Abstract
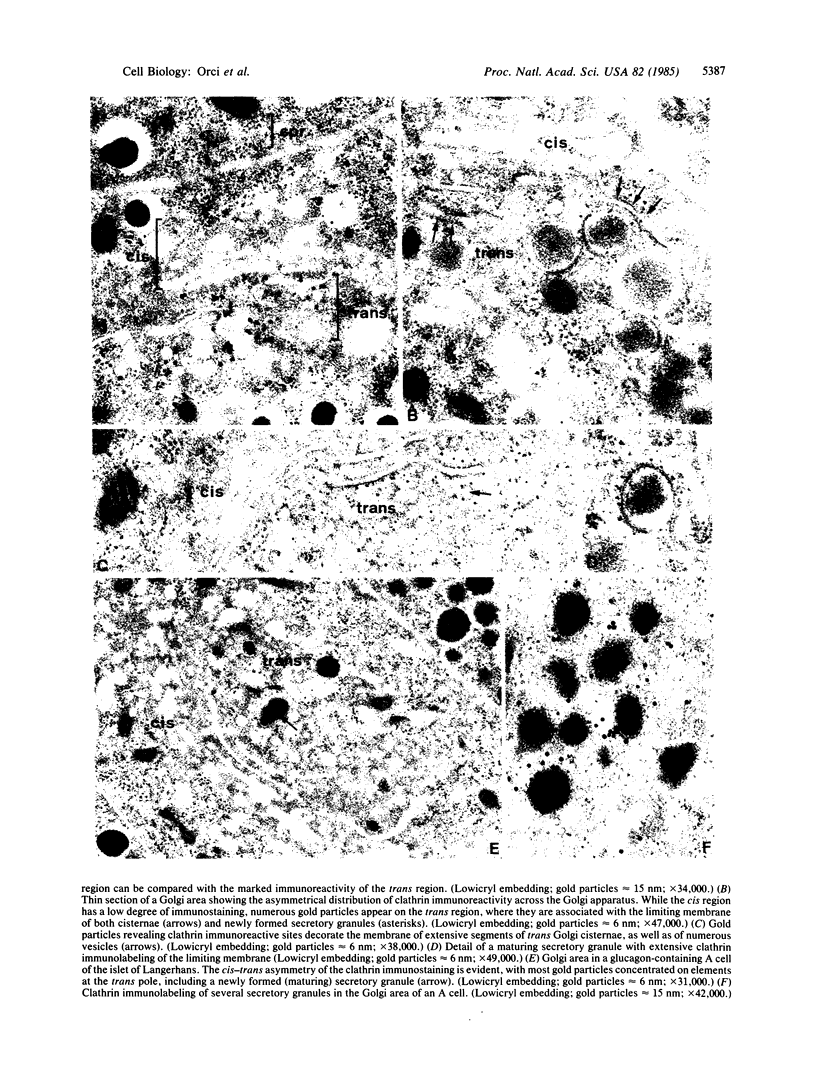
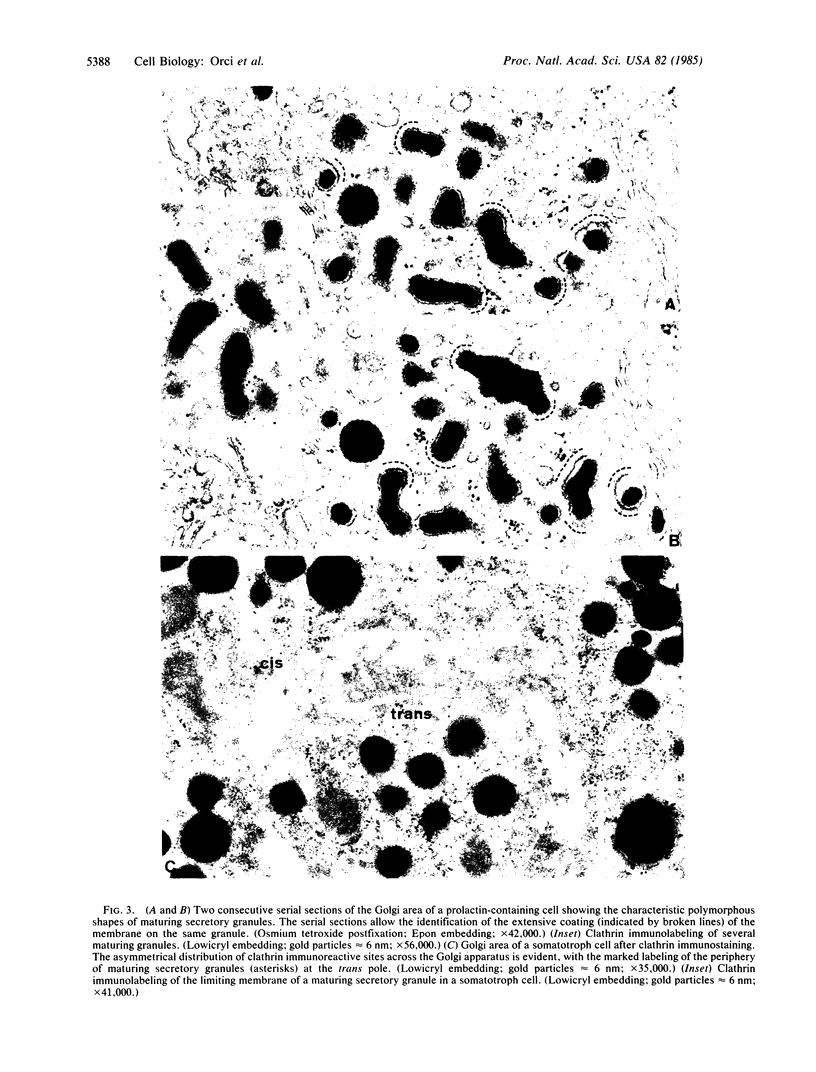
By analogy with receptor-mediated endocytosis, clathrin-coated membrane segments at locations other than at the plasma membrane have been implicated in intracellular membrane transport/sorting. The crucial role of the Golgi apparatus in these processes prompted us to study the distribution at this level of clathrin-immunoreactive sites in various polypeptide hormone-secreting cells. With a polyclonal antibody recognizing the polymerized form of clathrin (structural coats or cages), we found that the Golgi area had the highest degree of specific labeling of various cytoplasmic regions, including the subplasmalemmal web. Moreover, the Golgi labeling was not homogeneously distributed, most of the immunoreactive sites being associated with membrane segments at the trans pole. The labeled membranes comprised regions of trans cisternae with and without condensing secretory material, newly formed (maturing) secretory granules freshly released from trans cisternae, and typical coated vesicles. The cis Golgi cisternae, as well as the cytoplasmic region containing transfer (shuttle) vesicles and transitional cisternae of the rough endoplasmic reticulum, were only weakly labeled. Clathrin concentration, degree of assembly of triskelions into coats, duration of clathrin association with membranes, or presence of a coat protein different from clathrin could account for the low immunoreactivity observed at the cis pole as compared to the trans pole.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armbruster B. L., Carlemalm E., Chiovetti R., Garavito R. M., Hobot J. A., Kellenberger E., Villiger W. Specimen preparation for electron microscopy using low temperature embedding resins. J Microsc. 1982 Apr;126(Pt 1):77–85. doi: 10.1111/j.1365-2818.1982.tb00358.x. [DOI] [PubMed] [Google Scholar]
- COONS A. H., LEDUC E. H., CONNOLLY J. M. Studies on antibody production. I. A method for the histochemical demonstration of specific antibody and its application to a study of the hyperimmune rabbit. J Exp Med. 1955 Jul 1;102(1):49–60. doi: 10.1084/jem.102.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther R. A., Pearse B. M. Assembly and packing of clathrin into coats. J Cell Biol. 1981 Dec;91(3 Pt 1):790–797. doi: 10.1083/jcb.91.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Carroll R. J., Steiner D. F. Conversion of proinsulin to insulin: involvement of a 31,500 molecular weight thiol protease. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4613–4617. doi: 10.1073/pnas.79.15.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgac M., Cantley L., Wiedenmann B., Altstiel L., Branton D. Clathrin-coated vesicles contain an ATP-dependent proton pump. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1300–1303. doi: 10.1073/pnas.80.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman J., Croen K., Kelly S., Al-Awqati Q. Golgi membranes contain an electrogenic H+ pump in parallel to a chloride conductance. J Cell Biol. 1983 Oct;97(4):1303–1308. doi: 10.1083/jcb.97.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Louvard D., Morris C., Warren G., Stanley K., Winkler F., Reggio H. A monoclonal antibody to the heavy chain of clathrin. EMBO J. 1983;2(10):1655–1664. doi: 10.1002/j.1460-2075.1983.tb01640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvard D., Reggio H., Warren G. Antibodies to the Golgi complex and the rough endoplasmic reticulum. J Cell Biol. 1982 Jan;92(1):92–107. doi: 10.1083/jcb.92.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell M. H. Two rapid and simple methods used for the removal of resins from 1.0 micron thick epoxy sections. J Microsc. 1978 Mar;112(2):253–255. doi: 10.1111/j.1365-2818.1978.tb01174.x. [DOI] [PubMed] [Google Scholar]
- Orci L., Halban P., Amherdt M., Ravazzola M., Vassalli J. D., Perrelet A. A clathrin-coated, Golgi-related compartment of the insulin secreting cell accumulates proinsulin in the presence of monensin. Cell. 1984 Nov;39(1):39–47. doi: 10.1016/0092-8674(84)90189-2. [DOI] [PubMed] [Google Scholar]
- Orci L., Halban P., Amherdt M., Ravazzola M., Vassalli J. D., Perrelet A. Nonconverted, amino acid analog-modified proinsulin stays in a Golgi-derived clathrin-coated membrane compartment. J Cell Biol. 1984 Dec;99(6):2187–2192. doi: 10.1083/jcb.99.6.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L. Macro- and micro-domains in the endocrine pancreas. Diabetes. 1982 Jun;31(6 Pt 1):538–565. doi: 10.2337/diab.31.6.538. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Perrelet A. (Pro)insulin associates with Golgi membranes of pancreatic B cells. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6743–6746. doi: 10.1073/pnas.81.21.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Pearse B. M., Bretscher M. S. Membrane recycling by coated vesicles. Annu Rev Biochem. 1981;50:85–101. doi: 10.1146/annurev.bi.50.070181.000505. [DOI] [PubMed] [Google Scholar]
- Pearse B. M. Clathrin: a unique protein associated with intracellular transfer of membrane by coated vesicles. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1255–1259. doi: 10.1073/pnas.73.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pictet R., Orci L., Gonet A. E., Rouiller C., Renold A. E. Ultrastructural studies of the hyperplastic islets of Langerhans of spiny mice (Acomys cahirinus) before and during the development of hyperglycemia. Diabetologia. 1967 Apr;3(2):188–211. doi: 10.1007/BF01222197. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Bursztyn-Pettegrew H., Fine R. E. Transport of the membrane glycoprotein of vesicular stomatitis virus to the cell surface in two stages by clathrin-coated vesicles. J Cell Biol. 1980 Jul;86(1):162–171. doi: 10.1083/jcb.86.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Fine R. E. Coated vesicles transport newly synthesized membrane glycoproteins from endoplasmic reticulum to plasma membrane in two successive stages. Proc Natl Acad Sci U S A. 1980 Feb;77(2):780–784. doi: 10.1073/pnas.77.2.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. Sizing of protein A-colloidal gold probes for immunoelectron microscopy. J Cell Biol. 1981 Aug;90(2):533–536. doi: 10.1083/jcb.90.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly W. S., Fischer H. D. The phosphomannosyl recognition system for intracellular and intercellular transport of lysosomal enzymes. J Cell Biochem. 1982;18(1):67–85. doi: 10.1002/jcb.1982.240180107. [DOI] [PubMed] [Google Scholar]
- Stone D. K., Xie X. S., Racker E. An ATP-driven proton pump in clathrin-coated vesicles. J Biol Chem. 1983 Apr 10;258(7):4059–4062. [PubMed] [Google Scholar]
- Ungewickell E., Branton D. Assembly units of clathrin coats. Nature. 1981 Jan 29;289(5796):420–422. doi: 10.1038/289420a0. [DOI] [PubMed] [Google Scholar]