Abstract
In this paper, we propose a new method remMap — REgularized Multivariate regression for identifying MAster Predictors — for fitting multivariate response regression models under the high-dimension-low-sample-size setting. remMap is motivated by investigating the regulatory relationships among different biological molecules based on multiple types of high dimensional genomic data. Particularly, we are interested in studying the influence of DNA copy number alterations on RNA transcript levels. For this purpose, we model the dependence of the RNA expression levels on DNA copy numbers through multivariate linear regressions and utilize proper regularization to deal with the high dimensionality as well as to incorporate desired network structures. Criteria for selecting the tuning parameters are also discussed. The performance of the proposed method is illustrated through extensive simulation studies. Finally, remMap is applied to a breast cancer study, in which genome wide RNA transcript levels and DNA copy numbers were measured for 172 tumor samples. We identify a trans-hub region in cytoband 17q12–q21, whose amplification influences the RNA expression levels of more than 30 unlinked genes. These findings may lead to a better understanding of breast cancer pathology.
Keywords: sparse regression, MAP(MAster Predictor) penalty, DNA copy number alteration, RNA transcript level, v-fold cross validation
1 Introduction
In a few recent breast cancer cohort studies, microarray expression experiments and array CGH (comparative genomic hybridization) experiments have been conducted for more than 170 primary breast tumor specimens collected at multiple cancer centers (Sorlie et al. 2001; Sorlie et al. 2003; Zhao et al. 2004; Kapp et al. 2006; Bergamaschi et al. 2006; Langerod et al. 2007; Bergamaschi et al. 2008). The resulting RNA transcript levels (from microarray expression experiments) and DNA copy numbers (from CGH experiments) of about 20K genes/clones across all the tumor samples were then used to identify useful molecular markers for potential clinical usage. While useful information has been revealed by analyzing expression arrays alone or CGH arrays alone, careful integrative analysis of DNA copy numbers and expression data are necessary as these two types of data provide complimentary information in gene characterization. Specifically, RNA data give information on genes that are over/under-expressed, but do not distinguish primary changes driving cancer from secondary changes resulting from cancer, such as proliferation rates and differentiation state. On the other hand, DNA data give information on gains and losses that are drivers of cancer. Therefore, integrating DNA and RNA data helps to discern more subtle (yet biologically important) genetic regulatory relationships in cancer cells (Pollack et al. 2002).
It is widely agreed that variations in gene copy numbers play an important role in cancer development through altering the expression levels of cancer-related genes (Albertson et al. 2003). This is clear for cis-regulations, in which a gene’s DNA copy number alteration influences its own RNA transcript level (Hyman et al. 2002; Pollack et al. 2002). However, DNA copy number alterations can also alter in trans the RNA transcript levels of genes from unlinked regions, for example by directly altering the copy number and expression of transcriptional regulators, or by indirectly altering the expression or activity of transcriptional regulators, or through genome rearrangements affecting cis-regulatory elements. The functional consequences of such trans-regulations are much harder to establish, as such inquiries involve assessment of a large number of potential regulatory relationships. Therefore, to refine our understanding of how these genome events exert their effects, we need new analytical tools that can reveal the subtle and complicated interactions among DNA copy numbers and RNA transcript levels. Knowledge resulting from such analysis will help shed light on cancer mechanisms.
The most straightforward way to model the dependence of RNA levels on DNA copy numbers is through a multivariate response linear regression model with the RNA levels being responses and the DNA copy numbers being predictors. While the multivariate linear regression is well studied in statistical literature, the current problem bears new challenges due to (i) high-dimensionality in terms of both predictors and responses; (ii) the interest in identifying master regulators in genetic regulatory networks; and (iii) the complicated correlation relationships among response variables. Thus, the naive approach of regressing each response onto the predictors separately is unlikely to produce satisfactory results, as such methods often lead to high variability and over-fitting. This has been observed by many authors, for example, Breiman et al. (1997) show that taking into account of the relation among response variables helps to improve the overall prediction accuracy. More recently, Kim et al. (2008) propose a new statistical framework to explicitly incorporate the relationships among responses by assuming the linked responses depend on the predictors in a similar way. The authors show that this approach helps to select relevant predictors when the above assumption holds.
When the number of predictors is moderate or large, model selection is often needed for prediction accuracy and/or model interpretation. Standard model selection tools in multiple regression such as AIC and forward stepwise selection have been extended to multivariate linear regression models (Bedrick et al. 1994; Fujikoshi et al. 1997; Lutz and BÄuhlmann 2006). More recently, sparse regularization schemes have been utilized for model selection under the high dimensional multivariate regression setting. For example, Turlach et al. (2005) propose to constrain the coefficient matrix of a multivariate regression model to lie within a suitable polyhedral region. Lutz and BÄuhlmann (2006) propose an L2 multivariate boosting procedure. Obozinskiy et al. (2008) propose to use a ℓ1/ℓ2 regularization to identify the union support set in the multivariate regression. Moreover, Brown et al. (1998, 1999, 2002) introduce a Bayesian framework to model the relation among the response variables when performing variable selection for multivariate regression. Another way to reduce the dimensionality is through factor analysis. Related work includes Izenman (1975), Frank et al. (1993), Reinsel and Velu (1998), Yuan et al. (2007) and many others.
For the problem we are interested in here, the dimensions of both predictors and responses are large (compared to the sample size). Thus in addition to assuming that only a subset of predictors enter the model, it is also reasonable to assume that a predictor may affect only some but not all responses. Moreover, in many real applications, there often exist a subset of predictors which are more important than other predictors in terms of model building and/or scientific interest. For example, it is widely believed that genetic regulatory relationships are intrinsically sparse (Jeong et al. 2001; Gardner et al. 2003). At the same time, there exist master regulators — network components that affect many other components, which play important roles in shaping the network functionality. Most methods mentioned above do not take into account the dimensionality of the responses, and thus a predictor/factor influences either all or none responses, e.g., Turlach et al. (2005), Yuan et al. (2007), the L2 row boosting by Lutz and Bühlmann (2006), and the ℓ1/ℓ2 regularization by Obozinskiy et al. (2008). On the other hand, other methods only impose a sparse model, but do not aim at selecting a subset of predictors, e.g., the L2 boosting by Lutz and Bühlmann (2006). In this paper, we propose a novel method remMap — REgularized Multivariate regression for identifying MAster Predictors, which takes into account both aspects. remMap uses an ℓ1 norm penalty to control the overall sparsity of the coefficient matrix of the multivariate linear regression model. In addition, remMap imposes a “group” sparse penalty, which in essence is the same as the “group lasso” penalty proposed by Bakin (1999), Antoniadis and Fan (2001), Yuan and Lin (2006), Zhao et al. (2006) and Obozinskiy et al. (2008) (see more discussions in Section 2). This penalty puts a constraint on the ℓ2 norm of regression coefficients for each predictor, which controls the total number of predictors entering the model, and consequently facilitates the detection of master predictors. The performance of the proposed method is illustrated through extensive simulation studies.
We apply the remMap method on the breast cancer data set mentioned earlier and identify a significant trans-hub region in cytoband 17q12–q21, whose amplification influences the RNA levels of more than 30 unlinked genes. These findings may shed some light on breast cancer pathology. We also want to point out that analyzing CGH arrays and expression arrays together reveals only a small portion of the regulatory relationships among genes. However, it should identify many of the important relationships, i.e., those reflecting primary genetic alterations that drive cancer development and progression. While there are other mechanisms to alter the expression of master regulators, for example by DNA mutation or methylation, in most cases one should also find corresponding DNA copy number changes in at least a subset of cancer cases. Nevertheless, because we only identify the subset explainable by copy number alterations, the words “regulatory network” (“master regulator”) used in this paper will specifically refer to the subnetwork (hubs of the subnetwork) whose functions change with DNA copy number alterations, and thus can be detected by analyzing CGH arrays together with expression arrays.
The rest of the paper is organized as follows. In Section 2, we describe the remMap model, its implementation and criteria for tuning. In Section 3, the performance of remMap is examined through extensive simulation studies. In Section 4, we apply the remMap method on the breast cancer data set. We conclude the paper with discussions in Section 5. Technical details are provided in the supplementary material.
2 Method
2.1 Model
Consider multivariate regression with Q response variables y1, ⋯, yQ and P prediction variables x1, ⋯, xP:
| (1) |
where the error terms ε1, ⋯, εQ have a joint distribution with mean 0 and covariance Σε. In the above, we assume that, all the response and prediction variables are standardized to have zero mean and thus there is no intercept term in equation (1). The primary goal of this paper is to identify non-zero entries in the P × Q coefficient matrix B = (βpq) based on N i.i.d samples from the above model. Under normality assumptions, βpq can be interpreted as proportional to the conditional correlation Cor(yq, xp|x−(p)), where x−(p) ≔ {xp′ : 1 ≤ p′ ≠ p ≤ P}. In the following, we use to denote the sample of the qth response variable and that of the pth prediction variable, respectively. We also use Y = (Y1 : ⋯: YQ) to denote the N × Q response matrix, and use X = (X1 : ⋯: XP) to denote the N × P prediction matrix.
In this paper, we shall focus on the cases where both Q and P are larger than the sample size N. For example, in the breast cancer study discussed in Section 4, the sample size is 172, while the number of genes and the number of chromosomal regions are on the order of a couple of hundreds (after pre-screening). When P > N, the ordinary least square solution is not unique, and regularization becomes indispensable. The choice of suitable regularization depends heavily on the type of data structure we envision. In recent years, ℓ1-norm based sparsity constraints such as lasso (Tibshirani 1996) have been widely used under such high-dimension-low-sample-size settings. This kind of regularization is particularly suitable for the study of genetic pathways, since genetic regulatory relationships are widely believed to be intrinsically sparse (Jeong et al. 2001; Gardner et al. 2003). In this paper, we impose an ℓ1 norm penalty on the coefficient matrix B to control the overall sparsity of the multivariate regression model. In addition, we put constraints on the total number of predictors entering the model. This is achieved by treating the coefficients corresponding to the same predictor (one row of B) as a group, and then penalizing their ℓ2 norm. A predictor will not be selected into the model if the corresponding ℓ2 norm is shrunken to 0. Thus this penalty facilitates the identification of master predictors — predictors which affect (relatively) many response variables. This idea is motivated by the fact that master regulators exist and are of great interest in the study of many real life networks including genetic regulatory networks. Specifically, for model (1), we propose the following criterion
| (2) |
where Cp is the pth row of , which is a pre-specified P × Q 0–1 matrix indicating the coefficients on which penalization is imposed; Bp is the pth row of B; ‖ · ‖F denotes the Frobenius norm of matrices; ‖ · ‖1 and ‖ · ‖2 are the ℓ1 and ℓ2 norms for vectors, respectively; and “ ·” stands for Hadamard product (that is, entry-wise multiplication). The indicator matrix C is pre-specified based on prior knowledge: if we know in advance that predictor xp affects response yq, then the corresponding regression coefficient βpq will not be penalized and we set cpq = 0 (see Section 4 for an example). When there is no such prior information, C can be simply set to a constant matrix cpq ≡ 1. Finally, an estimate of the coefficient matrix B is B̂(λ1, λ2) ≔ arg minB L(B; λ1, λ2).
In the above criterion function, the ℓ1 penalty induces the overall sparsity of the coefficient matrix B. The ℓ2 penalty on the row vectors Cp · Bp induces row sparsity of the product matrix C · B. As a result, some rows are shrunken to be entirely zero (Theorem 1). Consequently, predictors which affect relatively more response variables are more likely to be selected into the model. We refer to the combined penalty in equation (2) as the MAP (MAster Predictor) penalty. We also refer to the proposed estimator B̂(λ1, λ2) as the remMap (REgularized Multivariate regression for identifying MAster Predictors) estimator. Note that, the ℓ2 penalty is a special case (with α = 2) of the more general penalty form: for a vector υ ∈ ℛQ and α > 1. In Turlach et al. (2005), a penalty with α = ∞ is used to select a common subset of prediction variables when modeling multivariate responses. In Yuan et al. (2007), a constraint with α = 2 is applied to the loading matrix in a multivariate linear factor regression model for dimension reduction. In Obozinskiy et al. (2008), the same constraint is applied to identify the union support set in the multivariate regression. In the case of multiple regression, a similar penalty corresponding to α = 2 is proposed by Bakin (1999) and by Yuan and Lin (2006) for the selection of grouped variables, which corresponds to the blockwise additive penalty in Antoniadis and Fan (2001) for wavelet shrinkage. Zhao et al. (2006) propose the penalty with a general α > 1. However, none of these methods takes into account the high dimensionality of response variables and thus predictors/factors are simultaneously selected for all responses. On the other hand, by combining the ℓ2 penalty and the ℓ1 penalty together in the MAP penalty, the remMap model not only selects a subset of predictors, but also limits the influence of the selected predictors to only some (but not necessarily all) response variables. Thus, it is more suitable for the cases when both the number of predictors and the number of responses are large. Lastly, we also want to point out a difference between the MAP penalty and the ElasticNet penalty proposed by Zou et al. (2005), which combines the ℓ1 norm penalty with the squared ℓ2 norm penalty. The ElasticNet penalty aims to encourage a group selection effect for highly correlated predictors under the multiple regression setting. However, the squared ℓ2 norm itself does not induce sparsity and thus is intrinsically different from the ℓ2 norm penalty discussed above.
In Section 3, we use extensive simulation studies to illustrate the effects of the MAP penalty. We compare the remMap method with two alternatives: (i) the joint method which only utilizes the ℓ1 penalty, that is λ2 = 0 in (2); (ii) the sep method which performs Q separate lasso regressions. We find that, if there exist large hubs (master predictors), remMap performs much better than joint in terms of identifying the true model; otherwise, the two methods perform similarly. This suggests that the “simultaneous” variable selection enhanced by the ℓ2 penalty pays off when there exist a small subset of “important” predictors, and it costs little when such predictors are absent. Moreover, by encouraging the selection of master predictors, the MAP penalty explicitly makes use of the correlations among the response variables caused by sharing a common set of predictors. We make a note that there are methods, such as Kim et al. (2008), that make more specific assumptions on how the correlated responses depend on common predictors. If these assumptions hold, it is possible that such methods can be more efficient in incorporating the relationships among the responses. In addition, both remMap and joint methods impose sparsity of the coefficient matrix as a whole. This helps to borrow information across different regressions corresponding to different response variables. It also amounts to a greater degree of regularization, which is usually desirable for the high-dimension-low-sample-size setting. On the other hand, the sep method controls sparsity for each individual regression separately and thus is subject to high variability and over-fitting. As can be seen by the simulation studies (Section 3), this type of “joint” modeling greatly improves the model efficiency. This is also noted by other authors including Turlach et al. (2005), Lutz and Bühlmann (2006) and Obozinskiy et al. (2008).
2.2 Model Fitting
In this section, we propose an iterative algorithm for solving the remMap estimator B̂(λ1, λ2). This is a convex optimization problem when the two tuning parameters are not both zero, and thus there exists a unique solution. We first describe how to update one row of B, when all other rows are fixed.
Theorem 1 Given {Bp}p≠p0 in (2), the solution for minBp0 L(B; λ1, λ2) is given by B̂p0 = (β̂p0,1, ⋯, β̂p0,Q) which satisfies: for 1 ≤ q ≤ Q
If cp0,q = 0, (OLS), where Ỹq = Yq − ∑p≠p0 Xpβpq;
- If cp0,q = 1,
where(3)
and(4)
The proof of Theorem 1 is given in the supplementary material (Appendix A).
Theorem 1 says that, when estimating the row of the coefficient matrix B with all other rows fixed: if there is a pre-specified relationship between the predictor and the qth response (i.e., cp0,q = 0), the corresponding coefficient βp0,q is estimated by the (univariate) ordinary least square solution (OLS) using current responses Ỹq; otherwise, we first obtain the lasso solution by the (univariate) soft shrinkage of the OLS solution (equation (4)), and then conduct a group shrinkage of the lasso solution (equation (3)). From Theorem 1, it is easy to see that, when the design matrix X is orthonormal: XTX = Ip and λ1 = 0, the remMap method amounts to selecting variables according to the ℓ2 norm of their corresponding OLS estimates.
Theorem 1 naturally leads to an algorithm which updates the rows of B iteratively until convergence. In particular, we adopt the active-shooting idea proposed by Peng et al. (2008) and Friedman et al. (2008), which is a modification of the shooting algorithm proposed by Fu (1998) and also Friedman et al. (2007) among others. The algorithm proceeds as follows:
- Initial step: for p = 1, …, P; q = 1, …, Q,
(5) - Define the current active-row set Λ = {p : current ‖B̂p‖2,C ≠ 0}.
- For each p ∈ Λ, update B̂p with all other rows of B fixed at their current values according to Theorem 1.
- Repeat (2.1) until convergence is achieved on the current active-row set.
For p = 1 to P, update B̂p with all other rows of B fixed at their current values according to Theorem 1. If no B̂p changes during this process, return the current B̂ as the final estimate. Otherwise, go back to step 2.
It is clear that the computational cost of the above algorithm is in the order of O(NPQ).
2.3 Tuning
In this section, we discuss the selection of the tuning parameters (λ1, λ2) by v-fold cross validation. To perform the v-fold cross validation, we first partition the whole data set into V non-overlapping subsets, each consisting of approximately 1/V fraction of total samples. Denote the ith subset as D(i) = (Y(i), X(i)), and its complement as D−(i) = (Y−(i), X−(i)). For a given (λ1, λ2), we obtain the remMap estimate: based on the ith training set D−(i). We then obtain the ordinary least square estimates as follows: for 1 ≤ q ≤ Q, define . Then set if p ∉ Sq; otherwise, define as the ordinary least square estimates by regressing . Finally, prediction error is calculated on the test set D(i):
| (6) |
The v-fold cross validation score is then defined as
| (7) |
The reason for using OLS estimates in calculating the prediction error is because the true model is assumed to be sparse. As noted by Efron et al. (2004), when there are many noise variables, using shrunken estimates in the cross validation criterion often results in over fitting. Similar results are observed in our simulation studies: if in (6) and (7), the shrunken estimates are used, the selected models are all very big which result in large numbers of false positive findings. In addition, we also try AIC and GCV for tuning and both criteria result in over fitting as well. These results are not reported in the next section due to space limitation.
In order to further control the false positive findings, we propose a method called cv.vote. The idea is to treat the training data from each cross-validation fold as a “bootstrap” sample. Then variables being consistently selected by many cross validation folds should be more likely to appear in the true model than the variables being selected only by few cross validation folds. Specifically, for 1 ≤ p ≤ P and 1 ≤ q ≤ Q, define
| (8) |
where Va is a pre-specified proportion. We then select edge (p, q) if spq(λ1, λ2) = 1. In the next section, we use Va = 0.5 and thus cv.vote amounts to a “majority vote” procedure. Simulation studies in Section 3 suggest that, cv.vote can effectively decrease the number of false positive findings while only slightly increase the number of false negatives.
An alternative tuning method is by a BIC criterion. Compared to v-fold cross validation, BIC is computationally cheaper. However it requires much more assumptions. In particular, the BIC method uses the degrees of freedom of each remMap model which is difficult to estimate in general. In the supplementary material, we derive an unbiased estimator for the degrees of freedom of the remMap models when the predictor matrix X has orthogonal columns (Theorem 2 of Appendix B in the supplementary materials). In Section 3, we show by extensive simulation studies that, when the correlations among the predictors are complicated, this estimator tends to select very small models. For more details see the supplementary material, Appendix B.
3 Simulation
In this section, we investigate the performance of the remMap model and compare it with two alternatives: (i) the joint model with λ2 = 0 in (2); (ii) the sep model which performs Q separate lasso regressions. For each model, we consider three tuning strategies, which results in nine methods in total:
remMap.cv, joint.cv, sep.cv: The tuning parameters are selected through 10-fold cross validation;
remMap.cv.vote, joint.cv.vote, sep.cv.vote: The cv.vote procedure with Va = 0.5 is applied to the models resulted from the corresponding * .cv approaches;
remMap.bic, joint.bic, sep.bic: The tuning parameters are selected by a BIC criterion. For remMap.bic and joint.bic, the degrees of freedom are estimated according to equation (S-6) in Appendix B of the supplementary material; for sep.bic, the degrees of freedom of each regression is estimated by the total number of selected predictors (Zou et al. 2007).
We simulate data as follows. Given (N, P, Q), we first generate the predictors (x1, ⋯, xP)T ~ NormalP (0, ΣX), where ΣX is the predictor covariance matrix (for simulations 1 and 2, ). Next, we simulate a P × Q 0–1 adjacency matrix A, which specifies the topology of the network between predictors and responses, with A(p, q) = 1 meaning that xp influences yq, or equivalently βpq ≠ 0. In all simulations, we set P = Q and the diagonals of A equal to one, which is viewed as prior information (thus the diagonals of C are set to zero). This aims to mimic cis-regulations of DNA copy number alternations on its own expression levels. We then simulate the P × Q regression coefficient matrix B = (βpq) by setting βpq = 0, if A(p, q) = 0; and βpq ~ Uniform([−5, −1] ∪ [1, 5]), if A(p, q) = 1. After that, we generate the residuals (ε1, ⋯, εQ)T ~ NormalQ(0, Σε), where . The residual variance is chosen such that the average signal to noise ratio equals to a pre-specified level s. Finally, the responses (y1, ⋯, yQ)T are generated according to model (1). Each data set consists of N i.i.d samples of such generated predictors and responses. For all methods, predictors and responses are standardized to have (sample) mean zero and standard deviation one before model fitting. Results reported for each simulation setting are averaged over 25 independent data sets.
For all simulation settings, C = (cpq) is taken to be cpq = 0, if p = q; and cpq = 1, otherwise. Our primary goal is to identify the trans-edges — the predictor-response pairs (xp, yq) with A(p, q) = 1 and C(p, q) = 1, i.e., the edges that are not pre-specified by the indicator matrix C. Thus, in the following, we report the number of false positive detections of trans-edges (FP) and the number of false negative detections of trans-edges (FN) for each method. We also examine these methods in terms of predictor selection. Specifically, a predictor is called a cis-predictor if it does not have any trans-edges; otherwise it is called a trans-predictor. Moreover, we say a false positive trans-predictor (FPP) occurs if a cis-predictor is incorrectly identified as a trans-predictor; we say a false negative trans-predictor (FNP) occurs if it is the other way around.
Simulation I
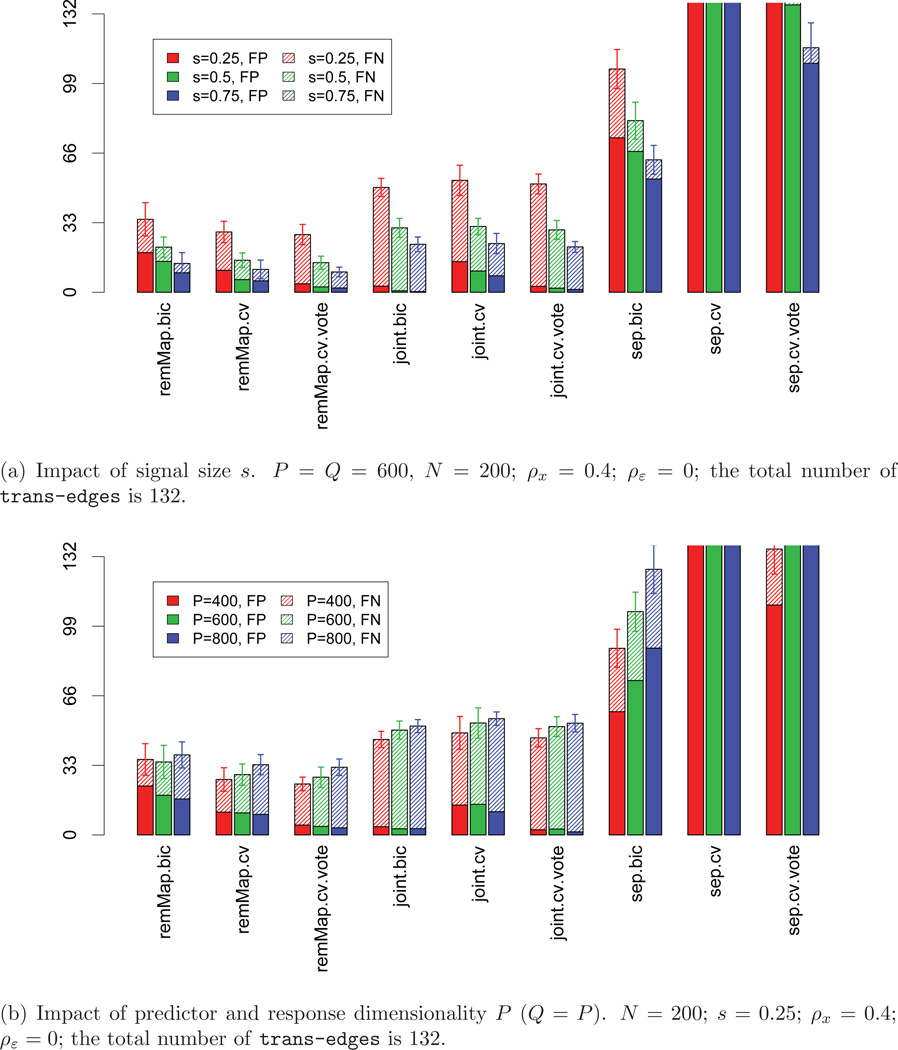
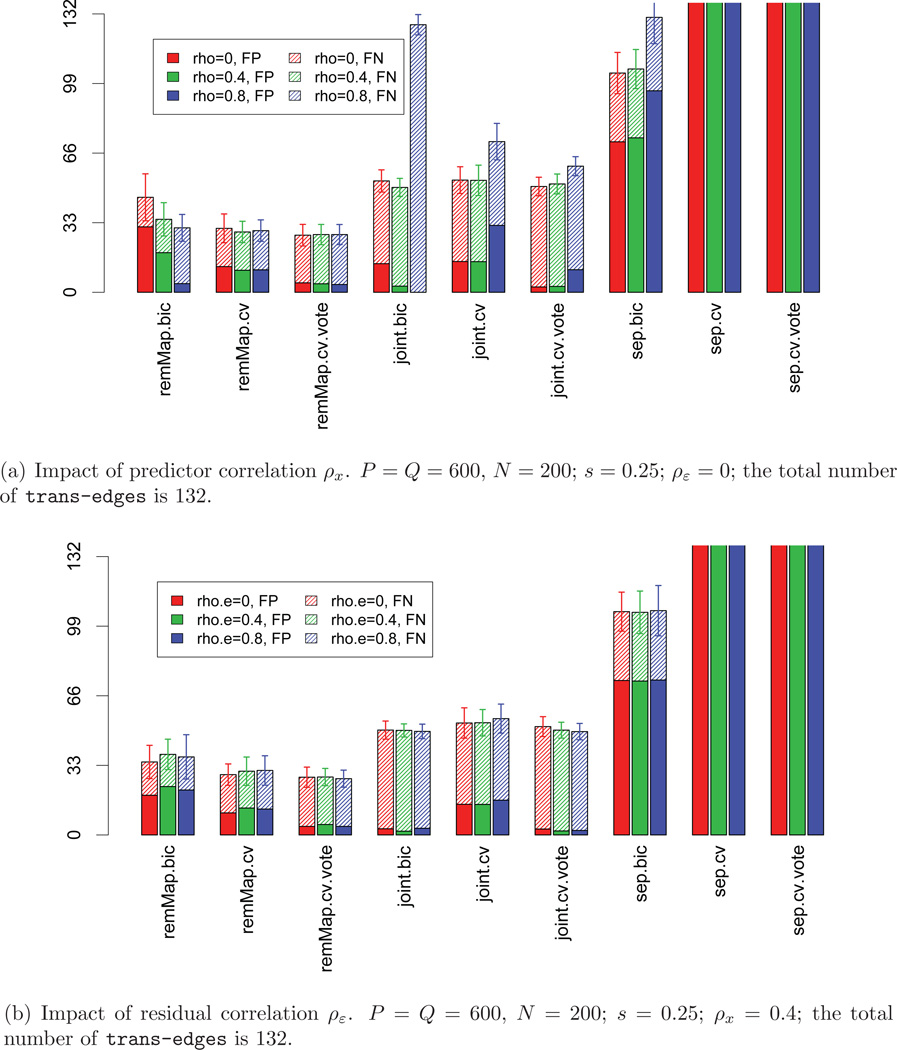
We first assess the performances of the nine methods under various combinations of model parameters. Specifically, we consider: P = Q = 400, 600, 800; s = 0.25, 0.5, 0.75; ρx = 0, 0.4, 0.8; and ρε = 0, 0.4, 0.8. For all settings, the sample size N is fixed at 200. The networks (adjacency matrices A) are generated with 5 master predictors (hubs), each influencing 20 ~ 40 responses; and all other predictors are cis-predictors. We set the total number of tran-edges to be 132 for all networks. Results on trans-edge detection are summarized in Figures 1 and 2. From these figures, it is clear that remMap.cv and remMap.cv.vote perform the best in terms of the total number of false detections (FP+FN), followed by remMap.bic. The three sep methods result in too many false positives (especially sep.cv). This is expected since there are in total Q tuning parameters selected separately, and the relations among responses are not utilized at all. This leads to high variability and over-fitting. The three joint methods perform reasonably well, though they have considerably larger number of false negative detections compared to remMap methods. This is because the joint methods incorporate less information about the relations among the responses caused by the master predictors. Finally, comparing cv.vote to cv, we can see that the cv.vote procedure effectively decreases the false positive detections and only slightly inflates the false negative counts.
Figure 1.
Impact of signal size and dimensionality. Heights of solid bars represent numbers of false positive detections of trans-edges (FP); heights of shaded bars represent numbers of false negative detections of trans-edges (FN). All bars are truncated at height=132.
Figure 2.
Impact of correlations. Heights of solid bars represent numbers of false positive detections of trans-edges (FP); heights of shaded bars represent numbers of false negative detections of trans-edges (FN). All bars are truncated at height=132.
As to the impact of different model parameters, signal size s plays an important role for all methods: the larger the signal size, the better these methods perform (Figure 1 (a)). Dimensionality (P, Q) also shows consistent impacts on these methods: the larger the dimension, the more false negative detections (Figure 1 (b)). With increasing predictor correlation ρx, both remMAP.bic and joint.bic tend to select smaller models, and consequently result in less false positives and more false negatives (Figure 2 (a)). This is because when the design matrix X is further away from orthogonality, (S-6) tends to overestimate the degrees of freedom and consequently smaller models are selected. The residual correlation ρε seems to have little impact on joint and sep, and some (though rather small) impacts on remMap (Figure 2 (b)). Moreover, remMap performs much better than joint and sep on master predictor selection, especially in terms of the number of false positive trans-predictors (results not shown). This is because the ℓ2 norm penalty is more effective than the ℓ1 norm penalty in excluding irrelevant predictors.
Simulation II
In this simulation, we study the performance of these methods on a network without big hubs. The data are generated similarly as before with P = Q = 600, N = 200, s = 0.25, ρx = 0.4, and ρε = 0. The network consists of 540 cis-predictors, and 60 trans-predictors with 1 ~ 4 trans-edges. This leads to 151 trans-edges in total. As can be seen from Table 1, remMap methods and joint methods now perform very similarly and both are considerably better than the sep methods. Indeed, under this setting, λ2 is selected (either by cv or bic) to be small in the remMap model, making it very close to the joint model.
Table 1.
Simulation II. Network topology: uniform network with 151 trans-edges and 60 trans-predictors. P = Q = 600, N = 200; s = 0.25; ρx = 0.4; ρε = 0.
| Method | FP | FN | TF | FPP | FNP |
|---|---|---|---|---|---|
| remMap.bic | 4.72(2.81) | 45.88(4.5) | 50.6(4.22) | 1.36(1.63) | 11(1.94) |
| remMap.cv | 18.32(11.45) | 40.56(5.35) | 58.88(9.01) | 6.52(5.07) | 9.2(2) |
| remMap.cv.vote | 2.8(2.92) | 50.32(5.38) | 53.12(3.94) | 0.88(1.26) | 12.08(1.89) |
| joint.bic | 5.04(2.68) | 52.92(3.6) | 57.96(4.32) | 4.72(2.64) | 9.52(1.66) |
| joint.cv | 16.96(10.26) | 46.6(5.33) | 63.56(7.93) | 15.36(8.84) | 7.64(2.12) |
| joint.cv.vote | 2.8(2.88) | 56.28(5.35) | 59.08(4.04) | 2.64(2.92) | 10.40(2.08) |
| sep.bic | 78.92(8.99) | 37.44(3.99) | 116.36(9.15) | 67.2(8.38) | 5.12(1.72) |
| sep.cv | 240.48(29.93) | 32.4(3.89) | 272.88(30.18) | 179.12(18.48) | 2.96(1.51) |
| sep.cv.vote | 171.00(20.46) | 33.04(3.89) | 204.04(20.99) | 134.24(14.7) | 3.6(1.50) |
FP: false positive; FN: false negative; TF: total false; FPP: false positive trans-predictor; FNP: false negative trans-predictor. Numbers in the parentheses are standard deviations
Simulation III
In this simulation, we try to mimic the true predictor covariance and network topology in the real data discussed in the next section. We observe that, for chromosomal regions on the same chromosome, the corresponding copy numbers are usually positively correlated, and the magnitude of the correlation decays slowly with genetic distance. On the other hand, if two regions are on different chromosomes, the correlation between their copy numbers could be either positive or negative and in general the magnitude is much smaller than that of the regions on the same chromosome. Thus in this simulation, we first partition the P predictors into 23 distinct blocks, with the size of the ith block proportional to the number of CNAI (copy number alteration intervals) on the ith chromosome of the real data (see Section 4 for the definition of CNAI). Denote the predictors within the ith block as xi1, ⋯, xigi, where gi is the size of the ith block. We then define the within-block correlation as: for 1 ≤ j, l ≤ gi; and define the between-block correlation as Corr(xij, xkl) ≡ ρik for 1 ≤ j ≤ gi, 1 ≤ l ≤ gk and 1 ≤ i ≠ k ≤ 23. Here, ρik is determined in the following way: its sign is randomly generated from {−1, 1}; its magnitude is randomly generated from . In this simulation, we set ρwb = 0.9, ρbb = 0.25 and use P = Q = 600, N = 200, s = 0.5, and ρε = 0.4. The heatmaps of the (sample) correlation matrix of the predictors in the simulated data and that in the real data are given by Figure S-2 in the supplementary material. The network is generated with five large hub predictors each having 14 ~ 26 trans-edges; five small hub predictors each having 3 ~ 4 trans-edges; 20 predictors having 1 ~ 2 trans-edges; and all other predictors being cis-predictors.
The results are summarized in Table 2. Among the nine methods, remMap.cv.vote performs the best in terms of both edge detectiion and master predictor prediction. remMAP.bic and joint.bic result in very small models due to the complicated correlation structure among the predictors. While all three cross-validation based methods have large numbers of false positive findings, the three cv.vote methods have much reduced false positive counts and only slightly increased false negative counts. These findings again suggest that cv.vote is an effective procedure in controlling false positive rates while not sacrificing too much in terms of power.
Table 2.
Simulation III. Network topology: five large hubs and five small hubs with 151 trans-edges and 30 trans-predictors. P = Q = 600, N = 200; s = 0.5; ρwb = 0.9, ρbb = 0.25; ρε = 0.4.
| Method | FP | FN | TF | FPP | FNP |
|---|---|---|---|---|---|
| remMap.bic | 0(0) | 150.24(2.11) | 150.24(2.11) | 0(0) | 29.88(0.33) |
| remMap.cv | 93.48(31.1) | 20.4(3.35) | 113.88(30.33) | 15.12(6.58) | 3.88(1.76) |
| remMap.cv.vote | 48.04(17.85) | 27.52(3.91) | 75.56(17.67) | 9.16(4.13) | 5.20(1.91) |
| joint.bic | 7.68(2.38) | 104.16(3.02) | 111.84(3.62) | 7(2.18) | 10.72(1.31) |
| joint.cv | 107.12(13.14) | 39.04(3.56) | 146.16(13.61) | 66.92(8.88) | 1.88(1.2) |
| joint.cv.vote | 63.80(8.98) | 47.44(3.90) | 111.24(10.63) | 41.68(6.29) | 2.88(1.30) |
| sep.bic | 104.96(10.63) | 38.96(3.48) | 143.92(11.76) | 64.84(6.29) | 1.88(1.17) |
| sep.cv | 105.36(11.51) | 37.28(4.31) | 142.64(12.26) | 70.76(7.52) | 1.92(1.08) |
| sep.cv.vote | 84.04(10.47) | 41.44(4.31) | 125.48(12.37) | 57.76 (6.20) | 2.4 (1.32) |
FP: false positive; FN: false negative; TF: total false; FPP: false positive trans-predictor; FNP: false negative trans-predictor. Numbers in the parentheses are standard deviations
We also carried out an additional simulation where some columns of the coefficient matrix B are related, and the results are reported in Table S-1 of Appendix C. The overall picture of the performances of different methods remains similar as other simulations.
4 Real application
In this section, we apply the proposed remMap method to the breast cancer study mentioned earlier. Our goal is to search for genome regions whose copy number alterations have significant impacts on RNA expression levels, especially on those of the unlinked genes, i.e., genes not falling into the same genome region. The findings resulting from this analysis may help to cast light on the complicated interactions among DNA copy numbers and RNA expression levels.
4.1 Data preprocessing
The 172 tumor samples were analyzed using cDNA expression microarray and CGH array experiments as described in Sorlie et al. (2001), Sorlie et al. (2003), Zhao et al. (2004), Kapp et al. (2006), Bergamaschi et al. (2006), Langerod et al. (2007), and Bergamaschi et al. (2008). In below, we outline the data preprocessing steps. More details are provided in the supplementary material (Appendix D).
Each CGH array contains measurements (log2 ratios) on about 17K mapped human genes. A positive (negative) measurement suggests a possible copy number gain (loss). After proper normalization, cghFLasso (Tibshirani and Wang 2008) is used to estimate the DNA copy numbers based on array outputs. Then, we derive copy number alteration intervals (CNAIs) — basic CNA units (genome regions) in which genes tend to be amplified or deleted at the same time within one sample — by employing the Fixed-Order Clustering (FOC) method (Wang 2004). In the end, for each CNAI in each sample, we calculate the mean value of the estimated copy numbers of the genes falling into this CNAI. This results in a 172 (samples) by 384 (CNAIs) numeric matrix.
Each expression array contains measurements for about 18K mapped human genes. After global normalization for each array, we also standardize each gene's measurements across 172 samples to median= 0 and MAD (median absolute deviation) = 1. Then we focus on a set of 654 breast cancer related genes, which is derived based on 7 published breast cancer gene lists (Sorlie et al. 2003; van de Vijver et al. 2002; Chang et al. 2004; Paik et al. 2004; Wang et al. 2005; Sotiriou et al. 2006; Saal et al. 2007). This results in a 172 (samples) by 654 (genes) numeric matrix.
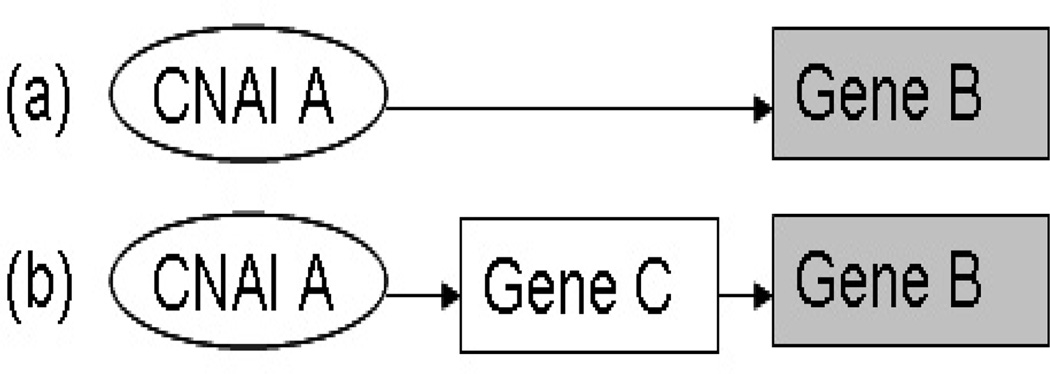
When the copy number change of one CNAI affects the RNA level of an unlinked gene, there are two possibilities: (i) the copy number change directly affects the RNA level of the unlinked gene; (ii) the copy number change first affects the RNA level of an intermediate gene (either linked or unlinked), and then the RNA level of this intermediate gene affects that of the unlinked gene. Figure 3 gives an illustration of these two scenarios. In this study, we are more interested in finding the relationships of the first type. Therefore, we first characterize the interactions among RNA levels and then account for these relationships in our model so that we can better infer direct interactions. For this purpose, we apply the space (Sparse PArtial Correlation Estimation) method to search for associated RNA pairs through identifying non-zero partial correlations (Peng et al. 2008). The estimated (concentration) network (referred to as Exp.Net.664 hereafter) has in total 664 edges — 664 pairs of genes whose RNA levels significantly correlate with each other after accounting for the expression levels of other genes.
Figure 3.
(a) Direct interaction between CNAI A and the expression of gene B; (b) indirect interaction between CNAI A and the expression of Gene B through one intermediate gene.
Another important factor one needs to consider when studying breast cancer is the existence of distinct tumor subtypes. Population stratification due to these distinct subtypes might confound our detection of associations between CNAIs and gene expressions. Therefore, we introduce a set of subtype indicator variables, which later on is used as additional predictors in the remMap model. Specifically, following Sorlie et al. (2003), we divide the 172 patients into 5 distinct groups based on their expression patterns. These groups correspond to the same 5 subtypes suggested by Sorlie et al. (2003) — Luminal Subtype A, Luminal Subtype B, ERBB2-overexpressing Subtype, Basal Subtype and Normal Breast-like Subtype.
4.2 Interactions between CNAIs and RNA expressions
We then apply the remMap method to study the interactions between CNAIs and RNA transcript levels. For each of the 654 breast cancer genes, we regress its expression level on three sets of predictors: (i) expression levels of other genes that are connected to the target gene (the current response variable) in Exp.Net.664; (ii) the five subtype indicator variables derived in the previous section; and (iii) the copy numbers of all 384 CNAIs. We are interested in whether any unlinked CNAIs are selected into this regression model (i.e., the corresponding regression coefficients are non-zero). This suggests potential trans-regulations (trans-edges) between the selected CNAIs and the target gene expression. The coefficients of the linked CNAI of the target gene are not included in the MAP penalty (this corresponds to cpq = 0, see Section 2 for details). This is because the DNA copy number changes of one gene often influence its own expression level, and we are less interested in this kind of cis-regulatory relationships (cis-edges) here. Furthermore, based on Exp.Net.664, no penalties are imposed on the expression levels of connected genes either. In another word, we view the cis-regulations between CNAIs and their linked expression levels, as well as the inferred RNA interaction network as “prior knowledge” in our study.
Note that, different response variables (gene expressions) now have different sets of predictors, as their neighborhoods in Exp.Net.664 are different. However, the remMap model can still be fitted with a slight modification. The idea is to treat all CNAI (384 in total), all gene expressions (654 in total), as well as five subtype indicators as nominal predictors. Then, for each target gene, we force the coefficients of those gene expressions that do not link to it in Exp.Net.664 to be zero. We can easily achieve this by setting those coefficients to zero without updating them throughout the iterative fitting procedure.
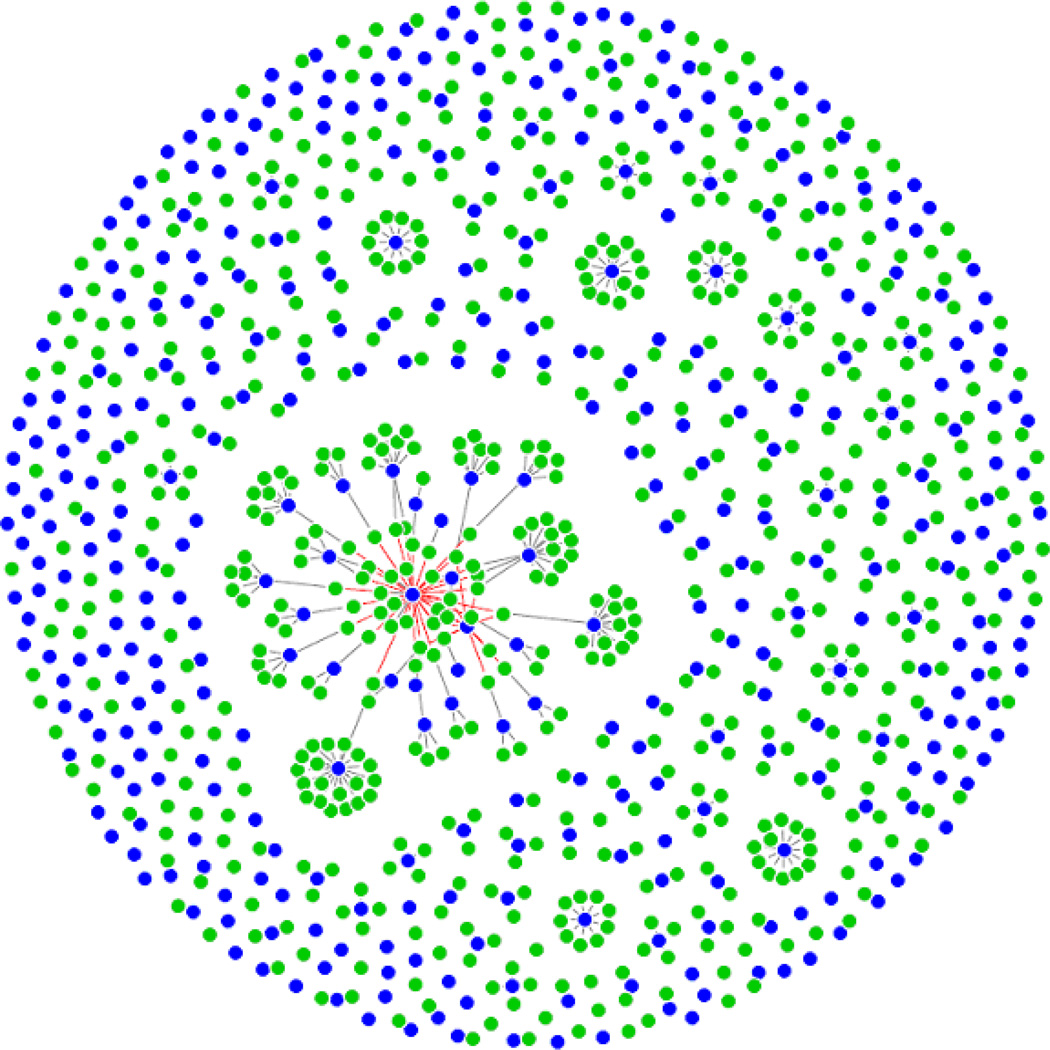
We select tuning parameters (λ1, λ2) in the remMap model through a 10-fold cross validation as described in Section 2.3. The optimal (λ1, λ2) corresponding to the smallest CV score from a grid search is (355.1, 266.7). The resulting model contains 56 trans-regulations in total. In order to further control false positive findings, we apply the cv.vote procedure with Va = 0.5, and filter away 13 out of these 56 trans-edges which have not been consistently selected across different CV folds. The remaining 43 trans-edges correspond to three contiguous CNAIs on chromosome 17 and 31 distinct (unlinked) RNAs. Figure 4 illustrates the topology of the estimated regulatory relationships. The detailed annotations of the three CNAIs and 31 RNAs are provided in Table 3 and Table 4. Moreover, the Pearson-correlations between the DNA copy numbers of CNAIs and the expression levels of the regulated genes/clones (including both cis-regulation and trans-regulation) across the 172 samples are reported in Table 4. As expected, all the cis-regulations have much higher correlations than the potential trans-regulations. In addition, none of the subtype indicator variables is selected into the final model. We also apply the remMap model while forcing these indicators in the model (i.e., not imposing the MAP penalty on these variables). Even though this results in a slightly different network, the hub CNAIs remain the same as before. These imply that the three hub CNAIs are unlikely due to the stratification of tumor subtypes.
Figure 4.
Network of the estimated regulatory relationships between the copy numbers of the 384 CNAIs and the expressions of the 654 breast cancer related genes. Each blue node stands for one CNAI, and each green node stands for one gene. Red edges represent inferred trans-regulations (43 in total). Grey edges represent cis-regulations.
Table 3.
Genome locations of the three CNAIs having (estimated) trans-regulations.
| Index | Cytoband | Begin1 | End 1 | # of clones2 | # of Trans-Reg3 |
|---|---|---|---|---|---|
| 1 | 17q12-17q12 | 34811630 | 34811630 | 1 | 12 |
| 2 | 17q12-17q12 | 34944071 | 35154416 | 9 | 30 |
| 3 | 17q21.1–17q21.2 | 35493689 | 35699243 | 7 | 1 |
Nucleotide position (bp).
Number of genes/clones on the array falling into the CNAI.
Number of unlinked genes whose expressions are estimated to be regulated by the CNAI.
Table 4.
| Clone ID | Gene symbol | Cytoband | Correlation |
|---|---|---|---|
| 753692 | ABLIM1 | 10q25 | 0.199 |
| 896962 | ACADS | 12q22-qter | −0.22 |
| 753400 | ACTL6A | 3q26.33 | 0.155 |
| 472185 | ADAMTS1 | 21q21.2 | 0.214 |
| 210687 | AGTR1 | 3q21–q25 | −0.182 |
| 856519 | ALDH3A2 | 17p11.2 | −0.244 |
| 270535 | BM466581 | 19 | 0.03 |
| 238907 | CABC1 | 1q42.13 | −0.174 |
| 773301 | CDH3 | 16q22.1 | 0.118 |
| 505576 | CORIN | 4p13-p12 | 0.196 |
| 223350 | CP | 3q23–q25 | 0.184 |
| 810463 | DHRS7B | 17p12 | −0.151 |
| 50582 | FLJ25076 | 5p15.31 | 0.086 |
| 669443 | HSF2 | 6q22.31 | 0.207 |
| 743220 | JMJD4 | 1q42.13 | −0.19 |
| 43977 | KIAA0182 | 16q24.1 | 0.259 |
| 810891 | LAMA5 | 20q13.2–q13.3 | 0.269 |
| 247230 | MARVELD2 | 5q13.2 | −0.214 |
| 812088 | NLN | 5q12.3 | 0.093 |
| 257197 | NRBF2 | 10q21.2 | 0.275 |
| 782449 | PCBP2 | 12q13.12–q13.13 | −0.079 |
| 796398 | PEG3 | 19q13.4 | 0.169 |
| 293950 | PIP5K1A | 1q22–q24 | −0.242 |
| 128302 | PTMS | 12p13 | −0.248 |
| 146123 | PTPRK | 6q22.2–q22.3 | 0.218 |
| 811066 | RNF41 | 12q13.2 | −0.247 |
| 773344 | SLC16A2 | Xq13.2 | 0.24 |
| 1031045 | SLC4A3 | 2q36 | 0.179 |
| 141972 | STT3A | 11q23.3 | 0.182 |
| 454083 | TMPO | 12q22 | 0.175 |
| 825451 | USO1 | 4q21.1 | 0.204 |
| 68400 | BM455010 | 17 | 0.748 |
| 756253,365147 | ERBB2 | 17q11.2–q12—17q21.1 | 0.589 |
| 510318,236059 | GRB7 | 17q12 | 0.675 |
| 245198 | MED24 | 17q21.1 | 0.367 |
| 825577 | STARD3 | 17q11–q12 | 0.664 |
| 7827562 | TBPL1 | 6q22.1–q22.3 | 0.658 |
The first part of the table lists the inferred trans-regulated genes. The second part of the table lists cis-regulated genes.
This cDNA sequence probe is annotated with TBPL1, but actually maps to one of the 17q21.2 genes.
The three CNAIs being identified as trans-regulators sit closely on chromosome 17, spanning from 34811630bp to 35699243bp and falling into cytoband 17q12–q21.2. This region (referred to as CNAI-17q12 hereafter) contains 24 known genes, including the famous breast cancer oncogene ERBB2, and the growth factor receptor-bound protein 7 (GRB7). The over expression of GRB7 plays pivotal roles in activating signal transduction and promoting tumor growth in breast cancer cells with chromosome 17q11–21 amplification (Bai and Louh 2008). In this study, CNAI-17q12 is highly amplified (normalized log2 ratio> 5) in 33 (19%) out of the 172 tumor samples. Among the 654 genes/clones considered in the above analysis, 8 clones (corresponding to six genes including ERBB2, GRB7, and MED24) fall into this region. The expressions of these 8 clones are all up-regulated by the amplification of CNAI-17q12 (see Table 4 for more details), which is consistent with results reported in the literature (Kao and Pollack 2006). More importantly, as suggested by the result of the remMap model, the amplification of CNAI-17q12 also influences the expression levels of 31 unlinked genes/clones. This implies that CNAI-17q12 may harbor transcriptional factors whose activities closely relate to breast cancer. Indeed, there are 4 transcription factors (NEUROD2, IKZF3, THRA, NR1D1) and 2 transcriptional co-activators (MED1, MED24) in CNAI-17q12. It is possible that the amplification of CNAI-17q12 results in the over expression of one or more transcription factors/co-activators in this region, which then influence the expressions of the unlinked 31 genes/clones. In addition, some of the 31 genes/clones have been reported to have functions directly related to cancer and may serve as potential drug targets (see Appendix D.5 of the supplementary material for more details). In the end, we want to point out that, besides RNA interactions and subtype stratification, there could be other unaccounted confounding factors. Therefore, caution must be applied when one tries to interpret these results.
5 Discussion
In this paper, we propose the remMap method for fitting multivariate regression models under the large P, Q setting. We focus on model selection, i.e., the identification of relevant predictors for each response variable. remMap is motivated by the rising needs to investigate the regulatory relationships between different biological molecules based on multiple types of high dimensional omics data. Such genetic regulatory networks are usually intrinsically sparse and harbor hub structures. Identifying the hub regulators (master regulators) is of particular interest, as they play crucial roles in shaping network functionality. To tackle these challenges, remMap utilizes a MAP penalty, which consists of an ℓ1 norm part for controlling the overall sparsity of the network, and an ℓ2 norm part for further imposing a row-sparsity of the coefficient matrix, which facilitates the detection of master predictors (regulators). This combined regularization takes into account both model interpretability and computational tractability. Since the MAP penalty is imposed on the coefficient matrix as a whole, it helps to borrow information across different regressions. As illustrated in Section 3, this type of “joint” modeling greatly improves model efficiency. Also, the combined ℓ1 and ℓ2 norm penalty further enhances the performance on both edge detection and master predictor identification. We also propose a cv.vote procedure to make better use of the cross validation results. As suggested by the simulation study, this procedure is very effective in decreasing the number of false positives while only slightly increases the number of false negatives. Moreover, cv.vote can be applied to a broad range of model selection problems when cross validation is employed. In the real application, we apply the remMap method on a breast cancer data set. The resulting model suggests the existence of a trans-hub region on cytoband 17q12–q21. This region harbors the oncogene ERBB2 and may also harbor other important transcriptional factors. While our findings are intriguing, clearly additional investigation is warranted. One way to verify the above conjecture is through a sequence analysis to search for common motifs in the upstream regions of the 31 RNA transcripts, which remains as our future work.
Besides the above application, the remMap model can be applied to investigate the regulatory relationships between other types of biological molecules. For example, it is of great interest to understand the influence of single nucleotide polymorphism (SNP) on RNA transcript levels, as well as the influence of RNA transcript levels on protein expression levels. Such investigation will improve our understanding of related biological systems as well as disease pathology. In addition, we can utilize the remMap idea to other models. For example, when selecting a group of variables in a multiple regression model, we can impose both the ℓ2 penalty (that is, the group lasso penalty), as well as an ℓ1 penalty to encourage within group sparsity. Similarly, the remMap idea can also be applied to vector autoregressive models and generalize linear models.
R package remMap is public available through CRAN (http : //cran.r-project.org/).
Supplementary Material
Acknowledgement
We are grateful to two anonymous reviewers for their valuable comments. Peng and Wang are partially supported by grant 1R01GM082802-01A1 from the National Institute of General Medical Sciences. Peng is also partially supported by grant DMS-0806128 from the National Science Foundation.
References
- Albertson DG, Collins C, McCormick F, Gray JW. Chromosome aberrations in solid tumors. Nature Genetics. 2003;34 doi: 10.1038/ng1215. [DOI] [PubMed] [Google Scholar]
- Antoniadis A, Fan J. Regularization of wavelet approximations. Journal of the American Statistical Association. 2001;96:939–967. [Google Scholar]
- Bai T, Luoh SW. GRB-7 facilitates HER-2/Neu-mediated signal transduction and tumor formation. Carcinogenesis. 2008;29(3):473–479. doi: 10.1093/carcin/bgm221. [DOI] [PubMed] [Google Scholar]
- Bakin S. PhD Thesis. Canberra: Australian National University; 1999. Adaptive regression and model selection in data mining problems. [Google Scholar]
- Bedrick E, Tsai C. Model selection for multivariate regression in small samples. Biometrics. 1994;50:226–231. [Google Scholar]
- Bergamaschi A, Kim YH, Wang P, Sorlie T, Hernandez-Boussard T, Lonning PE, Tibshirani R, Borresen-Dale AL, Pollack JR. Distinct patterns of DNA copy number alteration are associated with different clinicopathological features and gene-expression subtypes of breast cancer. Genes Chromosomes Cancer. 2006;45:1033–1040. doi: 10.1002/gcc.20366. [DOI] [PubMed] [Google Scholar]
- Bergamaschi A, Kim YH, Kwei KA, Choi YL, Bocanegra M, Langerod A, Han W, Noh DY, Huntsman DG, Jeffrey SS, Borresen-Dale AL, Pollack JR. CAMK1D amplification implicated in epithelial-mesenchymal transition in basal-like breast cancer. Mol Oncol. 2008 doi: 10.1016/j.molonc.2008.09.004. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman L, Friedman JH. Predicting multivariate responses in multiple linear regression (with discussion) J. R. Statist. Soc. B. 1997;59:3–54. [Google Scholar]
- Brown P, Fearn T, Vannucci M. The choice of variables in multivariate regression: a non-conjugate Bayesian decision theory approach. Biometrika. 1999;86:635–648. [Google Scholar]
- Brown P, Vannucci M, Fearn T. Multivariate Bayesian variable selection and prediction. J. R. Statist. Soc. B. 1998;60:627–641. [Google Scholar]
- Brown P, Vannucci M, Fearn T. Bayes model averaging with selection of regressors. J. R. Statist. Soc. B. 2002;64:519–536. [Google Scholar]
- Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: Similarities between tumors and wounds. PLoS Biol. 2004;2(2) doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, Hastie T, Johnstone I, Tibshirani R. Least Angle Regression. Annals of Statistics. 2004;32:407–499. [Google Scholar]
- Frank I, Friedman J. A statistical view of some chemometrics regression tools (with discussion) Technometrics. 1993;35:109–148. [Google Scholar]
- Fu W. Penalized regressions: the bridge vs the lasso. Journal of Computational and Graphical Statistics. 1998;7(3):417–433. [Google Scholar]
- Friedman J, Hastie T, Tibshirani R. Techniqual Report. Department of Statistics, Stanford University; 2008. Regularized Paths for Generalized Linear Models via Coordinate Descent. [Google Scholar]
- Friedman J, Hastie T, Tibshirani R. Pathwise coordinate optimization. The Annals of Applied Statistics. 2007;1(2):302–332. [Google Scholar]
- Fujikoshi Y, Satoh K. Modified AIC and Cp in multivariate linear regression. Biometrika. 1997;84:707–716. [Google Scholar]
- Gardner TS, DI Bernardo D, Lorenz D, Collins JJ. Inferring genetic networks and identifying compound mode of action via expression profiling. Science. 2003;301 doi: 10.1126/science.1081900. [DOI] [PubMed] [Google Scholar]
- Hyman E, Kauraniemi P, Hautaniemi S, Wolf M, Mousses S, Rozenblum E, Ringner M, Sauter G, Monni O, Elkahloun A, Kallioniemi O-P, Kallioniemi A. Impact of dna amplification on gene expression patterns in breast cancer. Cancer Res. 2002;62 [PubMed] [Google Scholar]
- Izenman A. Reduced-rank regression for the multivariate linear model. J. Multiv. Anal. 1975;5:248–264. [Google Scholar]
- Jeong H, Mason SP, Barabasi AL, Oltvai ZN. Lethality and centrality in protein networks. Nature. 2001;(411) doi: 10.1038/35075138. [DOI] [PubMed] [Google Scholar]
- Kapp AV, Jeffrey SS, Langerod A, Borresen-Dale AL, Han W, Noh DY, Bukholm IR, Nicolau M, Brown PO, Tibshirani R. Discovery and validation of breast cancer subtypes. BMC Genomics. 2006;7:231. doi: 10.1186/1471-2164-7-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao J, Pollack JR. RNA interference-based functional dissection of the 17q12 amplicon in breast cancer reveals contribution of coamplified genes. Genes Chromosomes Cancer. 2006;45(8):761–769. doi: 10.1002/gcc.20339. [DOI] [PubMed] [Google Scholar]
- Kim S, Sohn K-A, Xing EP. A multivariate regression approach to association analysis of quantitative trait network. 2008 doi: 10.1093/bioinformatics/btp218. http://arxiv.org/abs/0811.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langerod A, Zhao H, Borgan O, Nesland JM, Bukholm IR, Ikdahl T, Karesen R, Borresen-Dale AL, Jeffrey SS. TP53 mutation status and gene expression profiles are powerful prognostic markers of breast cancer. Breast Cancer Res. 2007;9:R30. doi: 10.1186/bcr1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz R, Bühlmann P. Boosting for high-multivariate responses in high-dimensional linear regression. Statist. Sin. 2006;16:471–494. [Google Scholar]
- Obozinskiy G, Wainwrighty MJ, Jordany MI. Union support recovery in high-dimensional multivariate regression. 2008 http://arxiv.org/abs/0808.0711. [Google Scholar]
- Paik S, Shak S, Tang G, Kim C, Baker J, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- Peng J, Wang P, Zhou N, Zhu J. Partial Correlation Estimation by Joint Sparse Regression Models. JASA. 2008;104(486) doi: 10.1198/jasa.2009.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack J, Srlie T, Perou C, Rees C, Jeffrey S, Lonning P, Tibshirani R, Botstein D, Brresen-Dale A, Brown P. Microarray analysis reveals a major direct role of dna copy number alteration in the transcriptional program of human breast tumors. Proc Natl Acad Sci. 2002;99(20) doi: 10.1073/pnas.162471999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinsel G, Velu R. Multivariate Reduced-rank Regression: Theory and Applications. New York: Springer; 1998. [Google Scholar]
- Saal LH, Johansson P, Holm K, Gruvberger-Saal SK, She QB, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci U S A. 2007;104(18):7564–7569. doi: 10.1073/pnas.0702507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lnning PE, Brresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lnning PE, Brown PO, Brresen-Dale A-L, Botstein D. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, et al. Gene expression profiling in breast cancer: Understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst. 2006;98(4):262–272. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]
- Tibshirani R. Regression shrinkage and selection via the lasso. J. R. Statist. Soc. B. 1996;58:267–288. [Google Scholar]
- Tibshirani R, Wang P. Spatial smoothing and hot spot detection for cgh data using the fused lasso. Biostatistics. 2008;9(1):18–29. doi: 10.1093/biostatistics/kxm013. [DOI] [PubMed] [Google Scholar]
- Turlach B, Venables W, Wright S. Simultaneous variable selection. Technometrics. 2005;47:349–363. [Google Scholar]
- Wang P. Ph.D. Thesis. Stanford University; 2004. Statistical methods for CGH array analysis. [Google Scholar]
- Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365(9460):671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347(25):1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- Yuan M, Ekici A, Lu Z, Monterio R. Dimension reduction and coefficient estimation in multivariate linear regression. J. R. Statist. Soc. B. 2007;69(3):329–346. [Google Scholar]
- Yuan M, Lin Y. Model Selection and Estimation in Regression with Grouped Variables. Journal of the Royal Statistical Society, Series B. 2006;68(1):49–67. [Google Scholar]
- Zhao H, Langerod A, Ji Y, Nowels KW, Nesland JM, Tibshirani R, Bukholm IK, Karesen R, Botstein D, Borresen-Dale AL, Jeffrey SS. Different gene expression patterns in invasive lobular and ductal carcinomas of the breast. Mol Biol Cell. 2004;15:2523–2536. doi: 10.1091/mbc.E03-11-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Rocha G, Yu B. Grouped and hierarchical model selection through composite absolute penalties. Annals of Statistics. 2006 Accepted. [Google Scholar]
- Zou H, Trevor H, Tibshirani R. On degrees of freedom of the lasso. Annals of Statistics. 2007;35(5):2173–2192. [Google Scholar]
- Zou H, Trevor T. Regularization and Variable Selection via the Elastic Net. Journal of the Royal Statistical Society, Series B. 2005;67(2):301–320. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.