Abstract
Background: Plasma exchange is used in the treatment of diseases mediated by pathogenic circulating proteins, or for transplant desensitization. Its non-targeted nature results in the depletion of physiologically important molecules, and it is often complicated by hypocalcaemia.
Aim: To determine the effects of plasma exchange on vitamin D binding protein (DBP) and associated vitamin D metabolites.
Design: Single-centre prospective cohort study of 11 patients.
Methods: DBP and vitamin D metabolites were measured before and immediately after five plasma exchanges, and 7 and 28 days after discontinuation of plasma exchange.
Results: Plasma exchange reduced plasma DBP concentration from 196.9 ± 53.2 to 98.5 ± 34 μg/ml (P = 0.0001), 1,25-dihydroxyvitamin D from 103 ± 52 to 42 ± 4 pmol/l (P = 0.003) and 25-hydroxyvitamin D from 49.7 ± 29 to 22 ± 9.4 nmol/l (P = 0.0017), through their removal in effluent. After 7 days, DBP and 1,25-dihydroxyvitamin D were not significantly different from baseline, but 25-hydroxyvitamin D remained significantly lower after 7 days (26.4 ± 9.8 nmol/l, P = 0.02) and 28 days (30.8 ± 15.5 nmol/l, P = 0.048). Corrected calcium decreased from 2.23 ± 0.11 to 1.98 ± 0.08 mmol/l (P = 0.0007) immediately after five treatments. Plasma calcium was significantly associated with 1,25-dihydroxyvitamin D (r2 = 0.79, P < 0.0001).
Conclusions: Plasma exchange induced an acute reversible decrease in plasma 1,25-dihydroxyvitamin D, DBP, calcium and a sustained decrease in plasma 25-hydroxyvitamin D.
Background
Plasma exchange is used for the treatment of diseases mediated by circulating pathogenic proteins and is a recommended therapy for auto-immune diseases such as severe anti-neutrophil cytoplasmic antibody-mediated vasculitis (AAV),1 anti-glomerular basement membrane disease (Goodpasture’s syndrome),2,3 immune-mediated neuropathies4,5 and myasthenia gravis6; dysproteinaemias7; thrombotic thrombocytopenic purpura and the haemolytic uremic syndrome;8,9 and in HLA-sensitized or blood group incompatible transplantation, for pre-operative desensitization and treatment of antibody-mediated organ rejection.10 Plasma exchange removes the plasma component of blood by extracorporeal circulation through a cell separator or semi-permeable filter. The cellular blood components are returned to the patient with the infusion of replacement fluid, usually electrolyte balanced human albumin solution or (less frequently) donor plasma. This approach effectively removes medium and large molecular weight proteins (up to 1000 kDa) including pathogenic antibodies.11 However, it results in the collateral depletion of many physiologically important molecules which may include vitamin binding protein, and its indiscriminate nature contributes to a number of well-characterized complications.
Common complications of plasma exchange include symptomatic hypocalcaemia, muscle cramp, urticaria and hypotension,12–14 and occur in up to 12% of plasma exchange treatments.12 Hypocalcaemia is conventionally attributed to the binding of ionized calcium to the citrate used as anticoagulant. Most patients require administration of intravenous calcium to treat or prevent symptomatic hypocalcaemia, although hypocalcaemia may still occur and persist after completion of plasma exchange.
Vitamin D may impact on patient outcomes in many plasma exchange-treated diseases. Its deficiency may potentiate steroid induced osteoporosis and increases fracture rates,15,16 results in impaired innate immunity and increased infection rates,17–19 and may be permissive to the development and activity of autoimmune diseases such as multiple sclerosis,20 AAV21 and systemic lupus erythematosus.22 Taken together, these data provide a strong rationale for the prevention of vitamin D deficiency in plasma exchange-treated disease.
Vitamin D metabolites are transported in plasma by carrier proteins.23 The most important of these is the 56 kDa vitamin D binding protein (DBP), which carries 99% of vitamin D metabolites in the circulation.18,24 DBP binding protects vitamin D metabolites from hydroxylase-mediated catabolism, and their half-life may therefore be decreased by a reduction in DBP. Furthermore, removal of DBP and associated vitamin D metabolites would reduce plasma vitamin D metabolite concentrations directly, similar to the situation in nephrotic syndrome where urinary losses of DBP result in vitamin D deficiency through concomitant urinary loss of vitamin D metabolites.25 The effect of plasma exchange on DBP is not known; however, since plasma exchange readily depletes proteins of a similar size, we hypothesized that plasma exchange would induce vitamin D deficiency through DBP depletion.
Methods
We performed a prospective cohort study of 11 patients receiving plasma exchange at Addenbrooke’s hospital, Cambridge, between June 2010 and March 2011. Patients aged 18 or older were enrolled in the study if they required five or more exchanges and were able to provide informed consent. Patients were excluded if they had received plasma exchange within the preceding 12 months, had known genetic defects of the vitamin D endocrine system, hypercalcaemia (defined as albumin-corrected calcium >2.6 mmol/l) or were receiving treatment with any vitamin D-containing compound including cholecalciferol, ergocalciferol, vitamin D-containing calcium supplements, alfacalcidol, 1,25-dihydroxyvitamin D or paricalcitol, vitamin D-containing over-the-counter supplements, calcimimetic agents or parathyroid hormone (PTH)-related compounds. The Cambridgeshire Regional Research Ethics Committee approved the study.
Demographic and clinical data including age, gender, ethnicity, diagnosis, co-morbidity and indication for plasma exchange were recorded. All patients received single plasma volume exchanges using continuous flow centrifugation with the COBE Spectra apheresis system (Terumo, Zaventem, Belgium). One volume of Acid Citrate Dextrose (ACD-A) was used with every 10 volumes of electrolyte-balanced human albumin solution volume to prevent coagulation. All patients received Zenalb® 4.5 human albumin solution exclusively as replacement fluid; no patient received donor plasma. Zenalb® 4.5 contains 4.5% human albumin to at least 95% purity, and contains sodium 140 mmol/l, potassium, chloride citrate and sodium n-octanoate. Zenalb® 4.5 contains no calcium, DBP or vitamin D metabolites (Rosalind Sackey, personal communication; Bio Products Laboratory, Hertfordshire, UK). Prophylaxis against hypocalcaemia was given with a minimum of 20 ml 10% intravenous calcium gluconate per treatment and was increased if hypocalcaemia occurred.
Non-fasting blood samples were collected in Lithium Heparin or EDTA bottles, and processed as described previously.26 Plasma exchange effluent was collected in Lithium Heparin bottles at the end of each treatment. Laboratory determinations included urea, creatinine, haemoglobin, albumin, calcium, phosphate, PTH, 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D and DBP. We determined these parameters before the onset of treatment and before and immediately after every plasma exchange for the first three patients, and subsequently before and immediately after the first and fifth treatment. After completion of plasma exchange, biochemical parameters were again measured after 7 and 28 days.
Vitamin D metabolites and DBP were also measured in plasma effluent after the first treatment, and the effluent volume recorded. Albumin corrected calcium (normal range 2.1–2.5 mmol/l), phosphate (normal range 0.8–1.4 mmol/l) and creatinine (normal range 35–125 μmol/l) were analysed using an autoanalyser (Dimension, Siemens Healthcare Diagnostics, Surrey, UK). Glomerular filtration rate was estimated from plasma creatinine (eGFR) using the four-variable Modified Diet in Renal Disease study formula. 1,25-Dihydroxyvitamin D, 25-hydroxyvitamin D and DBP analyses were conducted in duplicate. 1,25-Dihydroxyvitamin D was measured by radioimmunoassay (IDS Gamma-B, Tyne and Weir, UK) with inter- and intra-assay coefficient of variation (CV) of 4% and 9%, respectively. DBP concentration was determined by ELISA (R & D Systems, Oxford, UK) with both an inter- and intra-assay CV of 6%. 25-Hydroxyvitamin D was measured by chemiluminescent assay (LiasonDiaSorin, Stillwater, MN, USA) with an inter- and intra-assay CV of 3% and 6%, respectively. PTH (reference range 16–87 ng/l) was measured on an automatic chemiluminescent assay (Siemens Healthcare Diagnostics) with an inter-assay CV of 8%. Patients at risk of Vitamin D deficiency and insufficiency were defined as a 25-hydroxyvitamin D concentration of <25 or 50 nmol/l, respectively.
Data were analysed using Stata SE v12.1 (College Station, TX, USA) and are presented as mean ± SD or median (IQR) as appropriate for their distributions. The effects of plasma exchange on plasma 1,25-dihydroxyvitamin D, DBP and 25-hydroxyvitamin D concentrations were estimated using linear regression and are presented as coefficients ± standard error. Since the plasma concentration of PTH is influenced by renal function,27 changes in PTH were determined using mixed effects linear regression models for repeated measures of eGFR. Pre- and post-plasma exchange values were compared using a two-sided paired Student’s t-test for parametric data, and the Wilcoxon matched-pairs signed rank test for non-parametric data. Mean values at post-plasma exchange time points (1, 7 and 28 days) were compared using repeated measures ANOVA. P-values <0.05 for two-sided tests and <0.025 for one-sided tests were considered statistically significant.
Results
Eleven caucasian patients aged 59 ± 13 years were included in the study, of which seven were male. Indications for plasma exchange were AAV (n = 5), myasthenia gravis (n = 3), paraneoplastic neuropathy (n = 2) and voltage-gated potassium channel antibody-mediated encephalopathy (n = 1) (Table 1). All five patients with AAV had renal impairment (eGFR <60 ml/min/1.73 m2) at entry; three had an eGFR of <15, of which one was dialysis-dependent. The mean baseline plasma 1,25-dihydroxyvitamin D at entry was 119 (54–140) pmol/l. Baseline plasma DBP concentration was 206.5 ± 64.7 μg/ml, and 25-hydroxyvitamin D concentration was 49.7 ± 29 nmol/l. Vitamin D deficiency at entry of the study was identified in two, and insufficiency identified in four patients. Baseline PTH was 161 (98–343) ng/l, with the majority of values above the reference range. There was no correlation between baseline eGFR and 1,25-dihydroxyvitamin D (r2 = 0.37, P = 0.26) or 25-hydroxyvitamin D (r2 = −0.19, P = 0.57) concentrations, but PTH was inversely associated with eGFR (P = 0.005).
Table 1.
Patient characteristics
| Age | Gender | Diagnosis | Number of exchanges |
|---|---|---|---|
| 73 | M | ANCA-associated vasculitis (MPO) | 7 |
| 45 | M | Myasthenia gravis | 5 |
| 43 | F | ANCA-associated vasculitis (PR3) | 5 |
| 69 | M | ANCA-associated vasculitis (MPO) | 5 |
| 51 | M | Paraneoplastic neuropathy (neuro-endocrine) | 5 |
| 71 | M | Potassium channel antibody-associated encephalopathy (VGKC-Ab) | 5 |
| 64 | M | Myasthenia gravis | 5 |
| 63 | F | ANCA-associated vasculitis (PR3) | 7 |
| 73 | M | Myasthenia gravis | 5 |
| 63 | F | Paraneoplastic neuropathy (parotid acinic carcinoma associated anti-Ma2 antibody) | 5 |
| 38 | F | ANCA-associated vasculitis (PR3) | 7 |
MPO, myeloperoxidase; PR3, proteinase-3; VGKC-Ab, voltage gated potassium channel antibody.
Patients received a plasma exchange dose of 44.8 ± 10.8 ml/kg per treatment. All patients received purified human albumin solution exclusively as the exchange fluid. Three patients received seven treatments; eight received five treatments. There were no treatment withdrawals. One patient was lost to follow-up after completion of plasma exchange. Patient characteristics are shown in Table 2.
Table 2.
Patient characteristics and biochemical profile
| Entry | |
|---|---|
| Age (years) | 59 ± 13 |
| Renal function | |
| Creatinine (μmol/l) | 106 (71–379) |
| eGFR (ml/min/m2) | 56.9 ± 39.5 |
| 25-Hydroxyvitamin D (nmol/l) | 50.6 ± 30.1 |
| 1,25(OH)2 Vitamin D (pmol/l) | 103 ± 52 |
| Parathyroid hormone (pg/ml) | 161 (98–343) |
| DBP (μg/ml) | 196.9 ± 53.2 |
| Albumin (g/dl) | 32 ± 9 |
| Albumin corrected calcium (mmol/l) | 2.23 ± 0.11 |
| Phosphate (mmol/l) | 1.26 ± 0.28 |
| Haemoglobin (g/dl) | 11.4 ± 3.2 |
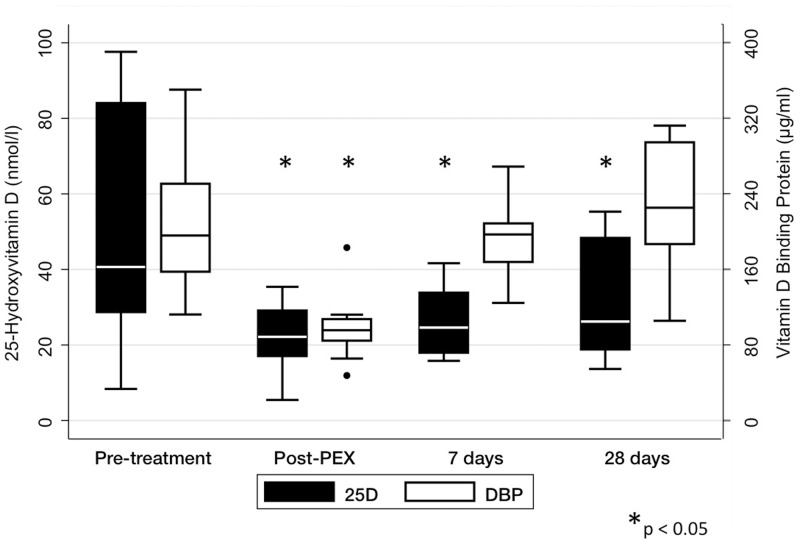
Five plasma exchange treatments reduced plasma 1,25-dihydroxyvitamin D from 103 ± 52 to 42 ± 4 pmol/l (P = 0.003). However, 1,25-dihydroxyvitamin D concentrations at 7 (83 ± 42 pmol/l, P = 0.38) and 28 days (75 ± 34 pmol/l, P = 0.46) after completion of plasma exchange did not differ significantly from baseline. Similarly, five treatments reduced plasma DBP levels (Figure 1) from 196.9 ± 53.2 to 98.5 ± 34 μg/ml (P = 0.0001), but after 7 (194 ± 47.8 μg/ml, P = 0.63) and 28 days (223.5 ± 69.9 μg/ml, P = 0.85), DBP did not differ from baseline. The plasma 25-hydroxyvitamin D concentration decreased from 49.7 ± 29 to 22 ± 9.4 nmol/l (P = 0.0017); however, in contrast to 1,25-dihydroxyvitamin D and DBP, it remained lower than baseline after 7 (26.4 ± 9.8 nmol/l, P = 0.02) and 28 days (30.8 ± 15.5 nmol/l, P = 0.048) (Figure 1). PTH was not significantly altered after five treatments (153 [118–230], P = 0.67), and did not differ from baseline after 7 (106 [50.8–124], P = 0.07) or 28 days (59 [37–208], P = 0.07). However, in a multilevel mixed effects regression model accounting for renal function, every plasma exchange resulted in a reduction in PTH of 28 ng/l (P = 0.001).
Figure 1.
Effect of plasma exchange on 25-hydroxyvitamin D and DBP. Statistical comparisons are made with baseline values for each variable. Plasma exchange significantly reduced DBP, although this had recovered to baseline after 7 days. In contrast, 25-hydroxyvitamin D concentrations remained significantly lower than baseline after 7 and 28 days.
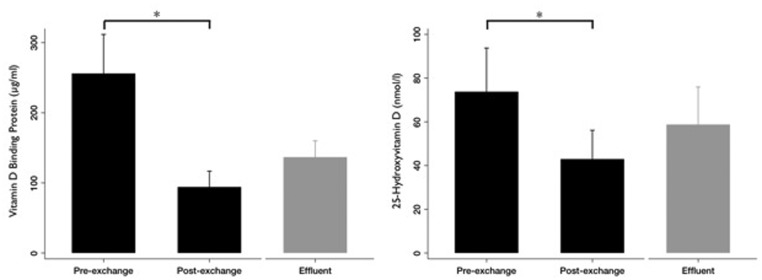
We next sought to determine whether the changes in plasma DBP and vitamin D metabolite concentrations were due to their removal in plasma. First, we tested this by measuring DBP and 25-hydroxyvitamin D in plasma exchange effluent in a subset consisting of the first three patients enrolled in our study (specified a priori). For these three patients, the pre-treatment plasma DBP and plasma 25-hydroxyvitamin D concentrations were 256 ± 97 μg/ml and 74 ± 35 nmol/l, respectively. The effluent contained 137 ± 40 μg/ml DBP and 59 ± 30 nmol/l 25-hydroxyvitamin D, confirming significant removal by plasma exchange (Figure 2). Second, we considered whether, for all patients, the number of plasma exchange treatments predicted post-treatment plasma concentrations of 1,25-dihydroxyvitamin D, DBP and 25-hydroxyvitamin D. Every plasma exchange treatment reduced plasma 1,25-dihydroxyvitamin D by 12.4 ± 3.4 pmol/l (P = 0.002), plasma DBP by 19.5 ± 4.8 μg/ml (P < 0.001) and plasma 25-hydroxyvitamin D by 5.7 ± 1.6 nmol/l (P = 0.001).
Figure 2.
Pre-exchange, effluent and post-exchange DBP and 25-hydroxyvitamin D values in a subset of three patients. Bars show mean (standard error) values of (A) DBP and (B) 25-hydroxyvitamin D levels in plasma before and after the first plasma exchange (black), and in the effluent from this exchange (grey).
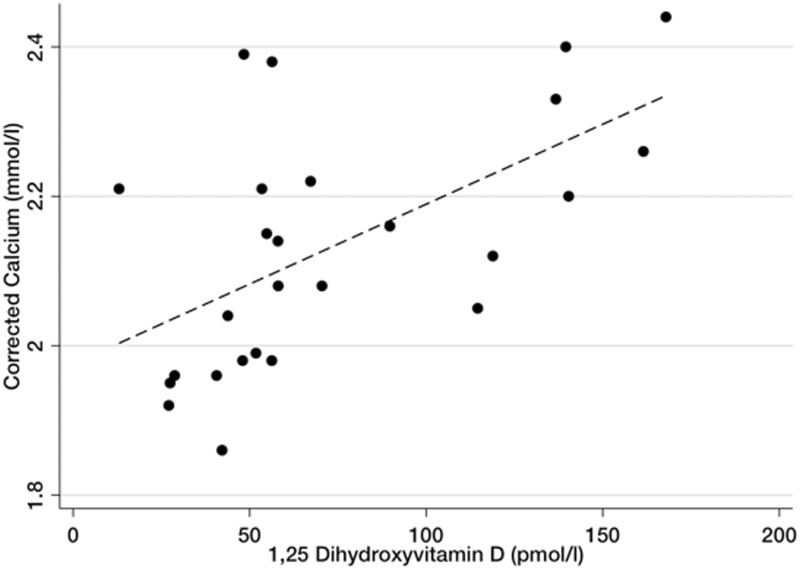
Given that we observed a marked decrease in 1,25-dihydroxyvitamin D and 25-hydroxyvitamin D concentrations and fluctuations in PTH with plasma exchange, we next asked whether depletion of vitamin D metabolites contributed to hypocalcaemia. In this study, we administered 28 ± 9 ml intravenous 10% calcium gluconate during each treatment. Despite this, we observed a decrease in albumin-corrected calcium from 2.23 ± 0.11 to 1.98 ± 0.08 mmol/l (P = 0.0007) after five plasma exchange treatments. Although corrected calcium tended to be lower 7 days after completion of plasma exchange (2.13 ± 0.18 mmol/l), this did not reach significance (P = 0.23). Across all time points, plasma 1,25-dihydroxyvitamin D concentrations correlated with corrected calcium (r2 = 0.79, P < 0.0001, Figure 3). Corrected calcium did not correlate with PTH (P = 0.67) or phosphate (P = 0.23).
Figure 3.
Relationship of plasma calcium and 1,25 dihydroxyvitamin D concentrations. Scatterplot and linear regression demonstrating the correlation of plasma corrected calcium and 1,25 dihydroxyvitamin D (r2 = 0.79, P = 0.0001, β-coefficient = 0.002). Values represent measurements for all patients from pre-treatment until immediately after the final plasma exchange.
Discussion
In this prospective cohort study, we evaluated the effect of plasma exchange on vitamin D status. A typical course of plasma exchange induced a sustained decrease in 25-hydroxyvitamin D concentration, and a transient decrease in 1,25-dihydroxyvitamin D, DBP and calcium concentrations. The mean 25-hydroxyvitamin D concentration of 22 ± 9.4 nmol/l after five plasma exchanges is within the range where clinical manifestations of vitamin D deficiency might be expected and, importantly, the 25-hydroxyvitamin D concentration remained depressed 4 weeks after plasma exchange. Furthermore, plasma exchange induced a marked reduction in 1,25-dihydroxyvitamin D, and plasma 1,25-dihydroxyvitamin D was closely associated with hypocalcaemia. Surprisingly, we found a decrease in PTH with plasma exchange and not association with hypocalcaemia. In addition to the direct removal of 25-hydroxyvitamin D with plasma effluent, two other factors are likely to contribute to a decrease in 25-hydroxyvitamin D: first, both hypocalcaemia and high PTH up-regulate the 25-hydroxyvitamin D 1α-hydroxylase (CYP27B1), leading to an increased conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D. Second, the half-life of 1,25-dihydroxyvitamin D and 25-hydroxyvitamin D is markedly reduced in DBP deficiency,28 through exposure of more unbound vitamin D metabolites to catabolism.
Consistent with previous reports, we observed a reduction in plasma calcium with plasma exchange. Hypocalcaemia is the commonest complication of plasma exchange and has been attributed to the use of citrate and, to a lesser extent, the removal of calcium in the plasma effluent.29,30 However, our data also demonstrate a strong association between post-plasma exchange hypocalcaemia and 1,25-dihydroxyvitamin D concentrations (Figure 3). 1,25-Dihydroxyvitamin D is indispensible for calcium homeostasis. Together with PTH, it is a key regulator of active intestinal trans-epithelial calcium absorption and renal tubular calcium reabsorption, and its deficiency results in hypocalcaemia. Our data suggest that acute 1,25-dihydroxyvitamin D deficiency may contribute to the hypocalcaemia observed with plasma exchange. This notion is supported by the finding that hypocalcaemia may persist for several days after completion of plasma exchange,31 whereas the plasma half-life of citrate is <40 min.32,33
Surprisingly, we found a decrease in PTH with plasma exchange despite reductions in calcium and 1,25 dihydroxyvitamin D and 25-hydroxyvitamin D concentrations. The expected increase in PTH is likely offset by the marked improvement in renal function observed in those subjects with significant renal impairment. Furthermore, PTH removal during plasma exchange, concomitant calcium gluconate infusion and fluctuation of other regulators of PTH secretion (such as phosphate) may have disrupted the normal parathyroid response to hypocalcaemia. Together with the removal of PTH by plasma exchange, these factors limit the inferences that can be drawn from the PTH response to plasma exchange.
Our findings should be interpreted within the limitations of our study. The study population was relatively small and heterogeneous in terms of diagnosis, renal function, season of recruitment and baseline vitamin D status. Our study did not include a control group not receiving plasma exchange. However, since we studied vitamin D status over a very short period of time and noted rapid changes in vitamin D concentrations within subjects coincident with plasma exchange, controlling for factors such as seasonality is unlikely to markedly affect our findings. There was some variation in the volume of plasma exchange and dose of calcium gluconate. Further, we did not follow patients beyond 4 weeks after plasma exchange.
The identification of a profound and sustained reduction in 25-hydroxyvitamin D and an acute but reversible reduction in 1,25-dihydroxyvitamin D reported here are of great clinical importance. The majority of patients receiving plasma exchange are also exposed to glucocorticoids and other immune modulating therapies that increase the risk of osteoporosis, fractures and infection. Vitamin D deficiency further increases these risks. Together with the mounting evidence that vitamin D deficiency predisposes to auto-immunity or to activity of auto-immune disease, our findings provide a strong rationale for both 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D supplementation during and after plasma exchange. However, the dose response in the setting of acute DBP depletion is not known. Studies to determine the optimal dosage regimen are urgently needed.
Acknowledgements
The Cambridgeshire Research Ethics Committee approved this study. All patients provided written informed consent. The study was supported by a project grant from the Addenbrooke’s Charitable Trust. T.F.H. is supported by the Cambridge Biomedical Research Centre and the National Institute of Health Research. We are grateful to Shailja Nigdikar, Janet Bennett and Ann Laidlaw for assistance with sample analysis, and to staff from the Addenbrooke’s hospital apheresis unit for their support of the study. The study was conceived and designed by T.F.H., D.R.W.J. and I.S. Patients were recruited by T.F.H., A.C. and D.R.W.J. P.B. carried out exchange treatments, and T.F.H., A.C. and P.B. collected and processed samples. Statistical analyses were carried out by T.F.H. T.F.H. drafted the manuscript, and all authors contributed to intellectual content and data interpretation.
Funding
This work was supported by Addenbrooke's Charitable Trust (ACT-9788).
Conflict of interest: None declared.
References
- 1.Jayne DR, Gaskin G, Rasmussen N, Abramowicz D, Ferrario F, Guillevin L, et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007;18:2180–8. doi: 10.1681/ASN.2007010090. [DOI] [PubMed] [Google Scholar]
- 2.Pusey CD, Levy JB. Plasmapheresis in immunologic renal disease. Blood Purif. 2012;33:190–8. doi: 10.1159/000334155. [DOI] [PubMed] [Google Scholar]
- 3.Pagnoux C. Plasma exchange for systemic lupus erythematosus. Transfus Apher Sci. 2007;36:187–93. doi: 10.1016/j.transci.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Yuki N, Hartung HP. Guillain-Barre syndrome. N Engl J Med. 2012;366:2294–304. doi: 10.1056/NEJMra1114525. [DOI] [PubMed] [Google Scholar]
- 5.Cortese I, Chaudhry V, So YT, Cantor F, Cornblath DR, Rae-Grant A. Evidence-based guideline update: plasmapheresis in neurologic disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2011;76:294–300. doi: 10.1212/WNL.0b013e318207b1f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gajdos P, Chevret S, Toyka K. Plasma exchange for myasthenia gravis. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD002275. CD002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drew MJ. Plasmapheresis in the dysproteinemias. Ther Apher. 2002;6:45–52. doi: 10.1046/j.1526-0968.2002.00393.x. [DOI] [PubMed] [Google Scholar]
- 8.Radhi M, Carpenter SL. Thrombotic microangiopathies. ISRN Hematol. 2012;2012:310596. doi: 10.5402/2012/310596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med. 1991;325:393–7. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 10.Roberts DM, Jiang SH, Chadban SJ. The treatment of acute antibody-mediated rejection in kidney transplant recipients—a systematic review. Transplantation. 2012;94:775–83. doi: 10.1097/TP.0b013e31825d1587. [DOI] [PubMed] [Google Scholar]
- 11.Derksen RH, Schuurman HJ, Meyling FH, Struyvenberg A, Kater L. The efficacy of plasma exchange in the removal of plasma components. J Lab Clin Med. 1984;104:346–54. [PubMed] [Google Scholar]
- 12.Sutton DM, Nair RC, Rock G. Complications of plasma exchange. Transfusion. 1989;29:124–7. doi: 10.1046/j.1537-2995.1989.29289146829.x. [DOI] [PubMed] [Google Scholar]
- 13.Huestis DW. Mortality in therapeutic haemapheresis. Lancet. 1983;1:1043. doi: 10.1016/s0140-6736(83)92664-8. [DOI] [PubMed] [Google Scholar]
- 14.Mokrzycki MH, Kaplan AA. Therapeutic plasma exchange: complications and management. Am J Kidney Dis. 1994;23:817–27. doi: 10.1016/s0272-6386(12)80135-1. [DOI] [PubMed] [Google Scholar]
- 15.Tse SM, Kelly HW, Litonjua AA, Van Natta ML, Weiss ST, Tantisira KG. Corticosteroid use and bone mineral accretion in children with asthma: effect modification by vitamin D. J Allergy Clin Immunol. 2012;130:53–60. doi: 10.1016/j.jaci.2012.04.005. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reginster JY, Kuntz D, Verdickt W, Wouters M, Guillevin L, Menkes CJ, et al. Prophylactic use of alfacalcidol in corticosteroid-induced osteoporosis. Osteoporos Int. 1999;9:75–81. doi: 10.1007/s001980050118. [DOI] [PubMed] [Google Scholar]
- 17.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 19.Bergman P, Norlin AC, Hansen S, Rekha RS, Agerberth B, Bjorkhem-Bergman L, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2:e001663. doi: 10.1136/bmjopen-2012-001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–8. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 21.Gatenby PA, Lucas RM, Engelsen O, Ponsonby AL, Clements M. Antineutrophil cytoplasmic antibody-associated vasculitides: could geographic patterns be explained by ambient ultraviolet radiation? Arthritis Rheum. 2009;61:1417–24. doi: 10.1002/art.24790. [DOI] [PubMed] [Google Scholar]
- 22.Amital H, Szekanecz Z, Szucs G, Danko K, Nagy E, Csepany T, et al. Serum concentrations of 25-OH vitamin D in patients with systemic lupus erythematosus (SLE) are inversely related to disease activity: is it time to routinely supplement patients with SLE with vitamin D? Ann Rheum Dis. 2010;69:1155–7. doi: 10.1136/ard.2009.120329. [DOI] [PubMed] [Google Scholar]
- 23.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol. 2005;289:F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 24.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63:954–9. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- 25.Barragry JM, France MW, Carter ND, Auton JA, Beer M, Boucher BJ, et al. Vitamin-D metabolism in nephrotic syndrome. Lancet. 1977;2:629–32. doi: 10.1016/s0140-6736(77)92498-9. [DOI] [PubMed] [Google Scholar]
- 26.Yan L, Schoenmakers I, Zhou B, Jarjou LM, Smith E, Nigdikar S, et al. Ethnic differences in parathyroid hormone secretion and mineral metabolism in response to oral phosphate administration. Bone. 2009;45:238–45. doi: 10.1016/j.bone.2009.04.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel S, Barron JL, Mirzazedeh M, Gallagher H, Hyer S, Cantor T, et al. Changes in bone mineral parameters, vitamin D metabolites, and PTH measurements with varying chronic kidney disease stages. J Bone Miner Metab. 2011;29:71–9. doi: 10.1007/s00774-010-0192-1. [DOI] [PubMed] [Google Scholar]
- 28.Safadi FF, Thornton P, Magiera H, Hollis BW, Gentile M, Haddad JG, et al. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest. 1999;103:239–51. doi: 10.1172/JCI5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinstein R. Hypocalcemic toxicity and atypical reactions in therapeutic plasma exchange. J Clin Apher. 2001;16:210–1. doi: 10.1002/jca.10000. [DOI] [PubMed] [Google Scholar]
- 30.Bolan CD, Greer SE, Cecco SA, Oblitas JM, Rehak NN, Leitman SF. Comprehensive analysis of citrate effects during plateletpheresis in normal donors. Transfusion. 2001;41:1165–71. doi: 10.1046/j.1537-2995.2001.41091165.x. [DOI] [PubMed] [Google Scholar]
- 31.Papari M, Tretiakova M, Fedson S, Richa E, Husain A, Baron JM, et al. Persistent hypocalcemia associated with therapeutic plasma exchange performed to reduce HLA antibody levels in cardiac transplant recipients. Transfus Apher Sci. 2011;44:243–8. doi: 10.1016/j.transci.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Apsner R, Schwarzenhofer M, Derfler K, Zauner C, Ratheiser K, Kranz A. Impairment of citrate metabolism in acute hepatic failure. Wien Klin Wochenschr. 1997;109:123–7. [PubMed] [Google Scholar]
- 33.Kramer L, Bauer E, Joukhadar C, Strobl W, Gendo A, Madl C, et al. Citrate pharmacokinetics and metabolism in cirrhotic and noncirrhotic critically ill patients. Crit Care Med. 2003;31:2450–5. doi: 10.1097/01.CCM.0000084871.76568.E6. [DOI] [PubMed] [Google Scholar]