Abstract
Progressive bone mineral loss and increasing bone fragility are hallmarks of osteoporosis. A combination of minerals isolated from the red marine algae, Lithothamnion sp. was examined for ability to inhibit bone mineral loss in female mice maintained on either a standard rodent chow (control) diet or a high-fat western diet (HFWD) for 5-, 12- and 18-months. At each time-point, femora were subjected to μ-CT analysis and biomechanical testing. A subset of caudal vertebrae was also analyzed. Following this, individual elements were assessed in bones. Serum levels of the 5b isoform of tartrate-resistant acid phosphatase (TRAP) and procollagen type I propeptide (P1NP) were also measured. Trabecular bone loss occurred in both diets (evident as early as 5-months). Cortical bone increased through month-5 and then declined. Cortical bone loss was primarily in mice on the HFWD. Inclusion of the minerals in the diet reduced bone mineral loss in both diets and improved bone strength. Bone mineral density (BMD) was also enhanced by these minerals. Of several cationic minerals known to be important to bone health, only strontium was significantly increased in bone tissue from animals fed the mineral diets, but the increase was large (5–10 fold). Serum levels of TRAP were consistently higher in mice receiving the minerals but levels of P1NP were not. These data suggest that trace minerals derived from marine red algae may be used to prevent progressive bone mineral loss in conjunction with calcium. Mineral supplementation could find use as part of an osteoporosis - prevention strategy.
Keywords: Bone, Bone mineral density, Bone mineral content, Calcium, Minerals, Osteoporosis, Red marine algae, Strontium, Trace elements
INTRODUCTION
Osteoporosis is a condition characterized by low bone mass, low bone mineral content, and micro-architectural deterioration leading to enhanced bone fragility and consequent increase risk of bone fracture [1]. Although in the Caucasian population, men account for up to 30% of the osteoporotic hip fractures [2], osteoporosis is widely regarded as a condition primarily affecting post-menopausal women [2,3]. Genetic factors underlie susceptibility, but environmental variables (including diet) [4] also suggested to play a role. In particular, the “typical” Western-style diet, with its high content of saturated fat and sugar and low levels of calcium and vitamin D, is thought to be a contributor to bone fragility in susceptible individuals. This is well-established based on epidemiological and interventional studies in humans [5,6]. In addition, studies in experimental animals have directly demonstrated the deleterious effects of the Western-style diet on bone structure/function [7,8] and age-related effects on the skeleton [9].
Since bone consists of a mineralized connective tissue, an adequate supply of inorganic minerals in the diet is critical throughout life. Calcium is the major cationic mineral incorporated into bone and a deficiency of calcium in the diet contributes to poor bone health [10–12]. In addition to calcium, other cationic minerals including boron, copper, iron, magnesium, manganese, potassium, selenium, silicon, strontium and zinc have all been suggested as being important for the formation and maintenance of strong, healthy bones [13–17]. How these various trace elements support bone formation and maintenance is not fully understood. Incorporation into the mineralized bone is, no doubt, important. In addition, however, certain trace elements regulate osteoblast and osteoclast metabolism [17–20] and are critical to the formation of the organic bone matrix as well as to its mineralization. While an adequate supply of the essential trace elements throughout life is important to formation and maintenance of healthy bones, whether or not dietary supplementation with individual trace elements or with groups of trace elements can be an effective strategy for improving bone formation and preventing bone mineral loss with age remains to be seen. To date, supplementation studies with only a relatively few trace elements have been conducted, and the results, while suggestive, are inconclusive [13–15].
Based on this, we hypothesized that including a wide variety of trace minerals along with calcium in the diet throughout life would help to prevent bone loss that commonly occurs with age. To test this hypothesis, we utilized a mineral preparation derived from the red marine algae, Lithothamnion sp. Previous studies have shown that this mineral preparation reduced colon polyp formation in mice [21,22], and also inhibited liver tumor formation [23] when included in the diet over the lifespan of the animals. As an unanticipated finding in the pilot study [24], our data suggested that mineral supplementation might also preserve bone structure, at least in female mice. However, in the pilot study, only a small number of animals were included, only a single time-point (15 months) was examined and only effects on long bones were assessed. In our pilot study, mineral supplementation was only included in a high-fat diet that is mineral-deficient and known to promote bone loss and bone fragility (6,7). Here, we have utilized the same mineral preparation to directly assess effects on bone structure and function in cohorts of female C57/BL6 mice at three different time-points (5, 12 and 18 months) in their lifespan. In this mouse strain, which is prone to early bone loss [9], mineral supplementation significantly increased bone mineralization at the early time-point (5-months) and preserved bone structure throughout life. Most importantly, beneficial effects were seen in animals maintained for the entire study on a rodent chow designed for optimal health as well as in mice fed the high-fat diet.
MATERIALS AND METHODS
Minerals
The minerals used in this study were obtained from the skeletal remains of Lithothamnion sp., the red marine algae [21,22] and consists entirely of the inorganic minerals (Marigot Ltd, Cork, IE). The product (GRAS 000028) contains approximately 12% calcium and 1% magnesium, but also has detectable levels of 72 other trace minerals. The mineral diets in this study made use of a single batch of the mineral product to avoid batch-to-batch variation.
Diets
A standard rodent chow (AIN76A - Control) and a variant of AIN76A, - i.e., the high-fat Western diet (HFWD) – were used in this study. The HFWD was prepared according to the formulation of Newmark et al [25] and is designed to mimic food consumption patterns of individuals in Western society [26]. Both diets were used as is, or supplemented with Lithothamnion sp. - derived minerals. The minerals were incorporated into the diet fed to the mice. The final concentrations of calcium in control and HFWD diets were 1.34 mg/kcal and 0.08 mg/kcal, respectively. With mineral supplementation, the control and HFWD diets contained 3.24 mg/kcal and 1.64 mg/kcal of calcium respectively. The slight increase in calcium in the supplemented-HFWD as compared to the unsupplemented control diet reflects the fact that mice consume food based on kcal. The diets are designed, therefore, to provide a comparable level of consumed calcium in these two groups. Diets were provided ad libitum. Diets were formulated and provided by Research Diets Incorporated (New Brunswick, NJ). The complete composition of each diet as fed is presented in Supplement Table 1. It should be noted that the control diet is formulated to contain a number of cationic minerals in addition to calcium that are known to be beneficial. All of these are included in the HFWD, as well. Supplement Table 2 provides comparative levels of important minerals in the four diets and shows the changes due to diet supplementation with the minerals.
Mice and experimental groups
A total of 140 female C57BL/6 mice (Charles River, Portage, MI) were put in four groups and started on either the control diet or the HFWD, both with and without the minerals beginning at 3-weeks of age. Diets were started at this age in order to observe early growth-related effects of the minerals on bone structure/function and subsequent effects on bone mineral content over the entire 18-month period of study. Separate cohorts of mice were euthanized after 5, 12 or 18 months on their respective diet. For the 5 and 12 month periods, there were 10 female mice per diet group. For the 18 month period, there were 15 mice in each group. In addition to these cohorts of mice, 5 female mice were euthanized at the start of the study for baseline values. All of the procedures were reviewed and approved by the University Committee on Use and Care of Animals (UCUCA) at the University of Michigan.
Preparation of skeletal tissue and micro-computed tomography (μ-CT)
The right femora were carefully dissected free of associated connective tissue, immediately placed in sealed containers with lactated Ringer’s solution, and frozen at −20°C until use. Three-dimensional images of the femora in Ringer’s solution were obtained using a μ-CT system (eXplore Locus SP, GE Healthcare Pre-Clinical Imaging, London, Ontario, Canada) as previously described and validated [24,27]. Whole bone was scanned and both trabecular and cortical regions of interest (ROI) were reconstructed from the scans as described previously [28]. A more complete description of the μ-CT procedure can be found in the Supplement under Methodology.
A subset of caudal vertebrae (C8) were identified and carefully dissected. Upon dissection, the vertebrae were immediately placed in lactated Ringer’s solution and frozen at −20°C until use. Whole vertebrae were scanned and ROIs through the cranial and middle isolateral surfaces were selected for analysis. μ-CT analysis was done exactly as with long bones.
Biomechanical testing
Long-bone mechanical properties were determined by loading the right femora to failure in 4-point bending, using a customized testing fixture attached to a servohydraulic materials testing machine (858 Mini Bionix II; MTS Systems, Eden Prairie, MN) [24,29]. Complete description of biomechanical testing is included in the Supplement under Methodology.
Whole-bone mechanical properties of intact caudal vertebrae were also measured by compressing the vertebral body with a 3 mm diameter platen attached to a servohydraulic materials testing machine (858 Mini Bionix II; MTS Systems, Eden Prairie, MN), as described previously [30].
Histological evaluation
The left femora were dissected, cleaned and fixed in 10% neutral buffered formalin for 24–48 hours and then demineralized in a formic acid based decalcifier (Immunocal; Decal, Tallman, NY) for 48 hours. Tissues were processed for histology, embedded in paraffin, sectioned at 5 μm thickness, and stained with hematoxylin and eosin. Histological parameters were evaluated by a board-certified veterinary pathologist blinded to the experimental groups. Evaluation was performed using an Olympus BX45 light microscope at total magnifications ranging from x40 to x600.
Tartrate-resistant acid phosphatase (TRAP) and N-terminal peptide of type I procollagen (P1NP)
Blood was obtained at the time of necropsy from each animal. TRAP and P1NP were assessed in serum samples using commercially-available enzyme-linked immunosorbant assays (ELISAs) (Immunodiagnostic Systems, Inc.; Fountain Hills, AZ). TRAP is produced by osteoclasts and macrophages and can be detected in serum. The ELISA used here measures TRAP 5b, the form specific to osteoclasts [31]. TRAP 5b is thought to be a measure of osteoclast number rather than activity [32]. P1NP is a measure of osteoblast function. Type I collagen is the major collagenous protein in bone [33].
Levels of individual trace elements in long bones
Following μ-CT and biomechanical testing, the long bones (one femur and tibia from all animals in each group) were “pooled” by group and time point, and analyzed for levels of trace metals. Bones were “pooled” in order to have a sufficient amount of material to obtain a detectable signal. Bones were digested in a concentrated nitric acid solution (10 ml) for approximately 30 minutes, after which they were cooled to room temperature. Concentrated hydrochloric acid (10 ml) was added and the sample was digested for an additional 15 minutes. After cooling and dilution with distilled water, levels of individual trace elements were determined by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) except Fluoride which was determined by Association of Analytical Communities (AOAC) 984.37 assay. Bone preparation and assays were done on a fee-for-service by Advanced Laboratories, Inc., (Salt lake City, UT).
Statistical evaluation
Data from μ-CT analysis, biomechanical testing and biochemical evaluations were obtained for individual mice. The data were presented as group averages and standard deviations at each time-point. Differences among groups were compared for statistical significance using ANOVA followed by paired group comparisons (two-tailed). Differences were considered significant at the p<0.05 value. While assessing the trace elements, individual “pooled” samples could not be analyzed statistically, the data (strontium only) were subjected to two-way factorial ANOVA to determine if the effect of diet or time was significant. Data from the three time-points were then grouped together and analyzed. Differences were considered significant at the p<0.05 value.
RESULTS
Animal weight and survival data
Animals were regularly monitored and weighed biweekly throughout the study period. At the initiation of the study, 3 weeks old animals had an average weight of 14.5±1 gram. Over the 18-month period of dietary intervention, animals gradually gained weight on both diets. Animals on the HFWD gained more weight than animals on the control diets, but the mineral supplement had no effect on weight gain in either diet. The progression in weights is provided in Supplement Table 3 at all time-points (5, 12 and 18 months).
Over the course of the 18-month maintenance period, there were 14 premature deaths. This included two animals between 6–12 months and 12 mice between months 13 and 18. The majority of the latter deaths occurred in months 15 to 17. Necropsies were done on all the animals that died prematurely and bones from these mice were included in their corresponding diet/time groups. Of these animals, 2 were on the control diet, 2 were on the mineral-supplemented control diet, 6 were on the HFWD and 4 were on the mineral-supplemented HFWD.
Bone Mineral Density (BMD)
Initially, we assessed BMD by μ-CT in trabecular and cortical ROIs of the femora. Femoral results are shown in Table 1, where it can be seen that in both the control diet and the HFWD, trabecular BMD fell significantly from baseline level. The drop could be seen as early as the 5-month time-point and continued throughout the study. By the 18-month time-point, trabecular BMD in mice on the control diet was 69% of the baseline value and in the HFWD was only 45% of the baseline level. In mice that received the minerals in either diet, BMD values at the 5-month time-point were substantially increased as compared to baseline level (35% and 16% increase). From thereon, BMD values fell. However, at both the 12-month and 18-month time-points, trabecular BMD values were higher in mice with the minerals than in those without (trabecular BMD was enhanced by minerals up to 82% at 18 months in HFWD). This was observed in both diets. Thus, the decrease in trabecular BMD that occurred over time was clearly mitigated in both diets by supplementation with the Lithothamnion sp. – derived minerals (Table 1).
Table 1.
Bone Mineral Density in femora and vertebrae.
| Diet group | Femoral BMD (mg/cc) | ||
|---|---|---|---|
| 5-months | 12-months | 18 months | |
| Trabecular | |||
| Baseline | 174 ± 18 | ||
| Control | 156 ± 12* | 131 ± 21* | 120 ± 38* |
| Control + minerals | 234 ± 15c | 158 ± 35 | 142 ± 40 |
| HFWD | 164 ± 54 | 146 ± 11* | 79 ± 29* |
| HFWD + minerals | 201 ± 11a, b | 171 ± 18a, b | 143 ± 30a |
| Cortical | |||
| Baseline | 151 ± 6 | ||
| Control | 347 ± 16 | 309 ± 36 | 303 ± 27 |
| Control + minerals | 378 ± 25c | 302 ± 31 | 299 ± 28 |
| HFWD | 314 ± 36 | 287 ± 30 | 260 ± 34 |
| HFWD + minerals | 339 ± 16 | 322 ± 21 | 322 ± 47a |
| Vertebral BMD (mg/cc) | ||
|---|---|---|
| 12-months | 18 months | |
| Trabecular | ||
| Control | 619 ± 63 | Not done |
| Control + minerals | 737 ± 83c | Not done |
| HFWD | 482 ± 34 | 452 ± 46 |
| HFWD + minerals | 687 ± 107a | 659 ± 108a |
| Cortical | ||
| Control | 961 ± 30 | Not done |
| Control + minerals | 942 ± 14 | Not done |
| HFWD | 731 ± 25 | 763 ± 146 |
| HFWD + minerals | 941 ± 33a | 928 ± 43 |
Femoral data are based on 5 mice at baseline (3 weeks of age), 10 mice at 5 and 12 months and 15 mice at 18 months in each diet group. Vertebral data are based on 6 mice at 12 months in each diet group and 6 mice in each of the two high-fat diets at 18 months. Values are means and standard deviations. Statistical significance was determined by ANOVA followed by paired group comparisons. “*” are placed to show the statistically significant drop in the BMD relative to baseline. “a” and “b” are placed on the HFWD+minerals group: “a” shows statistically significant increase relative to the HFWD group, “b” shows statistically significant increase relative to control; “c” is placed on the control+minerals group, and shows significant increase relative to the control (p <0.05). Data from baseline and 5 months in all groups and in control diets at 18 months for the vertebrae are not available.
In contrast to trabecular findings, cortical BMD increased significantly in both diets between baseline and 5-months of age (Table 1). Between the 5- and 18-month time-points, BMD values declined slightly. Similar trends were observed in both diets but the decline with time was more severe in the HFWD. Mineral-supplementation had little effect on BMD values in control diet mice, but preserved BMD in the HFWD (up to 24% increase at 18 months).
Although the primary focus of the study was on long bones, effects of mineral supplementation on vertebrae were also assessed. For this, a subset of caudal vertebrae from mice on both the HFWD and the control diet was analyzed at the 12-month time-point. An additional subset of caudal vertebrae from mice on the HFWD was also analyzed at the 18-month time-point. It can be seen from Table 1 that (similar to what was observed in long bones), mineral supplementation dramatically increased trabecular bone BMD in both diets. Increases ranged from 19% at 12-months in the control diet to 43% and 46% increases at 12-months and 18-months in the HFWD. Also similar to what was seen in long bones, there was little improvement in cortical bone BMD in the control diet but increases of 29% and 22% were seen in the HFWD at the 12-month and 18-month time-points, respectively with minerals inclusion in the diet as compared to the HFWD alone.
Femoral bone structure
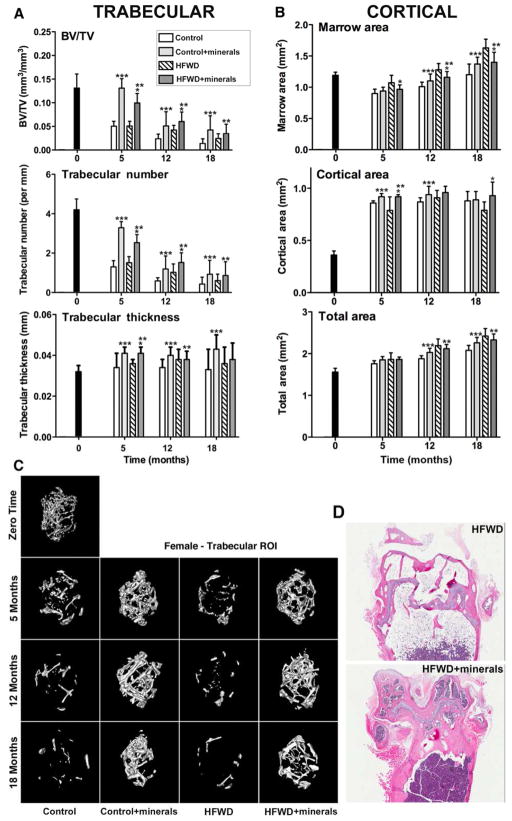
Given the significant effect of mineral supplementation on femoral BMD, a wide range of μ-CT parameters were assessed. Trabecular bone volume, number and thickness are shown for each of the three time-points in Figure 1A. To summarize, a rapid loss of trabecular bone was observed such that a decline from the beginning of the study (3-weeks of age) was apparent in these animals as early as the 5-month time-point. At the 5-month, trabecular number was reduced by 67% and 64% in the control and HFWD groups relative to what was seen at the initiation of the study. Trabecular bone loss was reduced in the presence of the minerals. With the addition of minerals, corresponding declines were only 17% and 34%. Trabecular bone loss continued over the 18-month observation period, but at all time-points, there was less bone loss in the mice receiving the minerals than in mice receiving unsupplemented diets (Figure 1A). The results were similar in both diets. While the largest changes were in trabecular number as opposed to thickness, the minerals beneficially influenced trabecular thickness as well. A loss of trabecular number concomitant with reduced trabecular thickness was associated, as expected, with an increase in trabecular space (Supplement Tables 4–6) and decreased BV/TV (Figure 1A). For example, bone volume was reduced to 85% in the HFWD group at 18 months from baseline but the mineral-containing HFWD enhanced bone volume up to 50% at 18 months. A representative 3D μ-CT image of the trabecular region (distal metaphysis) from the femur of a female mouse in each diet group is shown (Figure 1C). Histological images from distal femoral condyles from mice on the HFWD with or without the minerals (hematoxylin and eosin-stained sections of decalcified bone) at the 18 months-time point are shown (Figure 1D). The histological images are consistent with what was observed by μ-CT, i.e., increased bone preservation within the trabecular region in the presence of the minerals. We did not attempt to quantify trabecular bone by morphometry because of limitations inherent in 2–dimensional representation of trabeculae in a histological section. Additional histological findings included increased adipose tissue and decreased hematopoietic tissue in some sections of mice fed a HFWD in comparison to mice on the supplemented HFWD.
Figure 1. Structural features of femoral bone.
A: Trabecular/B: Cortical μ-CT parameters: Data are based on 5 mice at baseline (3 weeks of age), 10 mice at 5 and 12 months and 15 mice at 18 months in each diet group. Values are means and standard deviations. Statistical significance of each parameter was assessed by ANOVA followed by paired group comparisons. Statistical significance at the p<0.05 level is indicated by asterisks. * by the HFWD + minerals indicates statistically significant improvement relative to HFWD alone; ** by the HFWD + minerals indicates statistically significant improvement relative to control; *** by the control + minerals indicates statistically significant improvement relative to control. C: μ-CT images: A representative 3D μ-CT image of the trabecular region from the femur of a female mouse in each diet group is shown. D: Histological images: Hematoxylin and eosin-stained sections of decalcified bone (distal femoral condyles) from a mouse (at 18 months) in two high-fat diet groups are shown (Bars=200 μm).
Cortical bone properties, including marrow area and cortical area are shown in Figure 1B. Although the changes in cortical bone properties were not as dramatic as those observed in trabecular bone, mice on the HFWD had a larger marrow area value and smaller cortical area value than did mice on the control diet. The deleterious consequences of the HFWD were prevented by the minerals (Figure 1B). A representative μ-CT image of the cortical region (mid-diaphysis) from the femur of a female mouse in each diet group is shown (Supplement Figure 1). Tables 4–6 in the supplementary material present all of the femoral μ-CT parameters assessed at the three time-points for both trabecular and cortical ROIs.
Vertebral bone structure
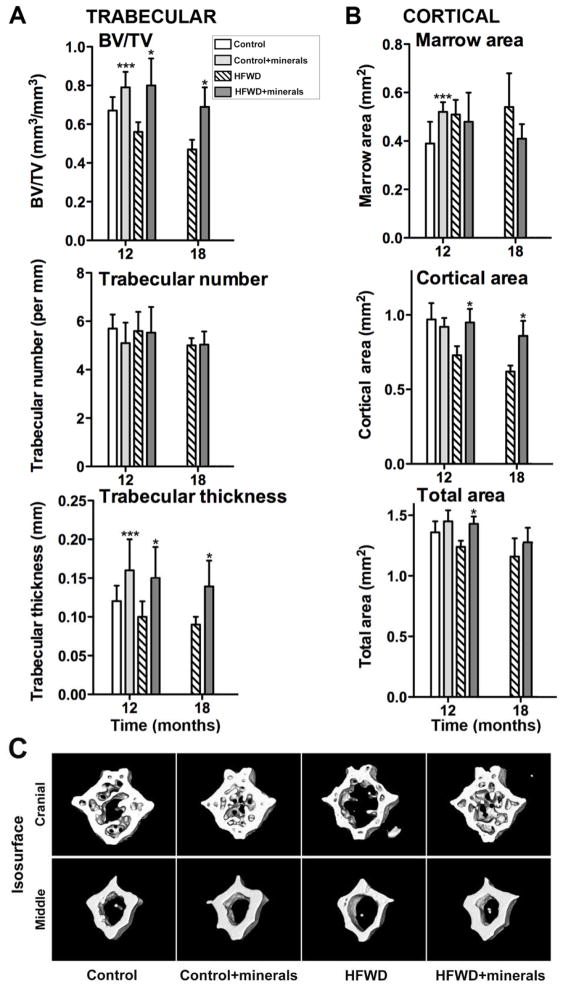
Figure 2 demonstrates effects of Lithothamnion sp. – derived minerals on vertebral bone structure (from a subset of vertebrae). Similarly as seen in femoral bone, where the major effect of the minerals was on the both trabecular region and the cortical region, in the vertebral bone, both trabecular and cortical properties were improved in the mineral-treated mice. In contrast to femoral bone, where the improvement in trabecular structure was a reflection of changes in both number and thickness, in the vertebral bone, the minerals’ effect was primarily on trabecular thickness and volume. Consistent with data from long bones, however, was the fact that improvement with minerals occurred in mice on either diet. Also consistent, results were more impressive in mice on the HFWD than in mice on the control diet. Representative μ-CT images of vertebrae from both the control and HFWD groups at the 12 month-time-point are shown in Figure 2C. All of the vertebral μ-CT parameters assessed at the two time-points are presented in Supplemental Tables 7 and 8.
Figure 2. Structural features of vertebral bone.
A: Trabecular/B: Cortical μ-CT parameters: Data are based on 6 mice at 12 months in each diet group and 6 mice in each of the two high-fat diets at 18 months. Values are means and standard deviations. Statistical significance of each parameter was assessed by ANOVA followed by paired group comparisons. Statistical significance at the p<0.05 level is indicated by asterisks. * by the HFWD + minerals indicates statistically significant improvement relative to HFWD alone; ** by the HFWD + minerals indicates statistically significant improvement relative to control; *** by the control + minerals indicates statistically significant improvement relative to control. C: μ-CT images: Representative 3D μ-CT images of the cranial and middle region from a C8 vertebra of a mouse in each diet group at 12 month-time-point.
Biomechanical properties of femora and vertebrae
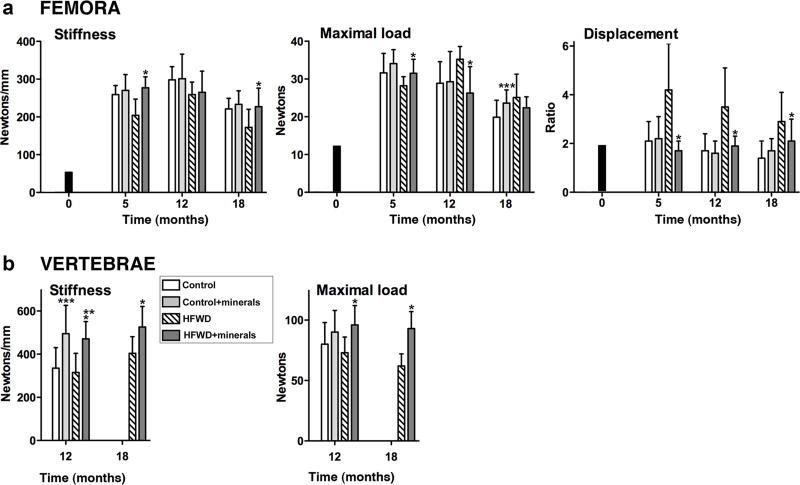
Biomechanical properties of long-bones were determined by testing femora to failure in 4-point bending. These measurements, which primarily reflect cortical bone mechanical properties [34], are presented in Figure 3A. Mice on the HFWD demonstrated an increase in ductility compared to mice on the control diet which can be seen by the decrease in stiffness and the increase in displacement ratio. The displacement ratio was especially sensitive to diet. Consistent with the decrease in bone stiffness, mice on the HFWD demonstrated an increase in maximum load value as compared to mice on the control diet [8]. In mice treated with the minerals, biomechanical properties were improved. Specifically, bone stiffness was enhanced (36 % at 5 months, 3% at 12 months and 32% at 18 months) in mice on the HFWD with minerals as compared to the HFWD alone mice.
Figure 3. Biomechanical properties.
A: Femora: Data are based on 5 mice at baseline (3 weeks of age), 10 mice at 5 and 12 months and 15 mice at 18 months in each diet group. B: Vertebrae: Data are based on 6 mice at 12 months in each diet group and 6 mice in each of the two high-fat diets at 18 months. Values are means and standard deviations. Statistical significance of each parameter was assessed by ANOVA followed by paired group comparisons. Statistical significance at the p<0.05 level is indicated by asterisks. * by the HFWD + minerals indicates statistically significant improvement relative to HFWD alone; ** by the HFWD + minerals indicates statistically significant improvement relative to control; *** by the control + minerals indicates statistically significant improvement relative to control.
Biomechanical properties were also assessed in a subset of vertebrae. Vertebrae were tested for resistance to compression loads. Results in the compression-damage assay are a reflection of both stiffness and bone strength, and depend on both trabecular and cortical properties [30]. As seen in Figure 3B, both stiffness and strength were substantially improved in mice receiving Lithothamnion sp. – derived minerals. Improvement was observed in both diets. Stiffness was increased by 48% and 49% at 12-months in the two supplemented diets as compared to the respective unsupplemented diet groups, and by 30% at 18-months in the mineral supplemented HFWD as compared to HFWD alone. Similarly, maximal load was increased by 12% and 32% at the 12-month time point in mineral-supplemented diets as compared to unsupplemeted diet groups, and by 51% at the 18-month time point in the supplemented HFWD compared to the HFWD alone.
Levels of individual trace elements in bone
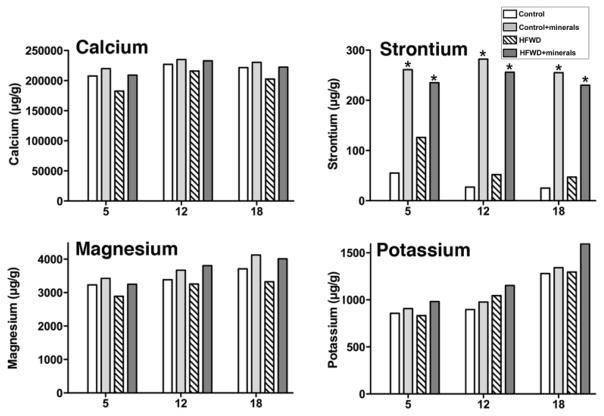
Following μ-CT analysis and biomechanical testing, bones were assessed for levels of 41 individual elements. To summarize, 28 of the 41 different trace elements assessed (including three that are known to be important for bone – i.e., boron, copper and selenium) were below detectable limits (0.5 μg/g) (Supplement Table 9). Among other minerals that play a role in bone health (including calcium, magnesium, potassium, iron, zinc, manganese and silicon), bone levels of each were largely unaffected by diet or (in the case of iron and manganese) actually higher in bones of mice on the high-fat diet (Supplement Table 9). In contrast, and perhaps most interesting, strontium levels were dramatically increased - up to 10-fold - in bones of mice on the mineral-supplemented diets relative to mice on the respective, unsupplemented diets. This was seen on both diets at all three time-points (Figure 4). Interestingly, bone calcium levels in mice on the control diet and HFWD with or without the minerals were similar at all the time points (Figure 4) and calcium was present as the major mineral in the bone. For example, there was a difference in the level of calcium of 12% at 5 months, 7% at 12 months and 9% at 18 months in bones of mice on the HFWD as compared to the HFWD with minerals, although there was 95 % less calcium in the unsupplemented high-fat diet, itself. In mice on the control diet with minerals (where the dietary calcium level was twice that of control), bone calcium levels were 4% higher at 5 months, 3% at 12 months and 4% at 18 months than in bones of mice on the control diet without supplementation. As shown in Figure 4, minimal differences in magnesium and potassium were also observed between mice with or without the minerals.
Figure 4. Strontium levels in bone; Comparison with calcium, magnesium and potassium.
Long bones from all mice in a group were pooled together, digested and analyzed by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) as one sample per diet group (10 mice per group at 5- and 12-months and 15 mice at 18-months). * is placed on diet groups with minerals and indicates statistically significant increase in the strontium level (using two-way factorial ANOVA) than the diet groups without minerals. (p<0.05)
Serum TRAP and P1NP
TRAP and P1NP levels were assessed as measures of bone turnover and bone formation, respectively. TRAP levels were consistently higher in mice on either diet with the minerals than in mice from groups without the minerals (Table 2). This was not seen with P1NP. In fact, levels of P1NP were slightly lower in mice from supplemented groups than in mice from the respective unsupplemented diet group (Table 2).
Table 2.
Serum TRAP and P1NP levels.
| Diet group | TRAP (U/ml) | ||
|---|---|---|---|
| 5-months | 12-months | 18 months | |
| Control | 1.1 ± 0.1 | 17.5 ± 2.6 | 10.3 ± 0.7 |
| Control + minerals | 1.4 ± 0.2 | 18.0 ± 2.0 | 19.6 ± 2.3c |
| HFWD | 1.5 ± 0.2 | 16.4 ± 2.1 | 15.1 ± 1.5 |
| HFWD + minerals | 1.5 ± 0.2 | 26.3 ± 3.8 | 24.4 ± 2.1a, b |
| P1NP (ng/ml) | ||
|---|---|---|
| 12-months | 18 months | |
| Control | 24.5 ± 0.9 | 21.3 ± 1.8 |
| Control + minerals | 22.2 ± 2.6 | 21.4 ± 2.0 |
| HFWD | 26.4 ± 2.3 | 32.7 ± 5.9 |
| HFWD + minerals | 19.6 ± 1.5 | 19.0 ± 2.6 |
Baseline TRAP = 1.2 ± 0.5 U/ml. Values are means and standard deviations. Statistical significance was determined by ANOVA followed by paired group comparisons. “a” and “b” are placed on the HFWD + minerals group: “a” shows statistically significant increase relative to the HFWD group, “b” shows statistically significant increase relative to control; “c” is placed on the control + minerals group, and shows significant increase relative to the control (p <0.05). P1NP data at baseline and at 5 month are not available.
DISCUSSION
This study demonstrates that bone structure and function in female mice are preserved by minerals obtained from marine red algae (Lithothamnion sp.) in a long-term dietary intervention study. The findings may be particularly significant since bone mineral loss was prevented in mice maintained on a rodent chow (control) as well as on a HFWD. Previous studies have demonstrated that the Western-style diet contributes to bone loss in people [4,5], and other studies have shown that a high fat diet leads to bone mineral loss in rodents and other experimental animals [7,8]. The control diet is routinely used for optimal health maintenance in mouse care. The current study confirmed more rapid and, ultimately, greater bone deterioration in mice on the HFWD, but, consistent with a previous study [9], demonstrated that significant bone mineral loss also occurred in mice on the rodent chow. Of interest, while both trabecular and cortical bone loss occurred in the HFWD, it was primarily trabecular bone that was lost in control diet mice. The minerals induced early bone built up (i.e., between 3 weeks of age and 5 months) and retarded subsequent bone mineral loss over an 18 month period. Bone mineral preservation was observed in both cortical and trabecular regions. However, the effects of the minerals were much greater in the trabecular bone. Thus, mineral addition had its greatest effect where bone loss was most rapid and severe.
While the consequences of bone mineral loss - increased susceptibility to fracture (especially in the head of the femur and the vertebral discs) are usually seen in later life, bone mineral loss typically begins during young adulthood and progresses over time [35]. Our findings suggest that inclusion of a minerals combination such as the one used here may provide a strategy for retarding progressive bone mineral loss. To the extent that BMD is a measure of resistance to fracture [36], reduced susceptibility to fracture could be expected. One might assume that effectiveness would be seen even with individuals who are consuming a “healthy” diet.
At this point, we do not know which minerals present in the mineral combination contribute to its beneficial effects. Calcium is, undoubtedly, important, but it should be noted that while mice on the mineral-supplemented control diet received a calcium dose equivalent to twice the level of control animals, and mice on the mineral-supplemented HFWD received an amount of calcium comparable to that received by control diet mice. In spite of this, mice on either diet with the minerals demonstrated several features that distinguished them from control mice. At the same time, bone calcium levels varied by only a few percent among the different groups. Although, while even the small differences in bone calcium level could be crucial to bone loss in high-fat diet mice, the implication is that while calcium is, a critical component of the mineral preparation, other trace elements in it also appear to play important role in maintaining bone structure and function, in conjunction with calcium.
Among the cationic minerals that have been suggested previously as important to bone health are boron, copper, iron, magnesium, manganese, selenium, silicon, strontium and zinc [13–17]. How the different minerals contribute to bone strength is not known and it is, perhaps, best to not speculate beyond noting that small amounts of these trace minerals are incorporated into the bone matrix along with calcium [37,38]. Strontium may be particularly important to the overall beneficial activity of the mineral combination. With each of the other minerals present in the mineral preparation, bone levels were either below detectable limits, comparable in all groups or actually lower in the presence of the HFWD. In contrast, the bone level of strontium was significantly increased (up to 10-fold) in mice receiving the minerals derived from marine red algae in either diet relative to the unsupplemented groups. Increased strontium was seen at 5-months in mice on either supplemented diet and persisted over the 18-month period of the study. Previous studies have convincingly demonstrated the beneficial effects of strontium on bone structure [17,39]. In Europe, a strontium-containing pharmaceutical (strontium ranelate) is an approved therapeutic for prevention of bone mineral loss and bone fragility [40]. A study in rats has suggested that long-term treatment with strontium ranelate increases bone mass, architecture and fracture-resistance [41]. The exact mechanism of action is still not fully understood. Strontium salts are more acid-insoluble than comparable calcium salts [42], and slower de-mineralization of strontium salts as compared to calcium salts may be part of the effect. This may be especially important for individuals consuming a typical western diet as this diet is known to produce an acidic environment [43].
Incorporation of trace elements such as strontium into the bone matrix may not be the entire explanation. In vitro studies have shown that strontium and several other trace elements influence the function of both osteoclasts and osteoblasts [17–20], leading to increased bone matrix synthesis and turn over. In this regard, however, it should be noted that we consistently saw increased TRAP activity but no increase in P1NP in the serum of mice on the mineral-supplemented diets relative to control mice. Without seeing increases in both P1NP and TRAP, it is difficult to postulate increased bone cell metabolism and increased bone turnover as the major mechanism. Additional experiments will be required to address this issue.
Finally, it should also be considered that the beneficial effects on bone may be secondary to other, more global actions of the minerals. An attractive (alternative) hypothesis is that the combination of minerals functions to help control systemic inflammation, a known risk factor for bone mineral loss [44]. In support of this, we observed in the same animal model that mice on the mineral-supplemented diets had fewer colonic polyps than control mice [21,22], and that liver tumor formation was almost completely absent in these animals [23]. Associated with both findings were reduced inflammatory lesions throughout the intestinal tract. Confounding the issue, however, is that inflammatory changes in the gastrointestinal tract did not appear until late - 12 months and beyond. In contrast, diet-induced changes in bone structure/function were seen within 5 month of starting the diet. The relative contribution of systemic changes versus bone-specific effects will require further study.
Ultimately, whether mineral supplementation will have a similar effect in humans as shown here with rodents is not known and controlled clinical studies will be necessary to address this question. Although the focus of this study was on the role of trace minerals in preservation of bone structure/function rather than on the source of minerals, per se, the natural product used here has already been examined in a previous small-scale clinical study related to osteoarthritis pain [45]. There is no reason why a study designed to assess biomarkers of bone health could not be undertaken with this natural product or with the minerals as present in it. Furthermore, while our data suggest an important role for strontium (among the trace elements and in the presence of calcium) in the beneficial activity of the natural product, the reality is that marine algae accumulate many different minerals from seawater. There are, undoubtedly, multiple trace elements present in the mineral product at amounts below detectable level. Any and all of these might be contributing to the beneficial activity of the natural product. Until the mechanisms of action of the mineral product are clearly defined, it will be difficult to elucidate which minerals are most important, singly or in combination.
In conclusion, this study shows that minerals obtained from marine red algae promote early (3-weeks to 5-months) bone mineral build-up and preserve bone mineralization over an 18-month period in female C57BL/6 mice. This occurs in mice on either a high-fat diet or a standard rodent chow. These findings support further effort to determine if mineral supplementation might provide an approach for maintenance of bone structure/function in the face of age and high-fat diet-related events that tend to reduce bone mineral content.
Supplementary Material
Acknowledgments
This study was supported in part by grant CA140760 from the National Institutes of Health, Bethesda, MD, and by grant 11-0577 from the Association for International Cancer Research, St. Andrews, Fife, Scotland. The authors would like to thank Marigot, Ltd. (Cork, Ireland) for providing the multi-mineral-rich supplement (Aquamin®) as a gift.
Abbreviations
- AIN76A
American Institute of Nutrition 76A
- ANOVA
Analysis of variance
- BMD
Bone mineral density
- GRAS
Generally regarded as safe
- HFWD
High-fat western-style diet
- μ-CT
Micro-computed tomography
- P1NP
N-terminal propeptide of type I procollagen
- TRAP
Tartrate-resistant acid phosphatase (5b)
- 2D
Two-dimensional
- 3D
Three-dimensional
Footnotes
Supplemental data included
Disclosure of Conflict: All authors state that they have no financial or personal conflict of interest (no disclosures).
References
- 1.Prentice A. Diet, Nutrition and the prevention of osteoporosis. Public Health Nutr. 2004;7(1A):227–243. doi: 10.1079/phn2003590. [DOI] [PubMed] [Google Scholar]
- 2.De Laet CE, Van Hout BA, Burger H, Weel AE, Hofman A, Pols HA. Hip fracture prediction in elderly men and women: validation in the Rotterdam study. J Bone Miner Res. 1998;13:1587–1593. doi: 10.1359/jbmr.1998.13.10.1587. [DOI] [PubMed] [Google Scholar]
- 3.Iskrant AP. The etiology of fractured hips in females. Am J Public Health. 1968;58:485–490. doi: 10.2105/ajph.58.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prentice A. Is nutrition important in osteoporosis? Proc Nutr Soc. 1997;56:357–367. doi: 10.1079/pns19970038. [DOI] [PubMed] [Google Scholar]
- 5.Rosen CJ, Klibanski A. Bone, Fat, and Body Composition: Evolving Concepts in the Pathogenesis of Osteoporosis. Am J Med. 2009;122:409–414. doi: 10.1016/j.amjmed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 6.Kato I, Toniolo P, Zeleniuch-Jacquotte A, Shore RE, Koenig KL, Akhmedkhanov A, Riboli E. Diet, smoking and anthropometric indices and postmenopausal bone fractures: a prospective study. Int J Epidemiol. 2000;29:85–92. doi: 10.1093/ije/29.1.85. [DOI] [PubMed] [Google Scholar]
- 7.Zernicke RF, Salem GJ, Barnard RJ, Schramm E. Long-term high-fat sucrose diet alters rat femoral neck and vertebral morphology, bone mineral content and mechanical properties. Bone. 1995;16:25–31. doi: 10.1016/s8756-3282(00)80007-1. [DOI] [PubMed] [Google Scholar]
- 8.Ionova-Martin SS, Do SH, Barth HD, Szadkowska M, Porter AE, Ager JW, 3rd, Ager JW, Jr, Alliston T, Vaisse C, Ritchie RO. Reduced size-independent mechanical properties of cortical bone in high-fat diet-induced obesity. Bone. 2010;46(1):217–225. doi: 10.1016/j.bone.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glatt V, Canalis E, stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Mineral Res. 2007;22:1197–1207. doi: 10.1359/jbmr.070507. [DOI] [PubMed] [Google Scholar]
- 10.Heaney RP, Weaver CM. Newer Perspectives on calcium nutrition and Bone Quality. J Am Coll Nutr. 2005;24:574S–581S. doi: 10.1080/07315724.2005.10719506. [DOI] [PubMed] [Google Scholar]
- 11.Peacock M, Liu G, Carey M, McClintock R, Ambrosius W, Hui S, Johnston CC. Effect of a calcium or 25OHD vitamin D3 dietary supplementation on bone loss at the hip in men and women over the age of 60. J Clin Endocrinol Metab. 2000;85(9):3011–3019. doi: 10.1210/jcem.85.9.6836. [DOI] [PubMed] [Google Scholar]
- 12.Royal College of Physicians. Osteoporosis: Clinical Guidelines for Prevention and Treatment. London, UK: Royal College of Physicians; 1999. [PMC free article] [PubMed] [Google Scholar]
- 13.Odabasi E, Turan M, Aydin A, Akay C, Kutlu M. Magnesium, zinc, copper, manganese and selenium levels in postmenopausal women with osteoporosis: Can magnesium play a key role in osteoporosis? Ann Acad Med Singapore. 2008;37(7):564–567. [PubMed] [Google Scholar]
- 14.Lowe NM, Lowe NM, Fraser WD, Jackson MJ. Is there potential therapeutic value of copper and zinc for osteoporosis? Proc Nutr Soc. 2002;61(2):181–185. doi: 10.1079/PNS2002154. [DOI] [PubMed] [Google Scholar]
- 15.Stause L, Saltman P, Smith KT, Bracker M, Andon MB. Spinal bone loss in post-menopausal women supplemented with calcium and trace metals. J Nutr. 1994;124(7):1060–1064. doi: 10.1093/jn/124.7.1060. [DOI] [PubMed] [Google Scholar]
- 16.Jugdaohsingh R. Silicon and bone health. J Nutr Health Aging. 2007;11(2):99–110. [PMC free article] [PubMed] [Google Scholar]
- 17.Marie PJ, Ammann P, Boivin G, Rey C. Mechanisms of action and therapeutic potential of strontium in bone. Calc Tissue Int. 2001;69(3):121–129. doi: 10.1007/s002230010055. [DOI] [PubMed] [Google Scholar]
- 18.Cashman K, Flynn A. Trace Elements and Bone Metabolism. In: Sandstrom B, Walter P, editors. Role of Trace Elements for Health Promotion and Disease Prevention. Vol. 54. Bibl Nutr Dieta; Karger, Basel: 1998. pp. 150–164. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi M. Role of Zinc in Bone Formation and Bone Resorption. J Trace Elem Exp Med. 1998;11:119–135. [Google Scholar]
- 20.Adluri RS, Zhan L, Bagchi M, Maulik N, Maulik G. Comparative effects of a novel plant-based calcium supplement with two common calcium salts on proliferation and mineralization in human osteoblast cells. Mol Cell Biochem. 2010;340(1–2):73–80. doi: 10.1007/s11010-010-0402-0. [DOI] [PubMed] [Google Scholar]
- 21.Aslam MN, Paruchuri T, Bhagavathula N, Varani J. A mineral- rich red algae extract inhibits polyp formation and inflammation in the gastrointestinal tract of mice on a high-fat diet. Integr Cancer Ther. 2010;9(1):93–99. doi: 10.1177/1534735409360360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aslam MN, Bergin I, Naik M, Paruchuri T, Hampton A, Rehman M, Dame MK, Rush H, Varani J. A Multimineral Natural Product from Red Marine Algae Reduces Colon Polyp Formation in C57BL/6 Mice. Nutr Cancer. 2012;64(7):1020–1028. doi: 10.1080/01635581.2012.713160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aslam MN, Bergin I, Naik M, Hampton A, Allen R, Kunkel SL, Rush H, Varani J. A Multi-Mineral Natural Product Inhibits Liver Tumor Formation in C57BL/6 Mice. Biol Trace Elem Res. 2012;147(1–3):267–274. doi: 10.1007/s12011-011-9316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aslam MN, Kreider JM, Paruchuri T, Bhagavathula N, DaSilva M, Zernicke RF, Goldstein SA, Varani J. A mineral-rich extract from the red marine algae lithothamnion calcareum preserves bone structure and function in female mice on a Western-style diet. Calcif Tissue Int. 2010;86(4):313–324. doi: 10.1007/s00223-010-9340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, Shinozaki H. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57BL/6 mice. Carcinogenesis. 2001;22(11):1871–1875. doi: 10.1093/carcin/22.11.1871. [DOI] [PubMed] [Google Scholar]
- 26.Lipkin M, Reddy B, Newmark H, Lamprecht SA. Dietary factors in human colorectal cancer. Annu Rev Nutr. 1999;19:545–586. doi: 10.1146/annurev.nutr.19.1.545. [DOI] [PubMed] [Google Scholar]
- 27.Meganck JA, Kozloff KM, Thornton MM, Broski SM, Goldstein SA. Beam hardening artifacts in micro-computed tomography scanning can be reduced by X-ray beam filtration and the resulting images can be used to accurately measure BMD. Bone. 2009;45(6):1104–1106. doi: 10.1016/j.bone.2009.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J Bone Miner Res. 2010;25(7):1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 29.Salem GJ, Zernicke RF, Martinez DA, Vailas AC. Adaptations of immature trabecular bone to moderate exercise: geometrical, biochemical, and biomechanical correlates. Bone. 1993;14(4):647–654. doi: 10.1016/8756-3282(93)90087-q. [DOI] [PubMed] [Google Scholar]
- 30.Tommasini SM, Wearne SL, Hof PR, Jepsen KJ. Percolation theory relates corticocancellaous architecture to mechanical function in vertebrae of inbred mouse strains. Bone. 2008;42(4):743–750. doi: 10.1016/j.bone.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hannon RA, Clowes JA, Eagleton AC, Al Hadari AA, Eastell R, Blumsohn A. Clinical performance of immunoreactive tartrate resistant acid phosphatase isoform 5b as a marker of bone resorption. Bone. 2004;34(1):187–194. doi: 10.1016/j.bone.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Alatalo SL, Halleen JM, Hentunen TA, Monkkonen J, Vaananen JH. Rapid screening method for osteoclast differentiation in vitro that measures tartrate-resistant acid phosphatase 5b activity secreted into the culture medium. Clin Chem. 2000;46(11):1751–1754. [PubMed] [Google Scholar]
- 33.Risteli J, Risteli L. Products of bone collagen metabolism. In: Seibel MJ, Robins SP, Bilezikian JP, Gl, editors. Dynamics of bone and cartilage metabolism: Principles and clinical applications. 2. Academic Press; London: 2006. pp. 391–405. [Google Scholar]
- 34.Peterlik H, Roschger P, Klauhofer K, Fratzl P. From brittle to ductile fracture of bone. Nat Mater. 2006;5(1):52–55. doi: 10.1038/nmat1545. [DOI] [PubMed] [Google Scholar]
- 35.Loro ML, Sayre J, Roe TF, Goran MI, Kaufman FR, Gilsanz V. Early identification of children predisposed to low peak bone mass and osteoporosis later in life. J Clin Endocrinol Metab. 2000;85(10):3908–3918. doi: 10.1210/jcem.85.10.6887. [DOI] [PubMed] [Google Scholar]
- 36.Khosla S. Surrogates for fracture endpoints in clinical trials. J Bone Miner Res. 2003;18(6):1146–1149. doi: 10.1359/jbmr.2003.18.6.1146. [DOI] [PubMed] [Google Scholar]
- 37.Roberts NB, Wassh HPJ, Klenerman L, Kelly SA, Helliwell TR. Determination of elements in human femoral bone using inductively coupled plasma atomic emission spectrmetry and inductively coupled plasma mass spectrometry. J Anal At Spectrom. 1996;11:133–138. [Google Scholar]
- 38.Zhang YX, Wang YS, Zhang YP, Zhang GL, Huang YY, He W. Investigation of elemental distribution in human femoral head by PIXE and SRXRF microprobe. Nucl Instrum Methods Phys Res. 2007;260:178–183. [Google Scholar]
- 39.Saidak Z, Marie PJ. Strontium signaling: Molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol Ther. 2012;136(2):216–226. doi: 10.1016/j.pharmthera.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD, Cannata J, Balogh A, Lemmel EM, Pors-Nielsen S, Rizzoli R, Genant HK, Reginster JY. The Effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004;350(5):459–68. doi: 10.1056/NEJMoa022436. [DOI] [PubMed] [Google Scholar]
- 41.Ammann P, Shen V, Robin B, Mauras Y, Bonjour JP, Rizzoli R. Strontium Ranelate Improves Bone Resistance by Increasing Bone Mass and Improving Architecture in Intact Female Rats. J Bone Miner Res. 2004;19(12):2012–20. doi: 10.1359/JBMR.040906. [DOI] [PubMed] [Google Scholar]
- 42.LeGeros RZ. Chemical and crystallographic events in the caries process. J Dent Res. 1990;69(Spec No):567–74. doi: 10.1177/00220345900690S113. discussion 634–6. [DOI] [PubMed] [Google Scholar]
- 43.Thornton JR. High colonic pH promotes colorectal cancer. Lancet. 1981;1(8229):1081–3. doi: 10.1016/s0140-6736(81)92244-3. [DOI] [PubMed] [Google Scholar]
- 44.Cashman KD. Altered bone metabolism in inflammatory disease: role for nutrition. Proc Nutr Soc. 2008;67(2):196–205. doi: 10.1017/S0029665108007039. [DOI] [PubMed] [Google Scholar]
- 45.Frestedt JL, Walsh M, Kuskowski MA, Zenk JL. A natural mineral supplement provides relief from knee osteoarthritis symptoms: a randomized controlled pilot trial. Nutr J. 2008;7:9. doi: 10.1186/1475-2891-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.