Abstract
BACKGROUND
Given accumulating evidence supporting postmastectomy radiotherapy (PMRT) in selected patients, it is important to evaluate patterns and correlates of PMRT utilization, including communication and attitudinal factors.
METHODS
The authors surveyed 2382 patients diagnosed with breast cancer in 2002 and reported to the Los Angeles and Detroit Surveillance, Epidemiology, and End Results registries (n = 1844, 77.4% response rate). Analyses were restricted to patients with nonmetastatic invasive breast cancer treated by mastectomy who had decided whether or not to undergo PMRT (n = 396). The authors assessed rates of explanation, recommendation, and receipt of radiation by indication grouping, defined primarily by the 2001 American Society of Clinical Oncology guidelines. They evaluated correlates of PMRT receipt, including tumor and sociodemographic characteristics. They also explored patients’ self-reported reasons for nonreceipt of PMRT.
RESULTS
The adjusted proportion in each indication group reporting that a provider had explained radiation was high (77% of those in whom PMRT was indicated, 76% of those in whom medical opinion was divided, and 73% of those in whom PMRT was not indicated; P = .10). The adjusted proportions reporting recommendations for radiation (86%, 35%, and 17%, respectively) and receipt (81%, 34%, and 10%, respectively) varied significantly by indication grouping (P < .001). On multivariate analysis, tumor size (P < .001), lymph node status (P < .001), comorbidity (P = .02), and chemotherapy receipt (P = .003) were found to be independent significant correlates of PMRT receipt. The most common reasons cited for not pursuing PMRT were lack of physician recommendation and perceived lack of need.
CONCLUSIONS
PMRT receipt is strongly correlated with clinical indication. The authors found no sociodemographic disparities in utilization. However, approximately one-fifth of patients with strong indications did not receive treatment.
Keywords: mastectomy, radiotherapy, breast neoplasms, guideline adherence, quality of healthcare
The use of postmastectomy radiotherapy (PMRT) to improve the locoregional control of breast cancer has been well established for many years.1,2 Early studies failed to demonstrate a survival benefit for PMRT, in part due to the use of techniques now considered to be outdated, which resulted in an increased incidence of late cardiac mortality that offset any potential survival benefit from improved cancer control.3,4 In 1997, randomized trials demonstrated, for the first time, an overall survival advantage from PMRT,5-7 with benefit observed not only among patients with ≥4 positive lymph nodes but also those with 1 to 3 positive lymph nodes. Nevertheless, because locoregional recurrence rates in patients with 1 to 3 positive lymph nodes were higher in these trials than typically observed in the US,8-10 the appropriate selection of patients for PMRT has remained a subject of debate.
In 2001, the American Society of Clinical Oncology (ASCO) consensus statement concluded that PMRT was “recommended” for patients with ≥4 positive axillary lymph nodes and “suggested” for patients with T3 tumors with positive axillary lymph nodes and patients with operable stage III tumors.11 The ASCO panel further concluded that there was insufficient evidence to make recommendations or suggestions for the routine use of PMRT in patients with T1 or T2 tumors with 1 to 3 positive lymph nodes, and physician opinion remained divided regarding the management of these patients.12 Similar guideline statements were also issued by other prominent groups contemporaneously.13-15
A limited number of studies have evaluated the use of PMRT since these guidelines were disseminated, and these have been limited by restriction to the elderly patients in the Surveillance, Epidemiology, and End Results (SEER)-Medicare database16,17 or to experiences at centers of excellence that may not be representative of patterns of care and outcomes among patients treated in more diverse settings.18 Furthermore, to our knowledge, none have examined clinician-patient communication factors that direct the use of therapy. To address these limitations, we examined patterns and correlates of PMRT use among a population-based sample of patients diagnosed with breast cancer in 2002 and reported to the SEER registries of Southern California and Detroit. We asked the following questions: 1) Did patients with clear indications for PMRT receive it? 2) How frequently was PMRT used in patients for whom medical opinion was divided? 3) Were there sociodemographic differences in utilization? 4) What communication and patient attitude factors influenced receipt of treatment?
MATERIALS AND METHODS
Study Population and Sampling
Details of the sampling strategy have been reported elsewhere.19 Women aged ≤79 years who were diagnosed with breast cancer and identified by the SEER Cancer Registries of the greater metropolitan areas of Detroit and Los Angeles during a 14-month period from December 2001 through January 2003 were eligible. The preliminary study sample (n = 2647) was accrued monthly during the study period. The sample included all patients with ductal carcinoma in situ. In addition, all African American women with invasive disease and a random sample of non–African American women with invasive disease were selected. A random number generator was used by field staff to select the non–African American invasive cases. Of the preliminary sample, 90% were eligible for the study (n = 2382). The study protocol was approved by the institutional review boards of the University of Michigan, the University of Southern California, and Wayne State University.
The survey was completed by 77.4% of eligible women (n = 1844). SEER data were merged with survey data for 98.2% of patients. For this study of PMRT, we selected patients with invasive disease (stage I-III) who received mastectomy (n = 447).
Measures
Copies of the full questionnaire are available to interested readers upon request to the corresponding author. The dependent variable was self-reported receipt of PMRT (“finished,” “started,” or “going to start”). Independent variables included tumor characteristics (tumor size, lymph node stage, grade, hormone receptor status), patient characteristics (age, race, income, education, insurance status, comorbidities), and other characteristics (distance to radiotherapy [RT] facility, chemotherapy receipt). Information regarding tumor characteristics was based exclusively on SEER data; information regarding patient and other characteristics was drawn from self-report. For age and race/ethnicity, SEER data were used for the few patients missing data by self-report.
We assessed rates of explanation, recommendation, and receipt of radiation by indication categories, defined primarily by reference to the ASCO guidelines that were published immediately before the period in which these patients were treated. We considered “RT indicated” for patients with any T classification and N2 or N3 disease, those with T3, lymph node–positive tumors, and those with T4 tumors of any lymph node stage. We considered “RT not indicated” for patients with T1-2N0 tumors (recognizing that the lack of margin information would lead to the utilization of RT in a small proportion of patients in this group whose mastectomies did not yield negative margins and therefore restricting our analysis to consider underuse rather than overuse). We considered “opinion divided” for patients with T1-2, N1 disease and those with T3N0 disease.
Patients who did not receive or plan to receive radiation were asked to indicate the reasons for this decision. Patient attitudes were also ascertained by asking women who perceived a choice of surgical treatment options the following question: “When you were deciding between mastectomy and lumpectomy, how much was your decision influenced by whether the treatment you chose …” From the 23 items that followed, we conducted factor analyses and subsequently constructed a number of scales, 1 of which addressed attitudes toward radiation and is considered in this analysis (3 items: “would allow you to avoid exposing yourself to radiation”; “would allow you to avoid the side effects of radiation therapy”; and “would allow you not to have to go back and forth to radiation treatment every day for weeks”; alpha = .95). Summary scores were interval measures that ranged from 1 (not influenced by attitude factor) to 4 (greatly influenced by attitude factor). We collapsed the score into 2 categories: not influenced or slightly influenced (scores from 1 to 2.3) and moderately to greatly influenced (scores from 2.4 to 4.0).
Statistical Analysis
We calculated proportions of patients who received PMRT by tumor, patient, and other characteristics. Univariate analysis was performed using chi-square testing. A multivariate logistic regression model of PMRT receipt was constructed with a backward stepwise approach, with tumor, patient, and other characteristics as independent variables. We repeated these analyses after including a separate category indicating a missing value for variables with >5% missing data (tumor size, tumor grade, age, education, income, and chemotherapy receipt). These secondary analyses yielded the same results. To examine the association between patient radiation attitude score and PMRT receipt, we regressed receipt of PMRT on the attitude toward RT scale, controlling for clinical and predisposing factors to calculate adjusted proportions, and testing for significance using Wald tests. All analyses were evaluated for second-order interactions between selected covariates, and none were observed. Point estimates were adjusted for design effects by using a sample weight that accounted for differential selection by stage, ethnicity, and nonresponse.
RESULTS
Sample Characteristics
Ten patients who reported that they were still considering whether to have radiation at the time of the survey were excluded, as were 41 patients not providing a response to the item asking whether they were considering RT, resulting in a sample size of 396 patients. Table 1 shows the characteristics of the 148 patients who reported having finished, started, or planned to start RT (hereinafter described as “receiving PMRT”) and the 248 patients who reported they were not considering radiation.
Table 1.
Univariate Correlates of Radiation Receipt After Mastectomy
| Characteristic | No. of Patients* | PMRT, % | P |
|---|---|---|---|
| T classification | <.001 | ||
| T1 | 175 | 18.9 | |
| T2 | 122 | 38.5 | |
| T3 | 43 | 81.4 | |
| T4 | 32 | 78.1 | |
| N classification | <.001 | ||
| No | 193 | 17.1 | |
| N1 | 104 | 42.3 | |
| N2 | 45 | 86.7 | |
| N3 | 23 | 82.6 | |
| Nx | 15 | 46.7 | |
| Tumor grade | .01 | ||
| Low | 59 | 23.7 | |
| Intermediate | 130 | 32.3 | |
| High | 154 | 46.0 | |
| Age, y | .003 | ||
| <40 | 29 | 62.1 | |
| 40-49 | 65 | 41.5 | |
| 50-59 | 95 | 42.1 | |
| 60-69 | 93 | 29.0 | |
| 70+ | 62 | 24.2 | |
| No. of comorbidities | .02 | ||
| 0 | 193 | 43.0 | |
| ≥1 | 203 | 32.0 | |
| Race | <.001 | ||
| White | 247 | 31.2 | |
| African American | 103 | 39.9 | |
|
Other (includes Asian,
Native American, other) |
43 | 62.8 | |
| Insurance | .001 | ||
| None | 9 | 77.8 | |
| Medicaid | 17 | 64.7 | |
| Medicare | 124 | 28.2 | |
| Private | 150 | 34.7 | |
| Other | 85 | 44.7 | |
| Marital status | .28 | ||
| Married or partnered | 220 | 35.0 | |
| Not married | 176 | 40.3 | |
| Education | .38 | ||
| Some high school or less | 50 | 48.0 | |
| High school graduate | 77 | 36.4 | |
| Some college or technical school | 142 | 35.2 | |
| College graduate and beyond | 82 | 34.2 | |
| Income | .08 | ||
| <30,000 | 139 | 42.5 | |
| 30,000-69,999 | 100 | 29.0 | |
| 70,000+ | 75 | 32.0 | |
| Received chemotherapy | <.001 | ||
| Yes | 225 | 51.6 | |
| No | 118 | 9.3 |
PMRT indicates postmastectomy radiotherapy.
Numbers may sum to <396 due to missing values. The P values tested differences in the receipt of radiation for the selected variable.
The median patient age was 59 years; 193 (48.7%) had lymph node–negative disease (11 of whom were stage T3N0 and 7 of whom were T4N0), 104 (26.3%) had N1 lymph node disease (14 of whom were T3N1 and 4 of whom were T4N1), 45 (11.4%) had N2 disease, and 23 (5.8%) had N3 disease. Fifteen patients had Nx disease, and lymph node status was unknown in another 16 patients. Overall, 175 patients (44.2%) were classified in the “RT not indicated” group, 97 (24.5%) were in the “opinion divided” group, and 99 (25.0%) were in the “RT indicated” group.
Factors Correlated With PMRT Receipt
On univariate analysis, tumor factors of T classification, N classification, and histologic grade were all found to be significantly associated with the receipt of PMRT, as was the receipt of chemotherapy. Young age, black race, and lack of insurance were also significantly associated with PMRT, but other demographic variables were not (Table 1). Patients with comorbidities were less likely to receive RT. On multivariate analysis, only tumor size (P < .001), lymph node status (P < .001), comorbidity (P = .02), and chemotherapy receipt (P = .003) were found to be independent significant correlates of PMRT receipt.
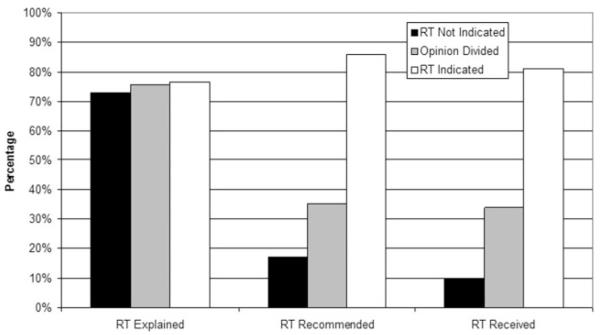
Figure 1 shows the proportions of patients who reported receiving explanations of radiation, receiving recommendations for radiation, and receiving radiation, by indication category and adjusted for clinical and socioeconomic factors. Similar proportions in each indication group reported receiving an explanation of radiation from their surgeon or another healthcare provider (P = .17); the proportions reporting recommendations for radiation and receipt of PMRT varied substantially by indication grouping (P < .001). Physician recommendation was strongly correlated with PMRT receipt; 80.2% of those reporting that a surgeon or other provider had recommended RT received PMRT, whereas only 3.4% reporting no recommendation received PMRT (P < .001).
FIGURE 1.
This figure demonstrates the rates with which patients reported that radiotherapy (RT) was explained (P = .10), recommended (P < .001), and received (P < .001) by indication grouping. Rates were adjusted for age, race, education, marital status, and comorbidities.
Attitudes Toward Radiation and Reasons for Declining PMRT
As shown in Table 2, few patients with strong indications for RT were not considering PMRT. Of note, the most common reason cited by those in this group for not considering RT was the lack of a physician recommendation. As expected, the majority of T1 or T2, N0 patients were not considering RT, and the most common reasons cited were that “there was no need” and that “my physician did not recommend it.” Among patients in whom medical opinion was divided (T3N0 or T1-2, N1 disease), approximately the same proportion of patients were not considering PMRT as those who were definitely undergoing PMRT. Again, the most common reasons cited for not pursuing PMRT in this group were lack of physician recommendation and a perceived lack of need. Few patients reported that concerns about side effects or inconvenience played a role, and similarly few reported that they perceived they could not have RT for medical reasons. Concern about cost was reported by only 1 patient.
Table 2.
Patient Reasons for Not Considering PMRT, by Indication Grouping
| RT Not Indicated | Opinion Divided | RT Indicated | |
|---|---|---|---|
| Total | 175 | 97 | 99 |
| No. not considering RT | 152 | 60 | 20 |
| No. not considering RTand indicating reasons | 138 | 49 | 17 |
| Reasons | |||
| My doctor(s) did not recommend it | 57.3% | 69.4% | 52.9% |
| There was no need | 54.4% | 42.9% | 23.5% |
| It was too inconvenient | 4.4% | 2.0% | 0.0% |
| I was worried about side effects or complications | 6.5% | 6.1% | 11.8% |
| I did not think it would be helpful | 5.8% | 4.1% | 17.7% |
| I was worried that it would cost too much | 0.7% | 0.0% | 0.0% |
| I did not know about it | 0.0% | 0.0% | 0.0% |
| I could not have it for medical reasons | 4.4% | 6.1% | 5.9% |
PMRT indicates postmastectomy radiotherapy; RT, radiotherapy.
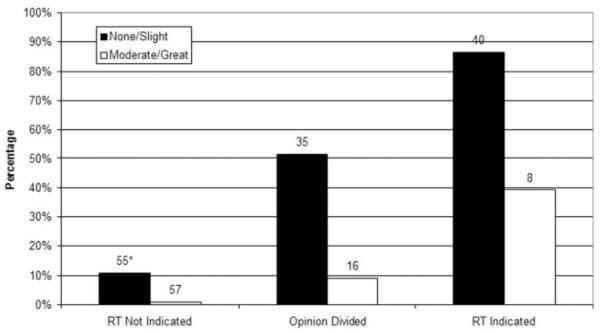
Figure 2 shows the association of patients’ concerns about RT with receipt of PMRT. Overall, 34% of the mastectomy patients reported concerns about radiation. Frequency of moderate to significant concern about radiation varied by indication grouping; 14% of those for whom PMRT was indicated, 28% of those for whom medical opinion was divided, and 50% of those for whom PMRT was not indicated (P < .001). Figure 2 shows that among all patients, those patients who were most concerned about radiation were less likely to receive radiation in each indication grouping (P < .001).
FIGURE 2.
This figure demonstrates rates of receipt of postmastectomy radiotherapy (RT) by indication grouping and concern regarding radiation effects. Rates were adjusted for age, race, education, marital status, and comorbidities (P < .001). *Indicates the number of patients in each cell.
DISCUSSION
This population-based study of patients who were diagnosed with breast cancer in 2002 in 2 large metropolitan areas and treated with mastectomy reveals that most patients with guideline-based indications for PMRT receive it. As one would hope, the tumor characteristics (primary tumor size and lymph node status) that define indications for PMRT treatment were found to be highly correlated with PMRT receipt, and patients without comorbidities were more likely to receive PMRT. Chemotherapy receipt was also found to be independently correlated with radiation receipt. Nonclinical factors appeared to be less important. We did not observe significant differences in receipt of treatment by socioeconomic or racial groups after controlling for clinical factors. Nevertheless, it was concerning that approximately one-fifth of patients with strong indications for treatment did not receive RT, and approximately one-fifth of patients in each indication category reported that their providers had never explained RT to them.
Few previous studies have addressed PMRT utilization. A 2002 survey of radiation oncologists found strong consensus regarding the need for PMRT in patients with ≥4 involved lymph nodes but divided opinion regarding the management of N1 disease.12 Although this study was valuable in illuminating radiation oncologists’ attitudes, to our knowledge actual patterns of utilization and reasons for nonutilization were not addressed. Further studies have therefore been necessary to explore whether PMRT utilization complies with consensus guidelines and radiation oncologists’ opinions (which may differ from those of surgeons, who serve as gatekeepers in these cases).
A large study using the SEER-Medicare database explored utilization of PMRT in women aged ≥65 years who were treated with mastectomy between 1991 and 1999.16 The study documented temporal trends toward increased utilization after the publication of the influential Danish and Canadian trials. It also documented differences between teaching and nonteaching institutions, as well as associations between PMRT use and patient age, race, distance to nearest RT facility, region of the country, socioeconomic status, comorbidity, and tumor characteristics. Receipt of PMRT ranged from only 30% to 50% among elderly patients with ≥4 positive lymph nodes who were treated in this period, during which clinical trial information was accumulating and national guidelines had not yet been promulgated. Although innovative, the study was limited by likely underreporting of radiation treatment in SEER, the necessity of relying upon proxy measures of socioeconomic status based upon zip code and census tract information, the omission of nonelderly patients, and the fact that the observation period preceded the release of clinical guidelines.
A subsequent study considering SEER-Medicare data through 2002 found that the rate of PMRT for patients with high-risk (defined by the authors as T3 or T4 or N2 or N3) disease stabilized after 1997, and from 1998 to 2002, only 53% of high-risk elderly patients received PMRT.17 In contrast, a study examining guideline concordance in the treatment of women at 8 National Comprehensive Cancer Network (NCCN) member institutions from 1997 to 2002 found high concordance with treatment guidelines (83.6% of those in whom PMRT was recommended by the NCCN guidelines received it, and 38.6% of those in whom it should be considered received it).18 In the “consider RT” group, the investigators found correlations with institution, tumor characteristics, age, and receipt of chemotherapy. Although the NCCN study is strengthened by including women of all ages, it may not be generalizable to the broader population of patients not treated at elite institutions.
Thus, the current study complements this previous work. Our findings suggest that PMRT utilization among patients with guideline-based indications in a population-based sample was higher than in studies focusing on elderly patients alone and very similar to that observed among patients at elite medical centers. As one would hope, PMRT receipt was highest among patients with advanced lymph node status and large primary tumors. This may reflect the impact of the 2001 ASCO consensus statement or general acceptance of the need for PMRT in patients with advanced disease. The finding that receipt of chemotherapy was an independent predictor of PMRT receipt may indicate that some patients or their physicians are more inclined toward aggressive treatment or perceive greater recurrence risks, potentially due to unmeasured differences in tumor characteristics, physician-patient interactions, or other factors.
This study also offers insights into patients’ decision processes regarding PMRT, including information regarding patient perceptions of provider discussions and recommendations, as well as reasons they did not receive PMRT. It is reassuring that patients did not appear to perceive cost or inconvenience to be important barriers to receiving PMRT. On the other hand, those who scored highest on the scale assessing general concerns about radiation were less likely to receive RT in each indication grouping—although this was observed least frequently for patients with guideline-based indications for treatment. Because patients undergoing mastectomy may have selected their surgery out of a desire to avoid RT and are more likely to express major concerns about radiation than patients undergoing lumpectomy,19 it is particularly important to ensure that adequate explanations of the nature of RT and its side effects are provided to patients with any indications for considering treatment. Of note, although we found that most patients in all risk groups reported that a provider had explained RT, this explanation of radiation may have been in the setting of discussion of initial therapeutic options, including breast conservation (and not necessarily a specific discussion of PMRT). Our finding that those with the strongest indications for PMRT were least concerned about radiation may indicate that discussions specifically focused on a recommendation for PMRT reassure patients regarding the risks of this modality of treatment.
Several aspects of the study merit comment. We note several strengths, including the diverse population-based sample derived from 2 large urban areas of the US: high response rates, valid measures of treatment use, patient-level clinical and sociodemographic variables, and patient report of clinician-patient communication. However, the study has several limitations. First, the fact that the population-based sample was drawn from 2 geographic areas (Detroit and Los Angeles) may limit the generalizability of our findings to other areas, such as rural regions. The modest number of patients undergoing mastectomy in this study may have limited the power to detect the effect of certain variables on patterns of PMRT utilization. Missing data for certain variables may also have affected the results. Patient report of discussions with clinicians may be subject to recall bias. In particular, we did not distinguish between discussions of RT in the context of breast conservation before the final surgical decision versus specific discussion of PMRT. Thus, a higher proportion of patients may not have appreciated the rationale for PMRT or actively participated in the decision to forgo this treatment than indicated by our findings regarding radiation explanation. Finally, timing of systemic therapy with respect to surgery was not known.
Our findings have important implications for clinical care. A nontrivial proportion of patients with clear indications for treatment (19%) did not receive PMRT. In addition, approximately one-fifth of patients studied reported that no provider ever explained RT to them. Increasing surgeons’ awareness of the importance of explaining the rationale for PMRT to their patients undergoing mastectomy, even in cases in which opinion is divided and the individual surgeon might not recommend treatment, is critical. These patients may nevertheless benefit from consultation with a radiation oncologist, so that they may participate actively in this important treatment decision.
As increasing evidence accumulates to support the use of PMRT in selected patients and to help select those patients whom PMRT is most likely to benefit,20-23 it becomes even more important to investigate its utilization. Future research should continue to track trends in the utilization of PMRT in the face of an evolving evidence base and clinical guidelines. Indeed, as Punglia et al. have suggested, receipt of PMRT may be a useful indicator of the quality of healthcare, just as postlumpectomy RT is.16 Correlations between PMRT utilization and surgeon characteristics, including practice factors, may be of particular interest. Future studies should seek to assess patients’ understanding of the risks and benefits of PMRT, the amount of time spent discussing PMRT with their surgeons, which patients are referred to have further discussion with radiation oncologists, and whether greater involvement in the PMRT decision is desired by patients or correlated with satisfaction. In this way, we may gain a much needed understanding of the quality of patient-physician communication in the setting of this complex decision.
Acknowledgments
Conflict of Interest Disclosures
Funded by a grant from the National Cancer Institute (RO1 CA8837) to the University of Michigan.
Dr. Katz was supported by an Established Investigator Award in Cancer Prevention, Control, Behavioral, and Population Sciences from the National Cancer Institute (K05 CA111340).
This project has been funded in part with Federal funds from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. N01-PC-35,139 and NO1-PC-65,064.
The collection of cancer incidence data used in this publication was supported by the California Department of Health Services as part of the state-wide cancer reporting program mandated by California Health and Safety Code Section 103885. The ideas and opinions expressed herein are those of the author, and no endorsement by the State of California, Department of Health Services is intended or should be inferred.
References
- 1.McArdle CS, Crawford D, Dykes EH, et al. Adjuvant radiotherapy and chemotherapy in breast cancer. Br J Surg. 1986;73:264–266. doi: 10.1002/bjs.1800730407. [DOI] [PubMed] [Google Scholar]
- 2.Griem KL, Henderson IC, Gelman R, et al. The 5-year results of a randomized trial of adjuvant radiation therapy after chemotherapy in breast cancer patients treated with mastectomy. J Clin Oncol. 1987;5:1546–1555. doi: 10.1200/JCO.1987.5.10.1546. [DOI] [PubMed] [Google Scholar]
- 3.Paszat LF, Mackillop WJ, Groome PA, Boyd C, Schulze K, Holowaty E. Mortality from myocardial infarction after adjuvant radiotherapy for breast cancer in the surveillance, epidemiology, and end-results cancer registries. J Clin Oncol. 1998;16:2625–2631. doi: 10.1200/JCO.1998.16.8.2625. [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative Group Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Lancet. 2000;355:1757–1770. [PubMed] [Google Scholar]
- 5.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 6.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 7.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–962. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- 8.Recht A, Gray R, Davidson NE, et al. Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: experience of the Eastern Cooperative Oncology Group. J Clin Oncol. 1999;17:1689–1700. doi: 10.1200/JCO.1999.17.6.1689. [DOI] [PubMed] [Google Scholar]
- 9.Katz A, Strom EA, Buchholz TA, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol. 2000;18:2817–2827. doi: 10.1200/JCO.2000.18.15.2817. [DOI] [PubMed] [Google Scholar]
- 10.Taghian A, Jeong JH, Mamounas E, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from 5 National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol. 2004;22:4247–4254. doi: 10.1200/JCO.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 11.Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1539–1569. doi: 10.1200/JCO.2001.19.5.1539. [DOI] [PubMed] [Google Scholar]
- 12.Ceilley E, Jagsi R, Goldberg S, et al. Radiotherapy for invasive breast cancer in North America and Europe: results of a survey. Int J Radiat Oncol Biol Phys. 2005;61:365–373. doi: 10.1016/j.ijrobp.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 13.Harris JR, Halpin-Murphy P, McNeese M, Mendenhall NP, Morrow M, Robert NJ. Consensus statement on postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44:989–990. doi: 10.1016/s0360-3016(99)00096-6. [DOI] [PubMed] [Google Scholar]
- 14.Taylor ME, Haffty BG, Shank BM, et al. Postmastectomy radiotherapy. American College of Radiology. ACR Appropriateness Criteria. Radiology. 2000;215(suppl):1153–1170. [PubMed] [Google Scholar]
- 15.Eifel P, Axelson JA, Costa J, et al. National Institutes of Health Consensus Development Conference statement. J Natl Cancer Inst. 2001;93:979–989. doi: 10.1093/jnci/93.13.979. [DOI] [PubMed] [Google Scholar]
- 16.Punglia RS, Weeks JC, Neville BA, Earle CC. Radiation therapy after mastectomy between 1991 and 1999 in elderly women: response to clinical trial information. J Clin Oncol. 2006;24:3473–3482. doi: 10.1200/JCO.2006.05.7844. [DOI] [PubMed] [Google Scholar]
- 17.Smith BD, Haffty BG, Smith GL, et al. Use of postmastectomy radiotherapy in older women. Int J Radiat Oncol Biol Phys. 2008;71:98–106. doi: 10.1016/j.ijrobp.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Punglia R, Hughes ME, Edge SB, et al. Factors associated with guideline-concordant use of radiotherapy after mastectomy in the National Comprehensive Cancer Network. Int J Radiat Oncol Biol Phys. 2008;72:1434–1440. doi: 10.1016/j.ijrobp.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz SJ, Lantz PM, Janz NK, et al. Patient involvement in surgery treatment decisions for breast cancer. J Clin Oncol. 2005;23:5526–5533. doi: 10.1200/JCO.2005.06.217. [DOI] [PubMed] [Google Scholar]
- 20.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 21.Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with 4 or more positive nodes, as recommended by international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol. 2007;82:247–253. doi: 10.1016/j.radonc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Cheng SH, Horng CF, West M, et al. Genomic prediction of locoregional recurrence after mastectomy in breast cancer. J Clin Oncol. 2006;24:4594–4602. doi: 10.1200/JCO.2005.02.5676. [DOI] [PubMed] [Google Scholar]
- 23.Kyndi M, Sorensen FB, Knudsen H, Overgaard M, Nielsen HM, Overgaard J, Danish Breast Cancer Cooperative Group Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:1419–1426. doi: 10.1200/JCO.2007.14.5565. [DOI] [PubMed] [Google Scholar]