Abstract
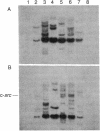
The effects of the src gene on the activity of protein kinase C and intercellular communication have been studied in transformed NIH/3T3 clones isolated from soft agar following transfection with the plasmid carrying the v-src gene (psrc-11). Six transformed clones that were studied contained newly incorporated v-src genes in the genome, had an increased amount of pp60src, and showed enhanced activities of protein kinase C. Intercellular communication, studied by observing with autoradiography the transfer of [3H]uridine nucleotide from prelabeled donor cells to recipient cells in contact, was found to be reduced in transformed clones as compared to parental NIH/3T3 cells. Treatment with phorbol 12-myristate 13-acetate was also found to increase protein kinase C activity and to reduce intercellular communication in normal NIH/3T3 cells. These results suggest that the v-src gene product, in a manner similar to some of the powerful tumor promoters, may directly or indirectly affect cell-cell communication.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. M., Menko A. S., Johnson R. G., Sheppard J. R., Sheridan J. D. Rapid and reversible reduction of junctional permeability in cells infected with a temperature-sensitive mutant of avian sarcoma virus. J Cell Biol. 1981 Nov;91(2 Pt 1):573–578. doi: 10.1083/jcb.91.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarnia R., Loewenstein W. R. Intercellular communication and the control of growth: XI. Alteration of junctional permeability by the src gene in a revertant cell with normal cytoskeleton. J Membr Biol. 1984;82(3):207–212. doi: 10.1007/BF01871630. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Blenis J., Erikson R. L. Phosphorylation of the ribosomal protein S6 is elevated in cells transformed by a variety of tumor viruses. J Virol. 1984 Jun;50(3):966–969. doi: 10.1128/jvi.50.3.966-969.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek C., Sachs L. The difference in contact inhibition of cell replication between normal cells and cells transformed by different carcinogens. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1705–1711. doi: 10.1073/pnas.56.6.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsaro C. M., Migeon B. R. Comparison of contact-mediated communication in normal and transformed human cells in culture. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4476–4480. doi: 10.1073/pnas.74.10.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mello W. C. Modulation of junctional permeability. Fed Proc. 1984 Sep;43(12):2692–2696. [PubMed] [Google Scholar]
- Decker S. Phosphorylation of ribosomal protein S6 in avian sarcoma virus-transformed chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4112–4115. doi: 10.1073/pnas.78.7.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F., Johnson M. S. Computer-based characterization of epidermal growth factor precursor. Nature. 1984 Feb 9;307(5951):558–560. doi: 10.1038/307558a0. [DOI] [PubMed] [Google Scholar]
- Enomoto T., Yamasaki H. Lack of intercellular communication between chemically transformed and surrounding nontransformed BALB/c 3T3 cells. Cancer Res. 1984 Nov;44(11):5200–5203. [PubMed] [Google Scholar]
- Fentiman I. S., Hurst J., Ceriani R. L., Taylor-Papadimitriou J. Junctional intercellular communication pattern of cultured human breast cancer cells. Cancer Res. 1979 Nov;39(11):4739–4743. [PubMed] [Google Scholar]
- Fujiki H., Tanaka Y., Miyake R., Kikkawa U., Nishizuka Y., Sugimura T. Activation of calcium-activated, phospholipid-dependent protein kinase (protein kinase C) by new classes of tumor promoters: teleocidin and debromoaplysiatoxin. Biochem Biophys Res Commun. 1984 Apr 30;120(2):339–343. doi: 10.1016/0006-291x(84)91259-2. [DOI] [PubMed] [Google Scholar]
- Jone C. M., Trosko J. E., Chang C., Fujiki H., Sugimura T. Inhibition of intercellular communication in Chinese hamster V79 cells by teleocidin. Gan. 1982 Dec;73(6):874–878. [PubMed] [Google Scholar]
- Kalimi G. H., Sirsat S. M. Phorbol ester tumor promoter affects the mouse epidermal gap junctions. Cancer Lett. 1984 Apr;22(3):343–350. doi: 10.1016/0304-3835(84)90173-3. [DOI] [PubMed] [Google Scholar]
- Keijzer W., Verkerk A., Bootsma D. Phenotypic correction of the defect in xeroderma pigmentosum cells after fusion with isolated cytoplasts. Exp Cell Res. 1982 Jul;140(1):119–125. doi: 10.1016/0014-4827(82)90163-x. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Krueger J. G., Wang E., Garber E. A., Goldberg A. R. Differences in intracellular location of pp60src in rat and chicken cells transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4142–4146. doi: 10.1073/pnas.77.7.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication and the control of growth. Biochim Biophys Acta. 1979 Feb 4;560(1):1–65. doi: 10.1016/0304-419x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- Macara I. G., Marinetti G. V., Balduzzi P. C. Transforming protein of avian sarcoma virus UR2 is associated with phosphatidylinositol kinase activity: possible role in tumorigenesis. Proc Natl Acad Sci U S A. 1984 May;81(9):2728–2732. doi: 10.1073/pnas.81.9.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Fitzgerald D. J. Tumor promoters inhibit metabolic cooperation in cocultures of epidermal and 3T3 cells. Biochem Biophys Res Commun. 1979 Nov 28;91(2):395–401. doi: 10.1016/0006-291x(79)91535-3. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Pitts J. D., Simms J. W. Permeability of junctions between animal cells. Intercellular transfer of nucleotides but not of macromolecules. Exp Cell Res. 1977 Jan;104(1):153–163. doi: 10.1016/0014-4827(77)90078-7. [DOI] [PubMed] [Google Scholar]
- Radke K., Martin G. S. Transformation by Rous sarcoma virus: effects of src gene expression on the synthesis and phosphorylation of cellular polypeptides. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5212–5216. doi: 10.1073/pnas.76.10.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins K. C., Antoniades H. N., Devare S. G., Hunkapiller M. W., Aaronson S. A. Structural and immunological similarities between simian sarcoma virus gene product(s) and human platelet-derived growth factor. Nature. 1983 Oct 13;305(5935):605–608. doi: 10.1038/305605a0. [DOI] [PubMed] [Google Scholar]
- Rohrschneider L. R. Adhesion plaques of Rous sarcoma virus-transformed cells contain the src gene product. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3514–3518. doi: 10.1073/pnas.77.6.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Permeability of cell junction depends on local cytoplasmic calcium activity. Nature. 1975 Mar 20;254(5497):250–252. doi: 10.1038/254250a0. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Ball E. H., Singer S. J. Vinculin: a cytoskeletal target of the transforming protein of Rous sarcoma virus. Cell. 1981 Apr;24(1):165–174. doi: 10.1016/0092-8674(81)90512-2. [DOI] [PubMed] [Google Scholar]
- Steinberg M., Defendi V. Patterns of cell communication and differentiation in SV40 transformed human keratinocytes. J Cell Physiol. 1981 Oct;109(1):153–159. doi: 10.1002/jcp.1041090117. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y., Whitman M., Cantley L. C., Erikson R. L. Evidence that the Rous sarcoma virus transforming gene product phosphorylates phosphatidylinositol and diacylglycerol. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2117–2121. doi: 10.1073/pnas.81.7.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trosko J. E., Chang C. C. Adaptive and nonadaptive consequences of chemical inhibition of intercellular communication. Pharmacol Rev. 1984 Jun;36(2 Suppl):137S–144S. [PubMed] [Google Scholar]
- Turin L., Warner A. Carbon dioxide reversibly abolishes ionic communication between cells of early amphibian embryo. Nature. 1977 Nov 3;270(5632):56–57. doi: 10.1038/270056a0. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Jay G., Pastan I. Localization of the ASV src gene product to the plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1979 Sep;18(1):125–134. doi: 10.1016/0092-8674(79)90361-1. [DOI] [PubMed] [Google Scholar]
- Yancey S. B., Edens J. E., Trosko J. E., Chang C. C., Revel J. P. Decreased incidence of gap junctions between Chinese hamster V-79 cells upon exposure to the tumor promoter 12-O-tetradecanoyl phorbol-13-acetate. Exp Cell Res. 1982 Jun;139(2):329–340. doi: 10.1016/0014-4827(82)90257-9. [DOI] [PubMed] [Google Scholar]
- Yotti L. P., Chang C. C., Trosko J. E. Elimination of metabolic cooperation in Chinese hamster cells by a tumor promoter. Science. 1979 Nov 30;206(4422):1089–1091. doi: 10.1126/science.493994. [DOI] [PubMed] [Google Scholar]