2005;46:280–293. doi: 10.1093/ilar.46.3.280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.