Abstract
Experiments reported here indicate a crude soybean extract, if defatted with acetone, effectively blocks cell transformation in vitro. An active component of this crude extract is the Bowman-Birk trypsin and chymotrypsin inhibitor. The chymotrypsin-inhibitory region of the Bowman-Birk inhibitor is responsible for suppressing in vitro transformation. Another low molecular weight soybean trypsin inhibitor does not significantly suppress transformation. The Bowman-Birk inhibitor (i) has an irreversible effect on the transformation process, (ii) can suppress radiation-induced transformation even when added to cultures many days after the carcinogen exposure, and (iii) is effective in its ability to suppress transformation when present in the medium at a concentration as low as 0.125 nM.
Full text
PDF
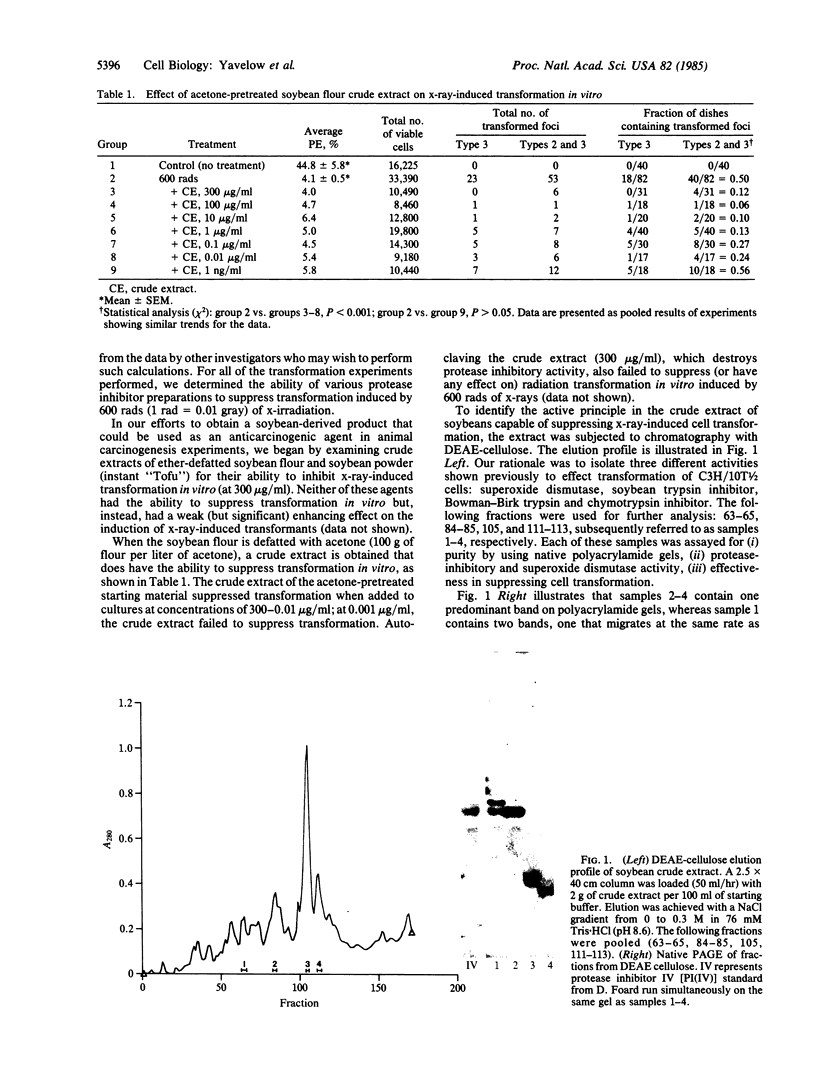

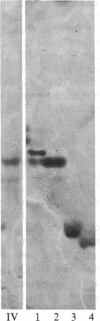
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramovitz A. S., Yavelow J., Randolph V., Troll W. Inhibition of superoxide production in human neutrophils by purified soybean polypeptides. Re-evaluation of the involvement of proteases. J Biol Chem. 1983 Dec 25;258(24):15153–15157. [PubMed] [Google Scholar]
- Andersen K. B. Leupeptin inhibits retrovirus infection in mouse fibroblasts. J Virol. 1983 Dec;48(3):765–769. doi: 10.1128/jvi.48.3.765-769.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Correa P. Epidemiological correlations between diet and cancer frequency. Cancer Res. 1981 Sep;41(9 Pt 2):3685–3690. [PubMed] [Google Scholar]
- Hwang D. L., Lin K. T., Yang W. K., Foard D. E. Purification, partial characterization, and immunological relationships of multiple low molecular weight protease inhibitors of soybean. Biochim Biophys Acta. 1977 Dec 20;495(2):369–382. doi: 10.1016/0005-2795(77)90392-0. [DOI] [PubMed] [Google Scholar]
- Kennedy A. R. Antipain, but not cycloheximide, suppresses radiation transformation when present for only one day at five days post-irradiation. Carcinogenesis. 1982;3(9):1093–1095. doi: 10.1093/carcin/3.9.1093. [DOI] [PubMed] [Google Scholar]
- Kennedy A. R., Cairns J., Little J. B. Timing of the steps in transformation of C3H 10T 1/2 cells by X-irradiation. Nature. 1984 Jan 5;307(5946):85–86. doi: 10.1038/307085a0. [DOI] [PubMed] [Google Scholar]
- Kennedy A. R., Fox M., Murphy G., Little J. B. Relationship between x-ray exposure and malignant transformation in C3H 10T1/2 cells. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7262–7266. doi: 10.1073/pnas.77.12.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A. R., Little J. B. Effects of protease inhibitors on radiation transformation in vitro. Cancer Res. 1981 Jun;41(6):2103–2108. [PubMed] [Google Scholar]
- Kennedy A. R., Little J. B. Investigation of the mechanism for enhancement of radiation transformation in vitro by 12-O-tetradecanoylphorbol-13-acetate. Carcinogenesis. 1980;1(12):1039–1047. doi: 10.1093/carcin/1.12.1039. [DOI] [PubMed] [Google Scholar]
- Kennedy A. R., Little J. B. Protease inhibitors suppress radiation-induced malignant transformation in vitro. Nature. 1978 Dec 21;276(5690):825–826. doi: 10.1038/276825a0. [DOI] [PubMed] [Google Scholar]
- Kuroki T., Drevon C. Inhibition of chemical transformation in C3H/10T1/2 cells by protease inhibitors. Cancer Res. 1979 Jul;39(7 Pt 1):2755–2761. [PubMed] [Google Scholar]
- Laskowski M., Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- O'Donnell-Tormey J., Quigley J. P. Detection and partial characterization of a chymostatin-sensitive endopeptidase in transformed fibroblasts. Proc Natl Acad Sci U S A. 1983 Jan;80(2):344–348. doi: 10.1073/pnas.80.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Smirnoff P., Khalef S., Birk Y., Applebaum S. W. A trypsin and chymotrypsin inhibitor from chick peas (Cicer arietinum). Biochem J. 1976 Sep 1;157(3):745–751. doi: 10.1042/bj1570745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavelow J., Finlay T. H., Kennedy A. R., Troll W. Bowman-Birk soybean protease inhibitor as an anticarcinogen. Cancer Res. 1983 May;43(5 Suppl):2454s–2459s. [PubMed] [Google Scholar]