Abstract
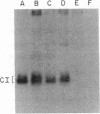
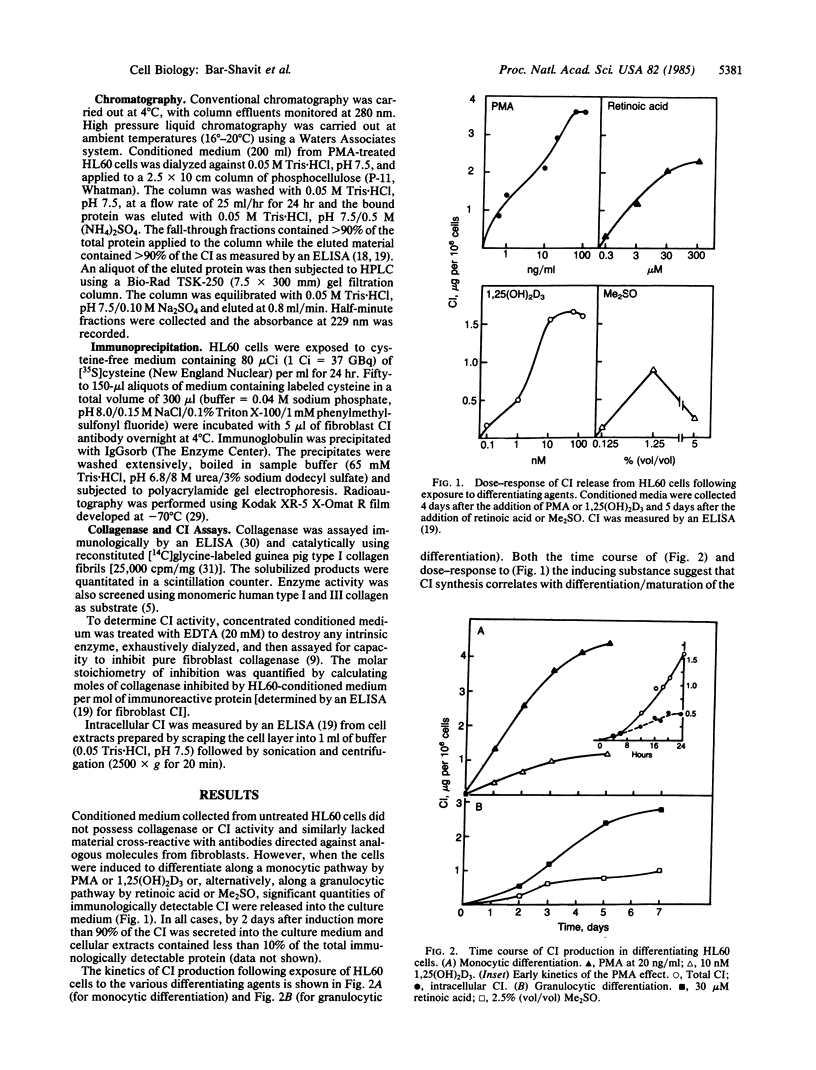
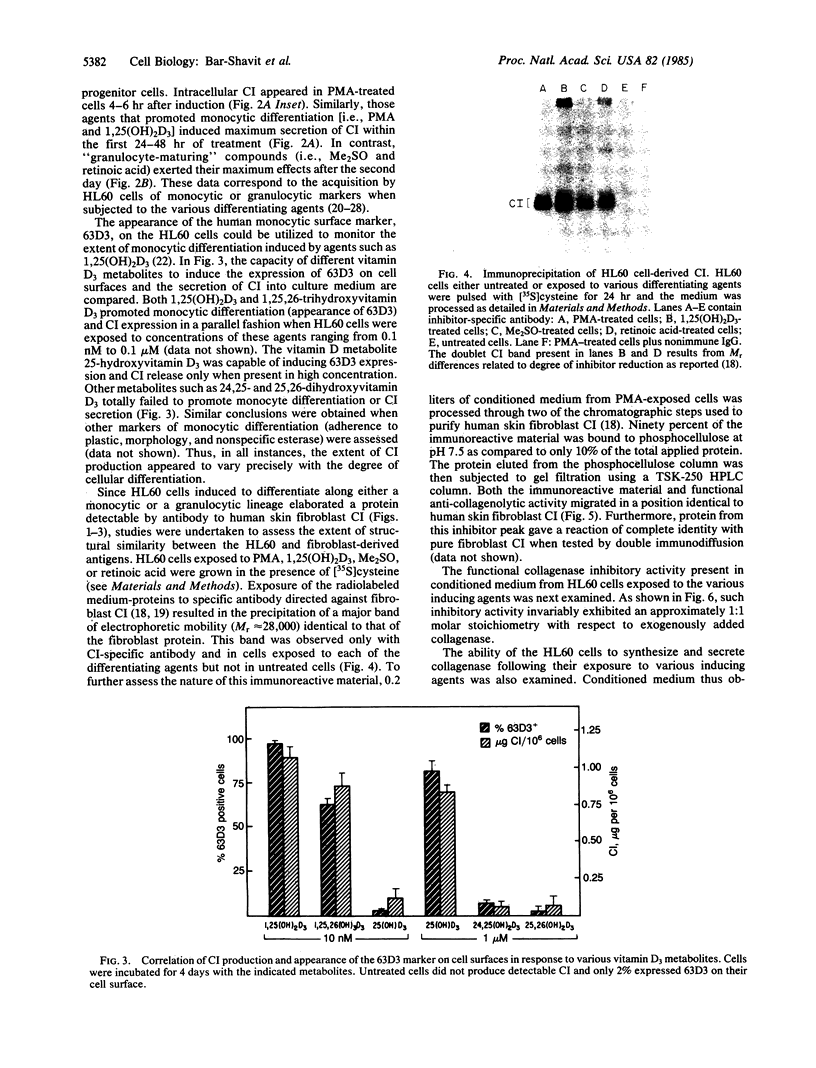
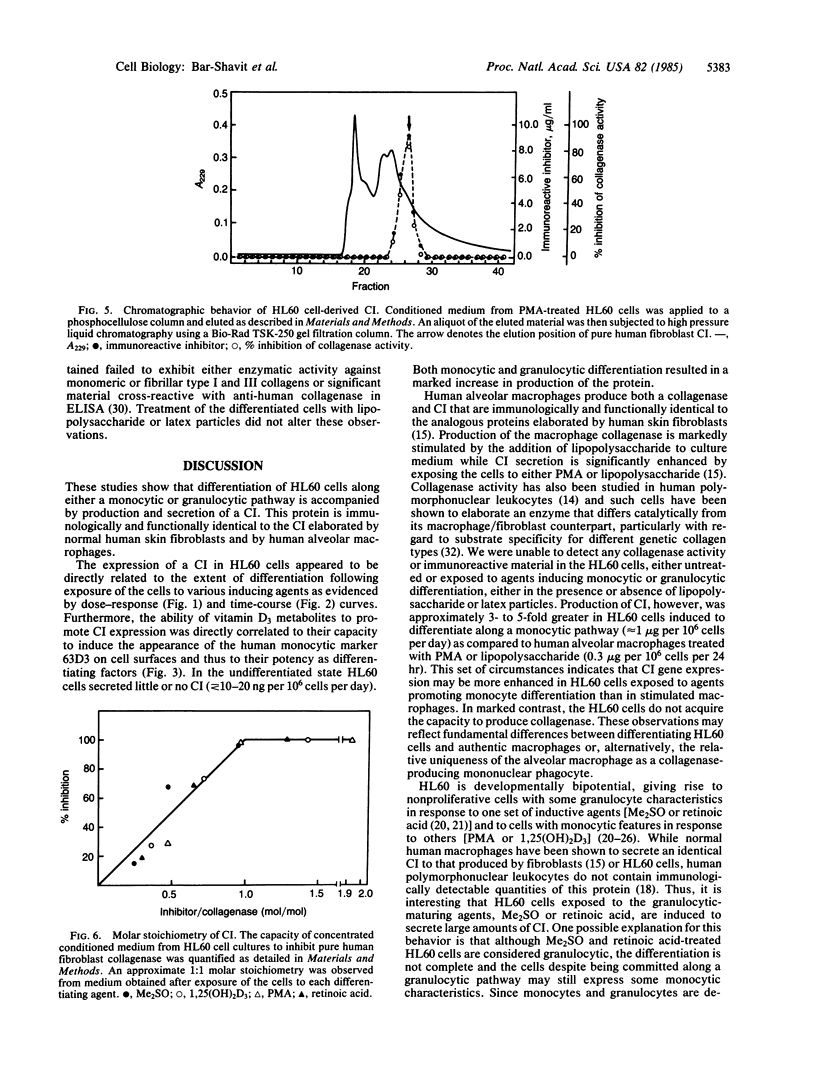
A human collagenase inhibitor (CI) of Mr 28,500 has been extensively characterized in skin fibroblasts and identified in a variety of connective tissues. Because human alveolar macrophages synthesize and secrete both a collagenase and CI that are immunologically and functionally identical to their counterparts in fibroblasts, we studied the production of such proteins by an immature human cell line (HL60) that can be induced to differentiate along monocytic or granulocytic pathways. The cells failed to synthesize collagenase under any culture condition tested. However, upon exposure to 1,25-dihydroxyvitamin D3 or phorbol esters (PMA), both of which promote monocytic differentiation of HL60, these cells synthesized and released CI in a dose-dependent manner. Furthermore, the extent of CI expression was paralleled by the acquisition by such cells of the monocytic marker 63D3, indicating that inhibitor production and differentiation are closely correlated. This CI was immunologically and functionally identical to that produced by human macrophages and human skin fibroblasts. The quantity of CI synthesized by PMA-stimulated cells was 3- to 5-fold greater than produced by human alveolar macrophages, approximately equal to 1 microgram per 10(6) cells per day. In contrast, undifferentiated HL60 cells produced little or no detectable CI (less than or equal to 10-20 ng per 10(6) cells per day). Interestingly, when HL60 cells were stimulated to undergo granulocytic differentiation by dimethyl sulfoxide or retinoic acid, they also produced the "monocytic" CI.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit Z., Teitelbaum S. L., Reitsma P., Hall A., Pegg L. E., Trial J., Kahn A. J. Induction of monocytic differentiation and bone resorption by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5907–5911. doi: 10.1073/pnas.80.19.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston T. E., Murphy G., Mercer E., Galloway W. A., Hazleman B. L., Reynolds J. J. The interaction of purified rabbit bone collagenase with purified rabbit bone metalloproteinase inhibitor. Biochem J. 1983 May 1;211(2):313–318. doi: 10.1042/bj2110313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston T. E., Tyler J. A. Purification of pig synovial collagenase to high specific activity. Biochem J. 1979 Dec 1;183(3):647–656. doi: 10.1042/bj1830647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. W., Bauer E. A., Eisen A. Z. Enzyme-linked immunosorbent assay for human skin collagenase. Coll Relat Res. 1983 May;3(3):205–215. doi: 10.1016/s0174-173x(83)80004-1. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Bréard J., Chess L., Krane S. M. Participation of monocyte-macrophages and lymphocytes in the production of a factor that stimulates collagenase and prostaglandin release by rheumatoid synovial cells. J Clin Invest. 1979 Nov;64(5):1386–1392. doi: 10.1172/JCI109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J., Nagai Y. Specific degradation of the collagen molecule by tadpole collagenolytic enzyme. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1197–1204. doi: 10.1073/pnas.54.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, Evanson J. M., DiBona D. R., Krane S. M. Collagenase and rheumatoid arthritis. Arthritis Rheum. 1970 Jan-Feb;13(1):83–94. doi: 10.1002/art.1780130109. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Collagenases (first of three parts). N Engl J Med. 1974 Sep 12;291(11):557–563. doi: 10.1056/NEJM197409122911105. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Collagenases (second of three parts). N Engl J Med. 1974 Sep 19;291(12):605–609. doi: 10.1056/NEJM197409192911205. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Collagenases (third of three parts). N Engl J Med. 1974 Sep 26;291(13):652–661. doi: 10.1056/NEJM197409262911305. [DOI] [PubMed] [Google Scholar]
- Hasty K. A., Hibbs M. S., Kang A. H., Mainardi C. L. Heterogeneity among human collagenases demonstrated by monoclonal antibody that selectively recognizes and inhibits human neutrophil collagenase. J Exp Med. 1984 May 1;159(5):1455–1463. doi: 10.1084/jem.159.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A. L., Hance A. J., Crystal R. G. Granulocyte collagenase: selective digestion of type I relative to type III collagen. Proc Natl Acad Sci U S A. 1977 Mar;74(3):897–901. doi: 10.1073/pnas.74.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Regulation of normal differentiation in mouse and human myeloid leukemic cells by phorbol esters and the mechanism of tumor promotion. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5158–5162. doi: 10.1073/pnas.76.10.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Koeffler H. P., Donaldson C. A., Pike J. W., Haussler M. R. 1,25-Dihydroxyvitamin D3-induced differentiation in a human promyelocytic leukemia cell line (HL-60): receptor-mediated maturation to macrophage-like cells. J Cell Biol. 1984 Feb;98(2):391–398. doi: 10.1083/jcb.98.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D. M., San Miguel J. F., Freake H. C., Green P. M., Zola H., Catovsky D., Goldman J. M. 1,25-dihydroxyvitamin D3 inhibits proliferation of human promyelocytic leukaemia (HL60) cells and induces monocyte-macrophage differentiation in HL60 and normal human bone marrow cells. Leuk Res. 1983;7(1):51–55. doi: 10.1016/0145-2126(83)90057-7. [DOI] [PubMed] [Google Scholar]
- Murao S., Gemmell M. A., Callaham M. F., Anderson N. L., Huberman E. Control of macrophage cell differentiation in human promyelocytic HL-60 leukemia cells by 1,25-dihydroxyvitamin D3 and phorbol-12-myristate-13-acetate. Cancer Res. 1983 Oct;43(10):4989–4996. [PubMed] [Google Scholar]
- Nagai Y., Lapiere C. M., Gross J. Tadpole collagenase. Preparation and purification. Biochemistry. 1966 Oct;5(10):3123–3130. doi: 10.1021/bi00874a007. [DOI] [PubMed] [Google Scholar]
- Reitsma P. H., Rothberg P. G., Astrin S. M., Trial J., Bar-Shavit Z., Hall A., Teitelbaum S. L., Kahn A. J. Regulation of myc gene expression in HL-60 leukaemia cells by a vitamin D metabolite. Nature. 1983 Dec 1;306(5942):492–494. doi: 10.1038/306492a0. [DOI] [PubMed] [Google Scholar]
- Rovera G., Santoli D., Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricklin G. P., Bauer E. A., Jeffrey J. J., Eisen A. Z. Human skin collagenase: isolation of precursor and active forms from both fibroblast and organ cultures. Biochemistry. 1977 Apr 19;16(8):1607–1615. doi: 10.1021/bi00627a013. [DOI] [PubMed] [Google Scholar]
- Stricklin G. P., Welgus H. G. Human skin fibroblast collagenase inhibitor. Purification and biochemical characterization. J Biol Chem. 1983 Oct 25;258(20):12252–12258. [PubMed] [Google Scholar]
- Vater C. A., Mainardi C. L., Harris E. D., Jr Inhibitor of human collagenase from cultures of human tendon. J Biol Chem. 1979 Apr 25;254(8):3045–3053. [PubMed] [Google Scholar]
- Wahl L. M., Wahl S. M., Mergenhagen S. E., Martin G. R. Collagenase production by endotoxin-activated macrophages. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3598–3601. doi: 10.1073/pnas.71.9.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Bar-Shavit Z., Senior R. M., Teitelbaum S. L. Human alveolar macrophages produce a fibroblast-like collagenase and collagenase inhibitor. J Clin Invest. 1985 Jul;76(1):219–224. doi: 10.1172/JCI111949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Jeffrey J. J., Eisen A. Z. The collagen substrate specificity of human skin fibroblast collagenase. J Biol Chem. 1981 Sep 25;256(18):9511–9515. [PubMed] [Google Scholar]
- Welgus H. G., Jeffrey J. J., Stricklin G. P., Roswit W. T., Eisen A. Z. Characteristics of the action of human skin fibroblast collagenase on fibrillar collagen. J Biol Chem. 1980 Jul 25;255(14):6806–6813. [PubMed] [Google Scholar]
- Welgus H. G., Stricklin G. P. Human skin fibroblast collagenase inhibitor. Comparative studies in human connective tissues, serum, and amniotic fluid. J Biol Chem. 1983 Oct 25;258(20):12259–12264. [PubMed] [Google Scholar]
- Werb Z., Gordon S. Secretion of a specific collagenase by stimulated macrophages. J Exp Med. 1975 Aug 1;142(2):346–360. doi: 10.1084/jem.142.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]