Abstract
Substantial progress has been made in recent years toward understanding the molecular mechanisms by which tumor cells, and the supporting stroma, degrade confining matrix during migration. Significant attention has been focused toward understanding the biology of several dynamic and distinct, but remarkably related, cell structures that include lamellipodia, focal adhesions, filopodia, podosomes, and invadopodia. How these invasive organelles assemble and function is a topic of intense study. Most exciting has been the recent progress made combining advanced microscopic technologies with a wide variety of different 3D matrices, tissue explants, or even living model organisms. From these approaches, it has become increasingly evident that the conventional definitions for these invasive structures may be less clear than was previously thought.
Keywords: Matrix remodeling, invadopodia, lamellipodia
Adhere and Degrade: Invasive Dissemination as a Key Component of the Metastatic Process
It is well documented that many malignant tumors are characterized by modest encapsulation that permits an aggressive dissemination from the site of origin into peripheral stroma, vessels, and subsequently, other organs. In addition to known defects in genomic stability, cell cycle check points, and contact inhibition, many aggressive tumors possess the capacity to actively degrade and remodel the surrounding stroma by the combined processes of matrix metalloprotease secretion [1-3] and chemotactic migration [4]. This invasive process is supported not only by the primary neoplastic lesion but by a complex stroma that includes cancer associated fibroblasts (CAFs), macrophages, and endothelial cells that contribute distinct motogenic cytokines and matrix components. This complicated mix forms what is commonly referred to as the tumor microenvironment and provides a supportive environment for tumor cell migration either individually or in a ‘collective’ fashion (Figure 1a)[1].
Figure 1.
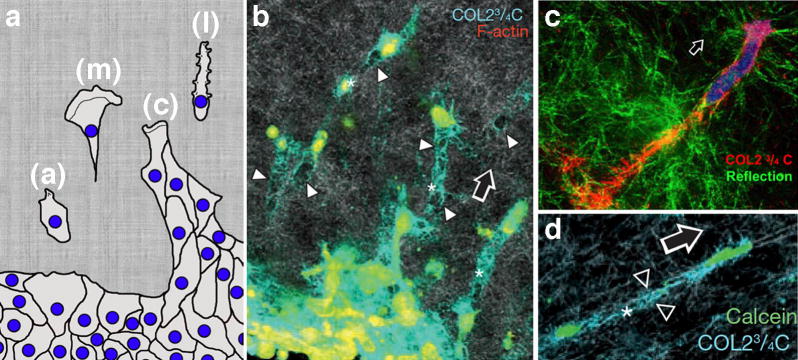
Breaking away: synergistic movement by tumor and stromal cells leaving a destructive path. a) Cartoon illustrating distinct classes of cellular movement from the site of the primary tumor. These include mesenchymal (m), lobopodial (l), amoeboid (a), and collective (c) cell motility. These cellular movements exhibit different morphologies and requirements for ROCK, Rho, myosin and other components while supporting tumor dissemination and matrix remodeling. (Modified from [1] with permission). b-d) Ht1080. Cells were fixed while migrating through a collagen lattice in vitro. The blue (b,d) or red (c) represents paths of degraded collagen using a specific antibody (COL2 ¾ C) while other markers indicate the cell body. Arrows indicate direction of migration, arrowheads point to degraded collagen trails. Republished with permission from [16] and [2].
Lamellipodia, focal adhesions, podosomes, filopodia, and invadopodia are known to share many components that are mixed and matched upon a central core of branched or bundled actin filaments. This core usually can sustain a protrusive deformation of a plasma membrane domain that may exhibit some enrichment in specific phosphoinositides. A large variety of different actin scaffolding proteins -- including WASP, N-WASP, VASP, and actin bundling/remodeling proteins such as cortactin, gelsolin, and many others -- can be attached upon this central scaffold. In turn, this network provides a platform for integrin binding and recruitment of small Rho GTPases, myosins, Src kinases, dynamin, and many other components (for helpful tables and illustrations that compare the content and characteristics of these structures see [5-7]). As lamellipodia, filopodia, focal adhesions and the bona fide matrix-degrading structures invadopodia and podosomes are similar in composition, it may not be surprising that these structures may interact, exchange, interconvert, and even coalesce at the leading edge of migrating tumor cells and/or accompanying fibroblasts. This essay does not provide a comprehensive review of the literature on invasive migration, which has been done nicely by others [5, 6, 8]. Instead, a focus on recent observations implicating the advancing lamellipodia as a multi-purpose, degradative, and contractile structure or ‘invadosome’ is provided.
Due to structural and functional similarities, the terms podosomes and invadopodia have been used interchangeably in the literature. More recently, the field has arrived at some clarity in the use of these descriptors. While perhaps identical in structural content, podosomes are now generally viewed as degradative organelles of more differentiated cell types that can include invading macrophages, vascular smooth muscle cells, bone remodeling osteoclasts, and more. In contrast, invadopodia could be viewed as renegade aberrations of neoplastic transformation in which oncogenic activation leads to an inappropriate mobilization of the actin cytoskeleleton and associated proteins. Indeed, normal epithelial cells of ductular-based organs such the liver, breast, or pancreas would seem to have little obvious need for these invasive structures. More likely, these are assembled from pre-existing components upon transformation as cells lose a characteristic polarized organization, and contacts with adjacent cells are compromised.
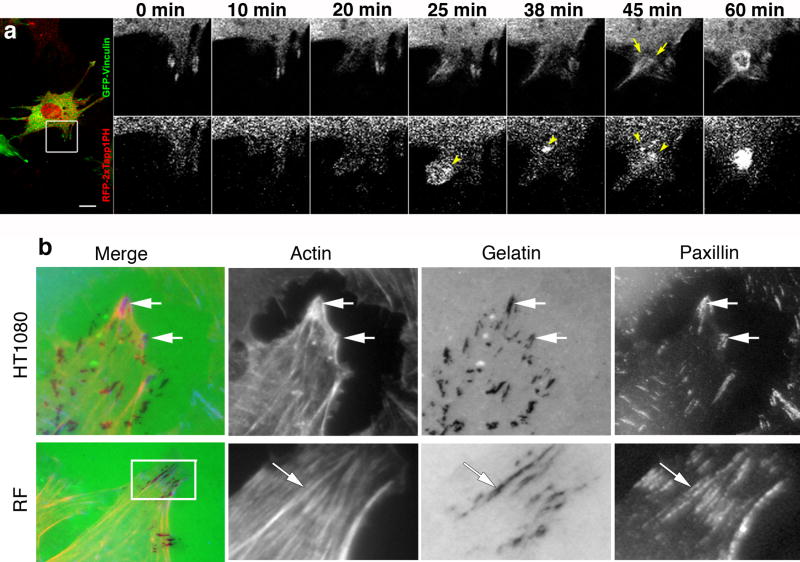
If most healthy differentiated epithelial cells do not normally degrade the surrounding matrix, then how are invadopodia formed upon neoplastic transformation? While many cultured tumor cell lines do not form bona fide invadopodia, those that do could utilize focal adhesions (FAs) or equivalent structures as nucleating precursors. As detailed in a recent review [6],these structures share a substantial, nearly complete set of components. In fact, it can be challenging to distinguish the two structures biochemically or by cell staining. There is strong evidence, however, for FAs functioning as precursors for invadopodia formation [9]. In this study, an accumulation of phosphoinositide (PtdIns(3,4)P2) at FAs was key to initiating a Src-induced formation of invadopodia in NIH3T3 cells (Figure 3a). These authors put forward a stepwise model for invadopodia formation that formats pre-existing FA sites by activation of Src and focal adhesion kinases (FAK) to stimulate the formation of (PtdIns(3,4)P2) that, in turn, recruit N-WASp, dynamin, cortactin and the invadopodia-specific adaptors Tks4/5.
Figure 3.
Focal adhesions as degradative structures. a) Focal adhesions as precursors for invadopodia. Live cell imaging of an NIH3T3 cell showing a conversion of focal adhesion structures into active podosome/invadopodia rosettes. The cells expressing a GFP-vinculin and an RFP biosensor to detect PtdIns(3,4)P2 (RFP-2x Tapp1PH) were transfected to express active Src that promotes rosette formation. The region outlined by a box is an example of a newly formed FA, which was associated with a podosome (magnified and shown with elapsed time points). Arrowheads indicate the accumulation of a PIP2 biosensor, and arrows indicate a developing podosome/invadopodia based on vinculin accumulation. Republished with permission from [9]. b) Focal adhesions degrade matrix. Cells plated on a fluorescent matrix to indicate site of degradations that colocalize with FA markers. These patterns are observed by many cell types including pancreatic tumor cells (not shown) as well as fibrosarcoma cells (HT1080) and rat fibroblasts. Actin stress fibers insert into the sites of focal adhesion matrix degradation. Following 10-16 hours on the matrix substrate cells were fixed, and the structural relationship between FAs (paxillin), actin stress fibers and sites of matrix degradation were monitored concomitantly. Boxed regions are enlarged on the right. Republished with permission from [12].
In addition to acting as nucleation sites for invadopodia formation, it is now believed that FAs can degrade matrix directly. This concept was first introduced from observations that exogenously expressed MT1-MMP was recruited to FAK-positive adhesive sites at the leading edge of migrating cells [10, 11]. This recruitment not only supported matrix degradation at FA sites but appeared to alter integrin clustering and thereby promote turnover of cell adhesions to facilitate motility. As HeLa cells express low levels of endogenous MT1-MMP, many of these observations used HeLa cells over-expressing exogenous MT1-MMP, or a human fibrosarcoma cell line (HT1080) that expresses high levels of endogenous MT1. Because of these elevated protease levels one could predict that the recruitment to FAs occurs upon saturation of the invadopodia machinery based on the similarity of these structures. These findings did, however, draw a functional comparison between FAs and invadopodia.
Very recently our group has made several observations that implicate FAs as bona fide matrix-degrading organelles [12]. Upon examination of several different human pancreatic adenocarcinoma cell lines (Panc-1, BxPC3, Panc 04.03), as well as human fibroblasts and HT-1080 cells, we observed a marked degradation of extracellular matrix at FA sites (Figure 3b). Because of the similarity between FAs and invadopodia, multiple criteria were utilized to discriminate between these structures in the cells examined. In general, FAs have an oblong shape, reside near the cell periphery, provide initiation sites for actin stress fibers, and are positive for FAK and p130Cas but negative for TKs4/5. In contrast, invadopodia were classified as centrally located and punctate in shape, have no apparent affinity for stress fibers, and stain intensely for cortactin and dynamin with little or no staining for FAK or p130Cas. Most importantly, this study provided molecular insights into how the MT1 protease could be recruited to FAs proper, mainly by an interaction with FAK. This interaction does not appear to be direct but is via binding to the adaptor protein p130Cas. Thus, the N-terminal SH3 domain of p130Cas binds directly to FAK while the central substrate domain interacts with the short cytoplasmic tail of the MT1-MMP. Finally, this interaction is initiated by a Src-dependent phosphorylation of a single tyrosine residue (Y573) within the Mt1 tail, because mutation of this tyrosine, or treatment of cells with Src inhibitors such as PP2, attenuates the formation of the FAK-p130Cas-MT1-MMP complex, and subsequently, matrix degradation and invasive migration.
Taken together, these findings implicate FAs as a key structure in both matrix attachment and degradation and provide support for the concept of a multi-purpose ‘invadosome’ at the leading edge of advancing cells. This hybrid structure would provide an economical function to extend, attach, compress, and degrade the surrounding stroma and clear the way for the lead cell and those that follow. A degradative role for FAs would be consistent with a role as precursor assembly sites for invadopodia formation [9] or providing a direct recruitment and activation of proteases to the adhesive site [12]. The adhesive regions of migrating cells are generally not considered to reside at the front-most edge of the advancing cell (lamellipodia) where actin dynamics occur. Instead, both adhesion and actomyosin-based contraction appear to ensue within a more proximal domain referred to as the lamella [13]. This structure/function demarcation of advancing cellular domains, as well as the distinct definitions of invadopodia versus FAs, has been generated largely from observations made in cultured cells residing upon a 2D glass substrate. As described below, these relationships appear to be both similar but also quite different when viewed in cells moving through a 3D environment.
Moving and Degrading in the Real World
With the development of novel cell imaging approaches coupled with a variety of different cell substrates to mimic conditions in situ, cell biologists are now able to observe the combined actions of tumor cell migration and extracellular proteolysis in 3-D [2, 14-21]. The observations from several laboratories performing sophisticated imaging of live cells, when combined with those from cells in culture, have led to the formulation of a five-step process supporting interstitial cell migration. Summarized nicely in recent reviews [2, 15], this process has been organized in spatial order from the leading edge of the cell to the distal tail and has been observed most often in individual migrating tumor cells or stromal cells that may act to lead the way for trailing cells by degrading a path through the surrounding stroma. Importantly, there is a concomitant forcible compression and alignment of adjacent collagen strands into larger fibrils that may act as retaining walls or ‘guardrails’ to confine the movement of trailing cells to the path of least resistance, thereby greatly facilitating migration.
Variations of this central theme will certainly occur depending upon the tumor type and as well as the specific microenvironment encountered. It has been observed that tumor cells or surrounding stromal cells exhibit several classes of migration; a linear, lamellipodial-based migration that is dependent upon concomitant matrix degradation, as well as a more rounded amoeboid-like movement (Figure 1a). It has been theorized that the more rigid, linear, and adhesive migration requires proteolytic matrix remodeling to allow the cell to navigate through its crosslinked surroundings. In contrast, the pliability of amoeboid cells would make them more amenable to squeezing through the tightest of spaces [15]. Several groups have observed this type of movement along collagen fibers that provide a path of least resistance without an obvious need for matrix degradation [15]. As suggested by others [19, 21, 22], the concentration, density, and crosslinking of the in vitro collagen matrices utilized could certainly change, reduce, or eliminate, perhaps artificially, the requirement for protease participation during invasion. Thus, by altering the characteristics of the experimental matrix, one might expect to change the mode of movement and matrix remodeling.
A graphic correlation between the biophysical properties of the surrounding matrix and migratory behavior has recently been observed [18]. In this study using primary human fibroblasts, they find that these cells undergo traditional polarized lamellipodial migration when placed on either a 2D-substrate or within a rigid 3D collagen matrix. Both environments appear to support polarized assembly of F-actin at the leading edge along with active Rac1, Cdc42, and PIP3. However, cells grown within a 3D cellular-derived matrix (CDM) exhibiting linear elasticity display a unique form of motility in which cells lack traditional lamellae and elongate with cylindrical protrusions. These ‘lobopodia’ are independent of RhoA, ROCK, or myosin II activity and appear to provide an alternative mechanism of translocation that is sensitive to the organization, linearity, and elasticity of the cell surroundings. It will be of interest to define how matrix degradation is altered by this unique mode of movement and if it is exhibited by cells of non-mesenchymal origin.
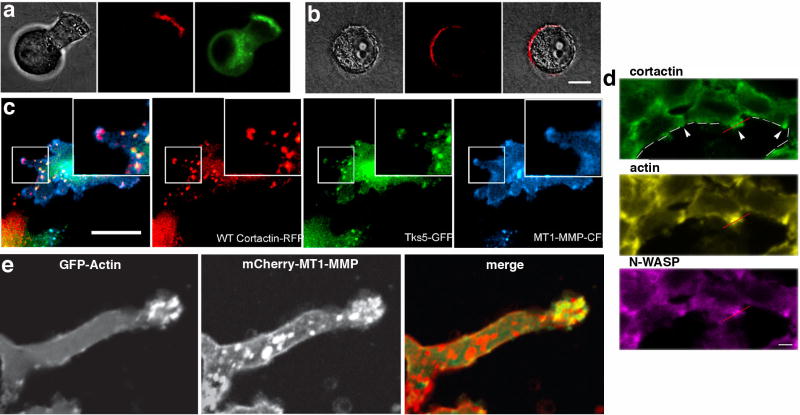
As the matrix environment is so influential to polarized cell migration, one can certainly expect alterations in the mode and pattern of the degradative process as well. A central question currently being addressed by cancer cell biologists is how do the many observations made from cells on a 2D substrate translate into a more relevant 3D environment; do invadopodia as they are currently defined actually play a role in situ, or might some modified structure be utilized that combines adhesion, protrusion and degradation in a concomitant way? This functional amalgamation has been observed previously. For example, a study of fibrosarcoma cells (HT1080) in a 3D matrix of native collagen has suggested that remodeling by these cells correlates spatially with protrusive activity at the leading edge and is protease-dependent [21]. Consistent with these findings are those employing a novel fluorescent biosensor that can be used in live cells to detect collagen cleavage [19]. With this probe the authors observe a graphic and prominent protease-dependent degradation of the fluorescent matrix almost exclusively at the protruding lamellipod of a variety of different migrating tumor cells (Figure 4a, b). The authors point out that this protease activity is essential for tumor cell translocation through 3D matrices composed of native crosslinked versus pepsin-treated gels. More recently, a detailed study has demonstrated a role for cortactin phosphorylation and cofilin activation in the dynamic protrusion of invadopodia from MDA-MB-231 breast carcinoma cells [23]. Equally interesting is the observation that the formation of canonical invadopodia at the base of these cells in 2D is altered significantly when the same cells are placed in a 3D Geltrex matrix mix. Under these conditions the authors report a merger of both invadopodia and lamellipodia into one invasive structure at the cell front that preserves the characteristics of an invadopodium, namely the presence of cortactin, cofilin, Tsk5 and proteolytic capacity (Figure 4c).
Figure 4.
Protease activity at the leading edge of migrating cells in 3D conditions. a,b) Imaging of protease activity on the surface of tumor cells migrating in a 3-dimensional collagen matrix using a novel fluorescent biosensor [19]. (a) GFP-MT1-MMP vesicles (green) appear to be transported to the leading edge of a breast tumor cell (A2) invading through the collagen matrix. Most protease activity (red) localizes to the leading edge. (b) A PC-3 prostate carcinoma cell invading a collagen gel showing localization to the leading edge. c) Representative images of a MDA-MB-231 breast tumor cell migrating through a 3D Geltrex matrix. The cell was transfected to express the invadopodial markers cortactin-RFP, Tks5-GFP, and MT1-MMP-CFP. Note the localization of the protease and other markers within the tips of the advancing lamellipodia [23]. d) Distribution of invadopodial actin components viewed in immunostained histological sections of mouse tumors formed by injection of human breast tumor cells expressing Cerulean–N-WASP. Leading edge protrusions are enriched in actin, cortactin, and N-WASP in cells advancing toward a blood vessel as indicated by a dashed white line[24]. e) Live cell imaging of a breast tumor cell (MDA-MB231) migrating through a 3D circular invasion assay. The cell is expressing GFP-actin and mCherry-MT1-MMP and shows an advancing invadosome enriched for both actin and the MT1 protease. Republished with permission from [17].
What cellular components might recruit and target nascent proteases to the protruding lamellipod to support matrix remodeling? The actin adaptor N-WASp that contributes to dendritic branched assembly of actin networks in a variety of cellular process is known to be upregulated in metastatic cancers. Once thought to mediate lamellipodial actin dynamics, it has most recently been found concentrated in invadopodia. Disruption of N-WASp expression or function in breast tumor cells (MTLn3) has been shown to attenuate invadopodia formation and subsequently matrix degradation and invasive migration both in vitro and in situ (Figure 4d) [24]. Most recently [17], an interesting extension of these initial observations was made. In this study the authors found that reducing N-WASp expression in invasive breast tumor cells (MDA-MB-231) has little negative impact on chemotactic cell migration. In contrast, N-WASp does appear to be required for cellular elongation and extension of long pseudopodia through 3D matrices. Most novel is the finding that these pseudopods provide an actin-rich cytoplasmic target for the trafficking of nascent membrane-associated MT1-MMP protease (Figure 4e). Upon arrival of the protease to this leading edge, perhaps by vesicle-mediated transport, a specific domain within the cytoplasmic tail (LLY) of the MT1 appears to bind directly to actin filaments thus facilitating its recruitment and stabilization.
These findings, coupled with those discussed earlier implicating a MT1-FAK interaction at focal adhesions [10, 12], provide mechanistic insights into how proteases could be targeted to actin-rich cellular domains. It is attractive to predict that nascent proteases, destined for the leading edge, might be transported from the Golgi to focal adhesions along microtubules in a kinesin- and Rab8-dependent process. As the dynamic, or plus ends, of microtubules are known to associate transiently with adhesions [25], this could provide a mechanism for targeting proteases to the leading edge for use [25]. Newly arrived MT1-MMP could bind to a p130Cas –FAK complex for subsequent stabilized recruitment via an interaction with actin filaments.
Concluding remarks
We speculate that the coalescence of components from focal adhesions, lamellipodia, and invadopodia results in the formation of an ‘invadosome’ or ‘invadatron’ that concomitantly adheres and extends a migrating cell forward as it remodels the matrix in its path. This model does not exclude the transport of proteases to conventional invadopodia at the base of migrating tumor cells, although there are remarkably few observations of these structures in situ. Indeed, after 30 years of study it has just become apparent that focal adhesions may actually form and function in a 3D environment [26]. Further, how invadopodia, situated around the cell center, would contribute to ‘clearing the way’ to support cellular migration is unclear. Whether invadopodia would form preferentially on any given surface of an invasive cell that is surrounded on all sides by matrix without defined ventral or dorsal surfaces is also unclear. It is possible that a cell could degrade centrally then repolarize to reform a nascent lamellipod that protrudes downward into the self-generated void. Alternatively, a series of leading vanguard cells, degrading from centrally situated invadopodia, could leave a path of matrix destruction for trailing cells to follow. An ‘invadatron’ at the advancing edge of invading cells seems most attractive as the concept is supported by several recent observations described above. Further, such a multipurpose organelle situated upfront to facilitate the ‘breaking away’ of a neoplastic lesion would function similar to an icebreaker ship specifically designed to clear a path for an ensuing convoy.
Some future challenges for those that wish to study and curtail this component of metastatic invasion are to further define the formation, composition, and regulation of the invadosome in situ. Are the cellular components utilized in 3D the same or different than on a flat surface? How is this ‘hybrid’ structure formed from other cellular organelles? How are proteases targeted to the leading edge and why might they exhibit a greater affinity for actin at the leading edge then other cellular locales? By what mechanisms would invading cells degrade matrix about the leading edge while concomitantly adhering to the same location?
Insight into these and many other challenges are key to moving the field forward.
Figure 2.
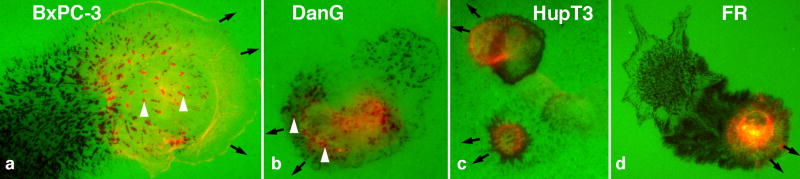
Migratory degradation by tumor and stromal cells in 2D culture. Images of cultured cells moving on top of a fluorescent gelatin matrix. a-c) Human pancreatic tumor cells, and (d) rat fibroblast (RF) transfected to express MT1-MMP. Cells were plated for 3-15 hours prior to fixation and staining for actin (a-c) or zyxin (d). Most striking are the numerous black voids that are left by the degradative action of actin-rich invadopodia (arrowheads), focal adhesions, and other structures. A trail of degradation that occurred prior to fixation reveals the migratory path made by each cell. The black arrows are situated at the advancing lamellipod of each cell and indicate the direction of movement. The white arrowheads point to sites of matrix degradation that correspond to actin structures, likely invadopodia.
Acknowledgments
Thanks to Dr. Yu Wang, Ph.D., who provided much of the initial observations of focal adhesion-based matrix degradation as part of his Ph.D. thesis in this laboratory. The McNiven program is supported by grants RO1CA104125 and DK044650. Several of the images provided in this review were by support from the Optical Microscopy Core of the Mayo Clinic Center for Cell Signaling in Gastroenterology (P30DK084567).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 2.Friedl P, Wolf K. Proteolytic interstitial cell migration: a five-step process. Cancer Metastasis Rev. 2009;28:129–135. doi: 10.1007/s10555-008-9174-3. [DOI] [PubMed] [Google Scholar]
- 3.Kessenbrock K, et al. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roussos ET, et al. Chemotaxis in cancer. Nat Rev Cancer. 2011;11:573–587. doi: 10.1038/nrc3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Block MR, et al. Podosome-type adhesions and focal adhesions, so alike yet so different. Eur J Cell Biol. 2008;87:491–506. doi: 10.1016/j.ejcb.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Sibony-Benyamini H, Gil-Henn H. Invadopodia: The leading force. Eur J Cell Biol. 2012 doi: 10.1016/j.ejcb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Oikawa T, et al. Sequential signals toward podosome formation in NIH-src cells. J Cell Biol. 2008;182:157–169. doi: 10.1083/jcb.200801042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takino T, et al. Inhibition of membrane-type 1 matrix metalloproteinase at cell-matrix adhesions. Cancer Res. 2007;67:11621–11629. doi: 10.1158/0008-5472.CAN-07-5251. [DOI] [PubMed] [Google Scholar]
- 11.Takino T, et al. Membrane-type 1 matrix metalloproteinase modulates focal adhesion stability and cell migration. Exp Cell Res. 2006;312:1381–1389. doi: 10.1016/j.yexcr.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, McNiven MA. Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J Cell Biol. 2012;196:375–385. doi: 10.1083/jcb.201105153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponti A, et al. Two distinct actin networks drive the protrusion of migrating cells. Science. 2004;305:1782–1786. doi: 10.1126/science.1100533. [DOI] [PubMed] [Google Scholar]
- 14.Tolde O, et al. The structure of invadopodia in a complex 3D environment. Eur J Cell Biol. 2010;89:674–680. doi: 10.1016/j.ejcb.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Wolf K, Friedl P. Extracellular matrix determinants of proteolytic and non-proteolytic cell migration. Trends Cell Biol. 2011;21:736–744. doi: 10.1016/j.tcb.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Wolf K, et al. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 17.Yu X, et al. N-WASP coordinates the delivery and F-actin mediated capture of MT1-MMP at invasive pseudopods to drive matrix remodeling and cancer cell invasion. doi: 10.1083/jcb.201203025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrie RJ, et al. Nonpolarized signaling reveals two distinct modes of 3D cell migration. J Cell Biol. 2012;197:439–455. doi: 10.1083/jcb.201201124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Packard BZ, et al. Direct visualization of protease activity on cells migrating in three-dimensions. Matrix Biol. 2009;28:3–10. doi: 10.1016/j.matbio.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harunaga JS, Yamada KM. Cell-matrix adhesions in 3D. Matrix Biol. 2011;30:363–368. doi: 10.1016/j.matbio.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloom RJ, et al. Mapping local matrix remodeling induced by a migrating tumor cell using three-dimensional multiple-particle tracking. Biophys J. 2008;95:4077–4088. doi: 10.1529/biophysj.108.132738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabeh F, et al. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol. 2009;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magalhaes MA, et al. Cortactin phosphorylation regulates cell invasion through a pH-dependent pathway. J Cell Biol. 2011;195:903–920. doi: 10.1083/jcb.201103045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gligorijevic B, et al. N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. J Cell Sci. 2012;125:724–734. doi: 10.1242/jcs.092726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akhmanova A, et al. Touch, grasp, deliver and control: functional crosstalk between microtubules and cell adhesions. Traffic. 2009;10:268–274. doi: 10.1111/j.1600-0854.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 26.Kubow KE, Horwitz AR. Reducing background fluorescence reveals adhesions in 3D matrices. Nat Cell Biol. 2011;13:3–5. doi: 10.1038/ncb0111-3. author reply 5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]