In human immunodeficiency virus–infected women, cervical cytomegalovirus (CMV) reactivation during pregnancy was correlated with higher CMV levels in breast milk. Low maternal CD4 count and high CMV levels in breast milk were independently associated with infant CMV infection.
Keywords: cytomegalovirus, human immunodeficiency virus, neonates, opportunistic infection, compartmentalization
Abstract
Background. Cytomegalovirus (CMV) infection is associated with adverse outcomes in human immunodeficiency virus (HIV)–exposed infants. Determinants of vertical CMV transmission in the setting of maternal HIV-1 infection are not well-defined.
Methods. CMV and HIV-1 levels were measured in plasma, cervical secretions, and breast milk of 147 HIV-1–infected women to define correlates of maternal CMV replication and infant CMV acquisition.
Results. Although few women had detectable CMV in plasma (4.8%), the majority had detectable CMV DNA in cervical secretions (66%) and breast milk (99%). There was a strong association between cervical CMV detection during pregnancy and later breast milk levels (β = 0.47; P = .005). Plasma HIV-1 level and CD4 counts were associated with CMV in the cervix and breast milk. However HIV-1 levels within the cervix and breast milk were not associated with CMV within these compartments. Maternal breast milk CMV levels (hazard ratio [HR], 1.4; P = .003) and maternal CD4 < 450 cells/mm3 (HR, 1.8; P = .008) were independently associated with infant CMV acquisition; each log10 increase in breast milk CMV was associated with a 40% increase in infant infection. The breast milk CMV level required to attain a 50% probability of CMV transmission increased with higher maternal CD4 counts, increasing from 3.55 log10 CMV DNA copies/mL at a CD4 count of 350 cells/mm3 to 5.50 log10 CMV DNA copies/mL at a CD4 count of 1000 cells/mm3.
Conclusions. Breast milk CMV levels and maternal CD4 count are major determinants of CMV transmission in the setting of maternal HIV-1. Maternal immune reconstitution or lowering breast milk CMV levels may reduce vertical CMV transmission.
Cytomegalovirus (CMV) infection can cause substantial morbidity in infancy. During maternal primary or reinfection, in utero CMV transmission is associated with a high risk of congenital disease and permanent sensorineural deficits [1]. Premature infants acquiring CMV by breast milk are at risk of developing symptomatic disease in the neonatal period [2] and may also experience long-term cognitive and psychomotor development [3, 4]. In the setting of infant human immunodeficiency virus type 1 (HIV-1) infection, both in utero and postnatally acquired CMV are associated with accelerated HIV-1 disease progression, neurologic disease, and death [5, 6].
CMV can be transmitted from mother-to-infant in utero, intrapartum, or postpartum by exposure to breast milk or saliva. Endothelial and epithelial cells support productive CMV infection and may facilitate systemic dispersion of virus [7]. CMV is frequently detected in saliva, urine, semen, cervical secretions, and breast milk, but detection in blood is rare in the absence of significant immunosuppression. In the cervix of healthy, HIV-negative women, CMV can be detected in approximately 10% of nonpregnant women and 11%–35% of pregnant women in the third trimester [8–10]. A small study of women with CMV cervicitis found inclusion bodies within glandular epithelial cells, endothelial cells, and mesenchymal stromal cells [11], suggesting these cells might be involved in CMV reactivation in the cervix. In breast milk, nearly all healthy women have detectable CMV DNA [12, 13]. CMV is found in both whey and cellular fractions of breast milk [13] and could have multiple sources, including mammary epithelial cells and migrating monocyte/macrophages and lymphocytes. In the setting of maternal HIV-1 infection, local HIV-1 replication and immunosuppression within tissues could increase CMV shedding at sites relevant for CMV transmission.
In Nairobi, Kenya, 97% of adult blood donors in the general population are CMV seropositive [14] and virtually all HIV-infected women are coinfected with CMV [15, 16]. We previously found the rate of congenital CMV infection was 6.3% in HIV-exposed but uninfected infants and 29% in HIV-infected infants; by 6 months of age, 90% of HIV-exposed but uninfected infants and 89% of HIV-infected infants had experienced plasma CMV DNAemia, and 46% of HIV-unexposed infants were CMV seropositive [16]. Preventing or delaying CMV infection may represent a novel strategy to improve the health of both HIV-infected and HIV-exposed uninfected infants in sub–Saharan Africa, but requires a better understanding of CMV replication and transmission in the setting of maternal HIV-1. Using specimens and data from a randomized trial of valacyclovir suppressive therapy in Nairobi, we examined associations between HIV-1 RNA level, immunosuppression, and CMV replication in different maternal compartments and identified maternal correlates of infant CMV infection.
METHODS
Study Participants and Sampling
Procedures were approved by the Institutional Review Board of the University of Washington and the Ethics and Research Committee of Kenyatta National Hospital. The clinical trial evaluated the efficacy of valacyclovir to reduce maternal HIV-1 RNA levels (NCT00530777, [17]). HIV/herpes simplex virus type 2 coinfected women were recruited at 28–32 weeks gestation, and treatment with antiretrovirals for the prevention of mother-to-child transmission (PMTCT) was initiated. The PMTCT regimen consisted of twice-daily zidovudine from 28 weeks, zidovudine every 3 hours during labor until delivery, and single-dose nevirapine at the onset of labor [17]. In June 2009, maternal lamivudine and twice-daily zidovudine for 1 week postpartum were also offered. At 34 weeks, women were randomized to twice-daily valacyclovir at 500 mg or placebo and continued until 1 year postpartum. Participants were evaluated at 34 and 38 weeks gestation, delivery, and 2, 6, 10, and 14 weeks and 6, 9, and 12 months postpartum. Blood was collected from mothers, and dried blood spots (DBSs) were collected from infants at all visits. Cervical swabs were collected at 34 weeks (prerandomization) and 38 weeks gestation, and breast milk was collected at all postpartum visits.
HIV-1 and CMV Testing
CD4 counts were measured by 32 weeks gestation. Plasma, cervical, and breast milk HIV-1 RNA levels were measured using the GenProbe assay [18]. The lower limit of detection was 150 copies/mL for plasma and 100 copies/mL for cervical secretions and breast milk. Undetectable specimens were recoded at half the limit of detection. Infant DBS were tested for HIV-1 at all time points, as previously described [19, 20].
Real-time quantitative polymerase chain reaction was used to detect the CMV glycoprotein B gene [21]. The limit of detection was 100 copies/mL for virus extracted from maternal plasma, cervical secretions, and breast milk; viral loads from infant DBS were normalized to copies/million cells against a β-globin standard [22] with a lower limit of detection of 100 copies/million cells.
Statistical Analysis
All analyses were conducted using Stata SE version 11.2 for Macintosh. All reported P values are 2-tailed, with alpha = 0.05. HIV-1 and CMV levels were log10-transformed to normalize distributions. CD4 count was dichotomized based on the cohort median at baseline (450 cells/mm3). For variables where >30% of assays were undetectable (CMV in plasma and cervix, HIV-1 in cervix and breast milk), data were dichotomized as detectable/undetectable. Plasma HIV-1 and breast milk CMV levels were treated as continuous variables.
Generalized estimating equations were used to examine associations between HIV-1 replication, CD4 count, and CMV. The binomial link function was used to model virus detection, and a Gaussian link was used to model CMV levels. All generalized estimating equations models used robust standard errors and exchangeable correlation matrices.
Cox proportional hazards regression was used to identify correlates of infant CMV acquisition. To reduce potential confounding by infant HIV-1, we excluded the 10 HIV-infected infants, leaving 131 HIV-exposed uninfected infants. The time of first positive DBS specimen was considered the time of infant CMV acquisition.
Because HIV-1 levels were reduced by valacyclovir [17], all generalized estimating equations and Cox regression models included adjustment for treatment allocation. Models examining CMV–CMV correlations between different compartments additionally adjusted for plasma HIV-1 RNA level.
We used logistic regression to predict the probability of CMV transmission by 1 year at different maternal baseline CD4 counts and 2-week breast milk CMV DNA levels. The logistic model was first fit with infant CMV acquisition as the outcome and with baseline CD4 count and 2-week breast milk CMV load as predictors. Then, resulting parameter estimates from this fitted model were used to predict infection rates for a range of 2-week breast milk CMV loads at 4 different CD4 counts (350, 450, 750, and 1000 cells/mm3). The corresponding confidence intervals were computed using delta method standard errors for each approximately normal predicted probability.
RESULTS
Study Population and Sampling
A total of 148 women were enrolled in the randomized trial; all were eligible for the CMV substudy. One participant declined CMV testing, leaving 147 women. Two women were lost to follow-up before delivery, and 4 infants died with no CMV testing, leaving 141 infants. CMV DNA levels were measured in 146 maternal plasma specimens, 246 cervical swabs, 542 breast milk specimens, and 964 infant DBSs.
Detection and Levels of CMV in Different Maternal Compartments
From 146 women, 7 (4.8%) had detectable CMV in their plasma at 34 weeks gestation; the median viral level in positive specimens was 1.8 log10 CMV copies/mL (interquartile range (IQR), 1.8–2.0) and 1.7 log10 CMV copies/mL overall (IQR, 1.7–1.7; Figure 1). CMV was detected in the cervix of 66% (n = 97/147) of women; the median cervical viral level was 3.2 log10 CMV copies/mL (IQR, 2.6–3.8) in positive specimens and 2.6 log10 CMV copies/mL overall (IQR, 1.7–3.5). Both the frequency of cervical CMV detection (P < .001) and cervical CMV DNA level increased during late pregnancy (P = .01). In breast milk, 99% of women (n = 142/143) had CMV detected at 1 or more study visits. The median viral level in breast milk was 5.5 log10 CMV copies/mL (IQR, 5.0–6.4) in positive specimens and 5.5 log10 CMV copies/mL overall (IQR, 4.9–6.4). Breast milk CMV DNA levels were highest early postpartum and declined slowly over time at a rate of −0.076 log10 CMV copies/mL per month (P = .002).
Figure 1.
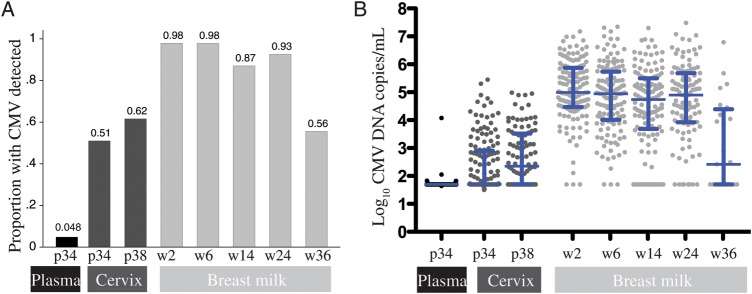
Cytomegalovirus (CMV) detection in human immunodeficiency virus–positive women. A, Bar chart showing the proportion of women with CMV detected at each visit. B, CMV DNA levels at each visit. Midlines show median. Bars show interquartile range. Abbreviations: CMV, cytomegalovirus; P, weeks gestation of pregnancy; W, weeks postpartum.
Correlations in CMV Replication Between Compartments
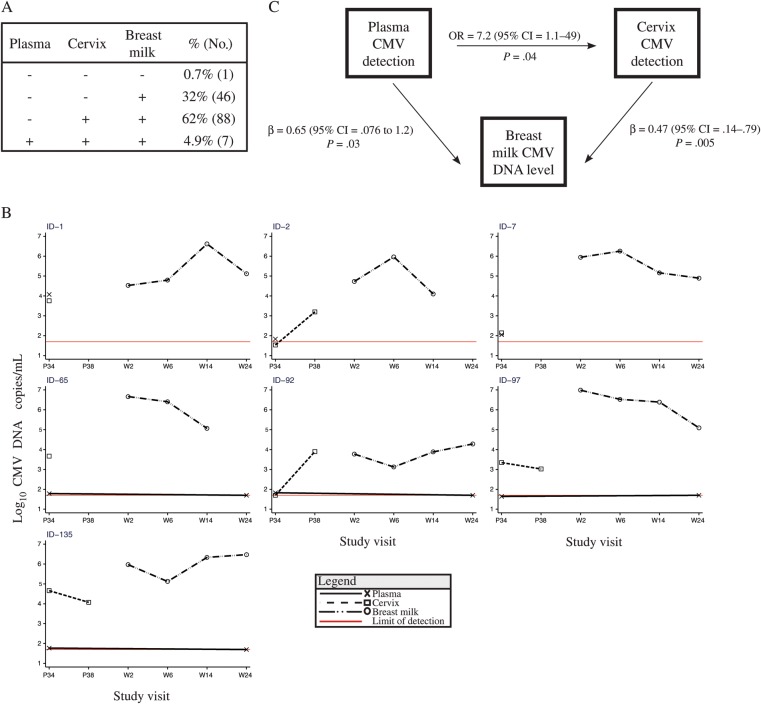
Four patterns of CMV detection were observed in the 142 women with samples collected from all 3 compartments (Figure 2A). All 7 women with CMV detected in plasma also had CMV detected in the cervix and breast milk. The most common pattern was CMV detection in both the cervix and the breast milk (62%). Only 1 woman had no detectable CMV in any compartment tested (0.7%); although we did not perform serology to confirm her CMV infection status, her infant became CMV DNA positive at 2 weeks of age, suggesting this mother was likely CMV infected. We examined associations between CMV replication in the different compartments, adjusting for both plasma HIV-1 RNA level and treatment allocation. Although there were few cases of CMV detected in the plasma, CMV detection in the plasma was associated with CMV detection in the cervix (odds ratio [OR], 7.2; P = .04), and CMV level in breast milk (β = 0.65; P = .03; Figure 2B). Women with CMV detected in the cervix had on average approximately 0.5 log10 higher viral load in their breast milk than women without CMV detected in the cervix (β = 0.47; P = .005). Sensitivity analyses excluding adjustment for plasma HIV-1 RNA level, treatment allocation, or alternatively stratifying by treatment allocation revealed very similar point estimates (data not shown).
Figure 2.
Patterns of cytomegalovirus (CMV) replication and associations between different maternal compartments. A, Patterns of CMV detection in 142 human immunodeficiency virus type 1 (HIV-1)–infected women with sampling in all compartments. B, Individual plots of the 7 subjects with CMV detected in all compartments. C, Schematic showing correlations between CMV replication in different maternal compartments. Odds ratios and beta coefficients (β) derived from regression models showing detection of CMV DNA in plasma at 34 weeks gestation as a predictor of cervical CMV DNA detection and CMV DNA levels in breast milk, and detection of CMV DNA in cervix 34 weeks gestation as a predictor of CMV DNA levels in breast milk. All estimates adjust for treatment allocation and plasma HIV-1 RNA level. Abbreviations: CI, confidence interval; CMV, cytomegalovirus; OR, odds ratio; P, weeks gestation of pregnancy; W, weeks postpartum.
Systemic and Compartmental Associations Between HIV-1, Immunosuppression, and CMV
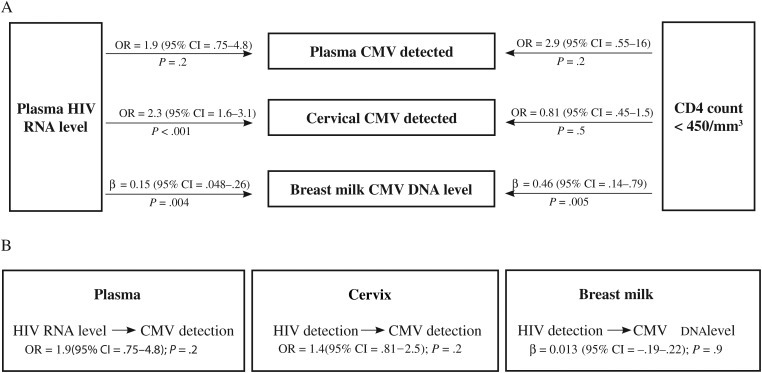
Plasma HIV-1 RNA level was correlated with both cervical CMV detection and breast milk CMV levels (Figure 3A). Each 1 log10 increase in plasma HIV-1 RNA level was associated with a >2-fold increased likelihood of detecting CMV in the cervix (OR, 2.3; P < .001) and a 0.15 log10 higher CMV level in breast milk (P = .004). We did not detect an association between plasma HIV-1 RNA level and the detection of CMV in plasma (OR, 1.9; P = .2).
Figure 3.
Associations between human immunodeficiency virus type 1 (HIV-1) and cytomegalovirus (CMV) replication in different maternal compartments. A, Relationships between plasma HIV-1 RNA level or CD4 count (dichotomized at the median) and CMV in different maternal compartments. Odds ratios (ORs) and beta coefficients (β) derived from regression models: concurrent plasma HIV-1 RNA level as a predictor of plasma CMV DNA detection, cervical CMV DNA detection, and CMV DNA level in breast milk; CD4 count < 450/mm3 at 34 weeks gestation as a predictor of plasma CMV DNA detection, cervical CMV DNA detection, or CMV DNA levels in breast milk. B, ORs and β coefficients from GEE models illustrate relationships between HIV-1 and CMV measured concurrently within different maternal compartments: HIV-1 level and CMV DNA detection in plasma, HIV-1 RNA and CMV DNA detection in the cervix, and HIV-1 RNA detection and CMV DNA level in breast milk. All GEE models include time, which was significant. Models including breast milk exclude time points after 6 months when very few women were still breastfeeding. All models are adjusted for treatment allocation. Abbreviations: CI, confidence interval; CMV, cytomegalovirus; GEE, generalized estimating equations; HIV, human immunodefiency virus; OR, odds ratio.
We detected no association between CD4 count and CMV detection in the plasma or cervix. However, CD4 < 450 cells/mm3 was associated with 0.46 log10 higher CMV levels in the breast milk (β = 0.46; P = .005).
Although plasma HIV-1 RNA level and immunosuppression were associated with CMV in discrete compartments, we found no association between HIV-1 replication and CMV replication within the cervix or within the breast milk (Figure 3B).
Predictors of Infant CMV Acquisition
Infant CMV acquisition was similar in the 2 treatment arms and has been detailed elsewhere (A. Roxby, submitted). In total, 87 of 131 (66%) infants had detectable CMV DNA during the first year of life. Breast milk CMV DNA level at 2 weeks postpartum was associated with earlier infant CMV detection (hazard ratio (HR), 1.5; P = .001; Table 1). Estimates were similar when using time-updated breast milk CMV DNA level in the model (data not shown). Plasma HIV-1 RNA levels in pregnancy and at 2 weeks postpartum were also significantly associated with CMV detection in the infants. Baseline maternal CD4 < 450 cells/mm3 was associated with approximately 80% increased risk of infant CMV detection (P = .008). There was also a trend for an association between the detection of CMV in the cervix at 38 weeks and the risk of infant CMV (HR, 1.5; P = .1). Cervical and breast milk HIV-1 detection were not associated with infant CMV acquisition (data not shown).
Table 1.
Maternal Correlates of CMV Acquisition in HIV-1–Exposed Uninfected Infants
| Correlates | Nontransmitters (n = 44) | CMV Transmitters (n = 87) | Hazard Ratio (95% CI)a | P Value |
|---|---|---|---|---|
| Pregnancy | ||||
| CD4 countb< 450 cells/mm3 | 30 (13/44) | 59 (51/87) | 1.8 (1.2–2.8) | .008 |
| HIV-1 RNA level in plasmac | 3.5 (2.7–3.9) | 4.0 (3.5–4.5) | 1.5 (1.2–1.9) | <.001 |
| CMV DNA detected in plasmac | 2.3% (1/44) | 4.7 (4/86) | 1.7 (.61–4.7) | .30 |
| CMV DNA detected in cervix at | ||||
| 34 wk gestation | 43 (19/44) | 54 (47/87) | 1.2 (.77–1.8) | .4 |
| 38 wk gestation | 47 (14/30) | 70 (43/61) | 1.5 (.88–2.7) | .1 |
| Postpartumd | ||||
| HIV-1 RNA level in plasma | 2.3 (1.9–3.3) | 2.9 (1.9–3.7) | 1.4 (1.1–1.7) | .005 |
| CMV DNA level in breast milk | 4.5 (4.0–5.3) | 5.4 (4.7–6.0) | 1.5 (1.2–1.9) | .001 |
Data are % (No./total No.) or median (interquartile range). CMV DNA and HIV RNA viral levels were log10-transformed and expressed as copies/mL; median CD4 count in the cohort was 450 cells/mm3.
Abbreviations: CI, confidence interval; CMV, cytomegalovirus; HIV-1, human immunodeficiency virus type 1.
a Hazard ratios are adjusted for randomization arm, which was not significant in any model.
b 32 weeks gestation.
c 34 weeks gestation.
d Measurements taken at 2 weeks postpartum. Regression model excludes cases with CMV detection in infant specimen prior to 2 weeks.
Breast milk CMV levels remained associated with infant CMV infection when adjusting for either maternal plasma HIV-1 RNA level or CD4 < 450 cells/mm3 (Table 2); in both models each 1 log10 increase in breast milk CMV DNA level was associated with an approximately 40% increase in the rate of infant CMV acquisition (Model 1: P = .008; Model 2: P = .003). Maternal CD4 was independently associated with infant CMV acquisition (Model 2: HR, 1.6; P = .05), but the association between plasma HIV-1 RNA level and infant CMV acquisition was attenuated (Model 1: HR, 1.2; P = .1).
Table 2.
Correlates of Cytomegalovirus Acquisition in HIV-1–Exposed but Uninfected Infants, Adjusted for Maternal HIV-1 RNA Level or CD4
| Models and Correlates | Adjusted HR (95% CI) | P Value |
|---|---|---|
| Model 1 | ||
| HIV-1 RNA level in plasma | 1.2 (.95–1.5) | .1 |
| CMV DNA level in breast milk | 1.4 (1.1–1.8) | .008 |
| Valacyclovira | 1.2 (.79–1.9) | .3 |
| Model 2 | ||
| CMV DNA level in breast milk | 1.4 (1.1–1.8) | .003 |
| CD4 count < 450 cells/mm3 | 1.6 (1.0–2.4) | .05 |
| Valacyclovira | 1.1 (.74–1.8) | .5 |
Model 1 was adjusted for week 2 plasma human immunodeficiency virus load. Model 2 was adjusted for baseline CD4 count at 32 weeks gestation. Both models include viral load measurements conducted at 2 weeks postpartum, excluding cases where infant cytomegalovirus was detected prior to 2 weeks.
Abbreviations: CI, confidence interval; CMV, cytomegalovirus; HIV-1, human immunodeficiency virus type 1; HR, hazard ratio.
a Randomization to valacyclovir 500 mg twice daily.
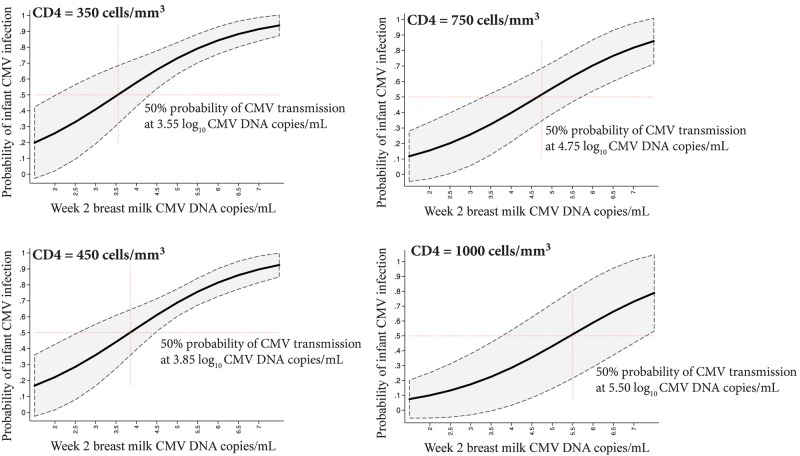
We next used logistic regression to estimate the probability of infant CMV infection as a function of breast milk viral level at a range of maternal CD4 counts. At a CD4 count of 450 cells/mm3, the model-estimated that 50% probability of CMV transmission occurs at a breast milk level of 3.85 log10 CMV DNA copies/mL (Figure 4). At higher CD4 counts, the breast milk viral level required to attain 50% transmission increases; for example, at a CD4 count of 1000 cells/mm3, the model-estimated 50% probability of CMV transmission occurs at a breast milk level of 5.50 log10 CMV DNA copies/mL.
Figure 4.
Estimated probability of cytomegalovirus (CMV) transmission according to maternal CD4 count and breast milk CMV DNA level. The bold black curves in each plot indicate model estimates of the probability of infant CMV infection by 1 year as a function of maternal breast milk CMV DNA level at 2 weeks postpartum, with 95% confidence intervals shown in gray. The 4 graphs show models fixed at different maternal CD4 counts. Red dashed lines indicate the breast milk viral level where the estimated probability of infant CMV infection is 50%. Abbreviation: CMV, cytomegalovirus.
DISCUSSION
In this study, we describe CMV levels in multiple compartments during the antenatal/postpartum period among HIV-infected women and their association with infant CMV acquisition. Cervical CMV detection in pregnancy was associated with later breast milk CMV levels, suggesting similar factors may influence CMV reactivation at these 2 sites. Plasma HIV-1 RNA level was associated with CMV replication in the cervix and breast milk; however, HIV-1 levels within the cervix and breast milk were not associated with CMV in these compartments. Finally, we found that breast milk CMV DNA level at 2 weeks postpartum and low maternal CD4 count were independently associated with infant CMV acquisition; the breast milk CMV DNA level required for CMV transmission increased with higher maternal CD4 counts.
To date, this is the only study examining the association between plasma, cervical, and breast milk CMV DNA levels and transmission in HIV-1 coinfected women. Consistent with earlier studies that found higher CMV levels in the breast milk of transmitting mothers [23, 24] we found a strong positive association between breast milk CMV DNA level and the timing of infant CMV acquisition. Maternal immunosuppression in pregnancy, measured by CD4 count, was also an important predictor of infant CMV acquisition. Using our study data to predict the probability of transmission, we demonstrate that higher breast milk CMV viral levels are required to attain the same probability of CMV transmission with increasing maternal CD4 count. These models help illustrate why CMV acquisition in the first year of life is less common among HIV-negative mother–infant pairs despite the common presence of CMV in breast milk. The association between maternal CD4 count and CMV transmission could be explained by several mechanisms. Reduced placental transfer of antibodies from immunosuppressed women could result in increased susceptibility of their infants to CMV infection, as observed with measles antibodies [25]. Transmission of CMV by saliva may also be an important, yet unmeasured, route of CMV transmission in this cohort; immunosuppressed mothers would be more likely to have CMV shedding in saliva [26] and be at a higher risk of CMV transmission. The association between blood CD4 counts and breast milk CMV levels could reflect a similar relationship within breast milk; higher CD4 levels in breast milk could potentially impact local containment of CMV reactivation by supporting adaptive immune responses in this compartment.
CMV viremia in blood is rare, and studies in HIV-negative individuals have shown no correlation between CMV in the genital tract and urine nor between breast milk and saliva, suggesting CMV reactivation may be compartmentalized in different sites [10, 12, 27]. We observed an increase in cervical CMV detection during late pregnancy, consistent with earlier reports in HIV-negative women [10]. In contrast, we observed a slow decline in breast milk CMV levels after delivery. Previous reports in HIV-negative women have reported the appearance of CMV in breast milk during the first days of life, peaking at approximately 1 month postpartum and declining to undetectable rapidly thereafter [28, 29]; our detection of high levels of CMV in the breast milk throughout lactation appears unique to HIV-infected women. We observed very different levels and patterns of detection in the 3 compartments studied; however, longitudinal analyses enabled us to detect associations between compartments despite these differences. In all cases, plasma CMV detection was accompanied by concurrent detection in the cervix and later detection in breast milk, suggesting systemic reactivation in a small number of women. The majority of women had CMV detected in both the cervix and the breast milk, and cervical CMV detection in pregnancy was strongly associated with later breast milk CMV DNA level. This association could be explained by CMV tropism for epithelial and endothelial cells, which are present in both breast and cervical tissue. Selection pressure from mucosal immune responses could also explain the correlation between CMV replication in the cervix and breast milk; CMV-specific memory T cells have been detected in breast milk [30, 31] and gut-associated lymphoid tissue [32], and although cervical data are lacking, CMV-specific T cells would be expected to migrate to genital mucosal sites as well. Importantly, Ehlinger and colleagues demonstrated a lack of correlation between CMV-specific cellular immune responses in blood and breast milk, suggesting that mucosal responses might be compartmentalized within tissues experiencing CMV reactivation [31].
Plasma HIV-1 RNA level was strongly associated with CMV in the cervix and breast milk, and maternal CD4 count at 32 weeks was associated with subsequent breast milk CMV levels. Surprisingly, localized HIV-1 and CMV replication (measured at concurrent time points) were not correlated in any compartments investigated. Previous studies have reported a correlation between CMV and HIV-1 in blood, cervical secretions, semen, and breast milk [15, 33–35]. These associations may result from immunosuppression and/or direct HIV/CMV interactions within cells or tissues. Unique to our study was the combination of antiretroviral prophylaxis for prevention of mother-to-child transmission and randomization protocol. We hypothesize that introduction of different maternal antiretrovirals caused rapid reductions in maternal HIV-1 viral replication, which may have disrupted steady-state relationships between HIV-1 and CMV. Valacyclovir was also found to have a substantial impact on HIV-1 RNA level in plasma and in breast milk [17], which may have attenuated our ability to detect an association between HIV-1 and CMV at these sites. However when we stratified by treatment allocation, point estimates were similar with the overall model and were also similar between treatment strata, suggesting valacyclovir was neither a confounder nor an effect modifier of this relationship.
A limitation of our study was a low prevalence of CMV detection in plasma, which reduced our power to examine systemic CMV reactivation. Because infant plasma was not collected, DBS were used to diagnose CMV. In our laboratory this method is less sensitive than plasma for diagnosis of acute infant infection (C. Atkinson, unpublished observation), and both are less sensitive than saliva. Because we likely underestimate the true number of infections, we also reduce our ability to detect associations with CMV transmission. Generalizability may be decreased because of the context of the randomized trial; highly active antiretroviral therapy and/or valacyclovir could have an effect on cervical CMV shedding by suppressing herpes simplex virus type 2 shedding at the same site [17], but we did not detect enough cases of active herpes simplex virus type 2 shedding in the cohort to examine this with adequate statistical power. We also acknowledge our limited ability to control for unmeasured confounders that could limit generalizability of our findings. Finally, because our focus was on CMV transmission, we used HIV-1 as the independent variable and CMV as the dependent variable in our analyses; however, true associations between HIV-1 and CMV may be bidirectional in nature.
Together these data help us to understand the factors contributing to CMV replication in discrete maternal compartments and their relevance for transmission. Our data demonstrate that CMV reactivation in mucosal compartments is common during late pregnancy and breastfeeding and is relevant for transmission. Our data additionally suggest an important role for immunosuppression in breast milk CMV transmission. In the absence of a commercially available CMV vaccine for neonates, restoring maternal immunity may offer an alternative approach to reducing or delaying infant CMV infection, an approach supported by decreasing rates of early infant CMV infection in the post–highly active antiretroviral therapy era [36, 37]. These findings provide additional support for expanding maternal access to highly active antiretroviral therapy in pregnancy because the prevention of other maternal–infant transmitted infections could further improve infant outcomes.
Notes
Acknowledgments. We are grateful for the contributions of the research personnel, laboratory staff, and the data management teams in Nairobi and Seattle. We are grateful to the Nairobi City Council Clinics for their participation and cooperation in the execution of these studies and to the Departments of Paediatrics and Medical Microbiology at Kenyatta National Hospital for providing facilities for laboratory and data analysis. We would like to acknowledge the Kizazi Working Group (UW Global Center for Integrated Health of Women, Adolescents and Children) for providing comments on the analysis and figures in this manuscript. Most of all, we thank the women and children who participated in this study.
Financial support. This study was supported by K01AI087369 (J. S.) from the National Institute of Allergy and Infectious Diseases (NIAID), and by an Emerging Opportunity Grant (J. S.) from the University of Washington Center for AIDS Research (CFAR), a National Institutes of Health (NIH)–funded program (P30 AI027757), which is supported by the following NIH Institutes and Centers: NIAID, National Cancer Institute, National Institute of Mental Health, National Institute on Drug Abuse, National Institute of Child Health and Human Development, NIH Heart, Lung, and Blood Institute, and the National Institute on Aging. A. D. was supported by a CFAR Training Grant (T32 AI07140-32), and A. R. was a scholar in the International AIDS Research and Training Program, funded by the Fogarty International Center, NIH (D43 TW000007) and was a Fogarty International Clinical Research Fellow (R24 TW007988). V. C. E. is supported through the MRC Centre for Clinical Virology. The randomized trial was conducted with the support of US NIH research grants (R03 HD 057773, R03 HD 057773-02S1, R01 AI076105; K24 AI087399 to C. F.; K24 HD 054314 to G. J. S.; K24 AI071113 and PO1 AI30731 to A. W.; K24 HL093294 to M. B.); a Puget Sound Partners for Global Health Research and Technology Grant; and a University of Washington Royalty Research Fund Grant (to C. F.). GlaxoSmithKline donated study drug and matched placebo but had no role in the study.
Role of the funding source. The funding sources were not involved in the analyses or interpretation of data.
Potential conflicts of interest. M. B. has served as a consultant to GlaxoSmithKline. V. E. holds a patent for CMV polymerase chain reaction. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev. 2013;26:86–102. doi: 10.1128/CMR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurath S, Halwachs-Baumann G, Muller W, Resch B. Transmission of cytomegalovirus via breast milk to the prematurely born infant: a systematic review. Clin Microbiol Infect. 2010;16:1172–8. doi: 10.1111/j.1469-0691.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- 3.Gompels UA, Larke N, Sanz-Ramos M, et al. Human cytomegalovirus infant infection adversely affects growth and development in maternally HIV-exposed and unexposed infants in Zambia. Clin Infect Dis. 2012;54:434–42. doi: 10.1093/cid/cir837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goelz R, Meisner C, Bevot A, Hamprecht K, Kraegeloh-Mann I, Poets CF. Long-term cognitive and neurological outcome of preterm infants with postnatally acquired CMV infection through breast milk. Arch Dis Child Fetal Neonatal Ed. 2013;98:F430–33. doi: 10.1136/archdischild-2012-303384. [DOI] [PubMed] [Google Scholar]
- 5.Kovacs A, Schluchter M, Easley K, et al. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1-infected women: Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study Group. N Engl J Med. 1999;341:77–84. doi: 10.1056/NEJM199907083410203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nigro G, Krzysztofiak A, Gattinara GC, et al. Rapid progression of HIV disease in children with cytomegalovirus DNAemia. Aids. 1996;10:1127–33. [PubMed] [Google Scholar]
- 7.Gerna G, Baldanti F, Revello MG. Pathogenesis of human cytomegalovirus infection and cellular targets. Hum Immunol. 2004;65:381–6. doi: 10.1016/j.humimm.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Chandler SH, Alexander ER, Holmes KK. Epidemiology of cytomegaloviral infection in a heterogeneous population of pregnant women. J Infect Dis. 1985;152:249–56. doi: 10.1093/infdis/152.2.249. [DOI] [PubMed] [Google Scholar]
- 9.Shen CY, Chang SF, Yen MS, Ng HT, Huang ES, Wu CW. Cytomegalovirus excretion in pregnant and nonpregnant women. J Clin Microbiol. 1993;31:1635–6. doi: 10.1128/jcm.31.6.1635-1636.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stagno S, Reynolds D, Tsiantos A, et al. Cervical cytomegalovirus excretion in pregnant and nonpregnant women: suppression in early gestation. J Infect Dis. 1975;131:522–7. doi: 10.1093/infdis/131.5.522. [DOI] [PubMed] [Google Scholar]
- 11.McGalie CE, McBride HA, McCluggage WG. Cytomegalovirus infection of the cervix: morphological observations in five cases of a possibly under-recognised condition. J Clin Pathol. 2004;57:691–4. doi: 10.1136/jcp.2004.016162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vochem M, Hamprecht K, Jahn G, Speer CP. Transmission of cytomegalovirus to preterm infants through breast milk. Pediatr Infect Dis J. 1998;17:53–8. doi: 10.1097/00006454-199801000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Hamprecht K, Maschmann J, Vochem M, Dietz K, Speer CP, Jahn G. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet. 2001;357:513–8. doi: 10.1016/S0140-6736(00)04043-5. [DOI] [PubMed] [Google Scholar]
- 14.Njeru DG, Mwanda WO, Kitonyi GW, Njagi EC. Prevalence of cytomegalovirus antibodies in blood donors at the National Blood Transfusion Centre, Nairobi. East Afr Med J. 2009;86:S58–61. doi: 10.4314/eamj.v86i12.62903. [DOI] [PubMed] [Google Scholar]
- 15.Slyker JA, Lohman-Payne BL, Rowland-Jones SL, et al. The detection of cytomegalovirus DNA in maternal plasma is associated with mortality in HIV-1-infected women and their infants. AIDS. 2009;23:117–24. doi: 10.1097/QAD.0b013e32831c8abd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slyker JA, Lohman-Payne BL, John-Stewart GC, et al. Acute cytomegalovirus infection in Kenyan HIV-infected infants. Aids. 2009;23:2173–81. doi: 10.1097/QAD.0b013e32833016e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drake AL, Roxby AC, Ongecha-Owuor F, et al. Valacyclovir suppressive therapy reduces plasma and breast milk HIV-1 RNA levels during pregnancy and postpartum: a randomized trial. J Infect Dis. 2012;205:366–75. doi: 10.1093/infdis/jir766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emery S, Bodrug S, Richardson BA, et al. Evaluation of performance of the Gen-Probe HIV type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J Clin Microbiol. 2000;38:2688–95. doi: 10.1128/jcm.38.7.2688-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panteleeff DD, John G, Nduati R, et al. Rapid method for screening dried blood samples on filter paper for human immunodeficiency virus type 1 DNA. J Clin Microbiol. 1999;37:350–3. doi: 10.1128/jcm.37.2.350-353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wamalwa DC, Obimbo EM, Farquhar C, et al. Predictors of mortality in HIV-1 infected children on antiretroviral therapy in Kenya: a prospective cohort. BMC Pediatr. 2010;10:33. doi: 10.1186/1471-2431-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattes FM, Hainsworth EG, Hassan-Walker AF, et al. Kinetics of cytomegalovirus load decrease in solid-organ transplant recipients after preemptive therapy with valganciclovir. J Infect Dis. 2005;191:89–92. doi: 10.1086/425905. [DOI] [PubMed] [Google Scholar]
- 22.Wamalwa D, Benki-Nugent S, Langat A, et al. Survival benefit of early infant antiretroviral therapy is compromised when diagnosis is delayed. Pediatr Infect Dis J. 2012;31:729–731. doi: 10.1097/INF.0b013e3182587796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Strate BW, Harmsen MC, Schafer P, et al. Viral load in breast milk correlates with transmission of human cytomegalovirus to preterm neonates, but lactoferrin concentrations do not. Clin Diagn Lab Immunol. 2001;8:818–21. doi: 10.1128/CDLI.8.4.818-821.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jim WT, Shu CH, Chiu NC, et al. High cytomegalovirus load and prolonged virus excretion in breast milk increase risk for viral acquisition by very low birth weight infants. Pediatr Infect Dis J. 2009;28:891–4. doi: 10.1097/INF.0b013e3181a55c52. [DOI] [PubMed] [Google Scholar]
- 25.Farquhar C, Nduati R, Haigwood N, et al. High maternal HIV-1 viral load during pregnancy is associated with reduced placental transfer of measles IgG antibody. J Acquir Immune Defic Syndr. 2005;40:494–7. doi: 10.1097/01.qai.0000168179.68781.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fidouh-Houhou N, Duval X, Bissuel F, et al. Salivary cytomegalovirus (CMV) shedding, glycoprotein B genotype distribution, and CMV disease in human immunodeficiency virus-seropositive patients. Clin Infect Dis. 2001;33:1406–11. doi: 10.1086/322630. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds DW, Stagno S, Hosty TS, Tiller M, Alford CA., Jr Maternal cytomegalovirus excretion and perinatal infection. N Engl J Med. 1973;289:1–5. doi: 10.1056/NEJM197307052890101. [DOI] [PubMed] [Google Scholar]
- 28.Hamprecht K, Witzel S, Maschmann J, et al. Rapid detection and quantification of cell free cytomegalovirus by a high-speed centrifugation-based microculture assay: comparison to longitudinally analyzed viral DNA load and pp67 late transcript during lactation. J Clin Virol. 2003;28:303–316. doi: 10.1016/s1386-6532(03)00074-x. [DOI] [PubMed] [Google Scholar]
- 29.Yasuda A, Kimura H, Hayakawa M, et al. Evaluation of cytomegalovirus infections transmitted via breast milk in preterm infants with a real-time polymerase chain reaction assay. Pediatrics. 2003;111:1333–6. doi: 10.1542/peds.111.6.1333. [DOI] [PubMed] [Google Scholar]
- 30.Sabbaj S, Ghosh MK, Edwards BH, et al. Breast milk-derived antigen-specific CD8+ T cells: an extralymphoid effector memory cell population in humans. J Immunol. 2005;174:2951–6. doi: 10.4049/jimmunol.174.5.2951. [DOI] [PubMed] [Google Scholar]
- 31.Ehlinger EP, Webster EM, Kang HH, et al. Maternal cytomegalovirus-specific immune responses and symptomatic postnatal cytomegalovirus transmission in very low-birth-weight preterm infants. J Infect Dis. 2011;204:1672–82. doi: 10.1093/infdis/jir632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shacklett BL, Cox CA, Sandberg JK, Stollman NH, Jacobson MA, Nixon DF. Trafficking of human immunodeficiency virus type 1-specific CD8+ T cells to gut-associated lymphoid tissue during chronic infection. J Virol. 2003;77:5621–31. doi: 10.1128/JVI.77.10.5621-5631.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gantt S, Carlsson J, Shetty AK, et al. Cytomegalovirus and Epstein-Barr virus in breast milk are associated with HIV-1 shedding but not with mastitis. Aids. 2008;22:1453–60. doi: 10.1097/QAD.0b013e32830184f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheth PM, Danesh A, Sheung A, et al. Disproportionately high semen shedding of HIV is associated with compartmentalized cytomegalovirus reactivation. J Infect Dis. 2006;193:45–8. doi: 10.1086/498576. [DOI] [PubMed] [Google Scholar]
- 35.Lurain NS, Robert ES, Xu J, et al. HIV type 1 and cytomegalovirus coinfection in the female genital tract. J Infect Dis. 2004;190:619–23. doi: 10.1086/422533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frederick T, Homans J, Spencer L, et al. The effect of prenatal highly active antiretroviral therapy on the transmission of congenital and perinatal/early postnatal cytomegalovirus among HIV-infected and HIV-exposed infants. Clin Infect Dis. 2012;55:877–84. doi: 10.1093/cid/cis535. [DOI] [PubMed] [Google Scholar]
- 37.Guibert G, Warszawski J, Le Chenadec J, et al. Decreased risk of congenital cytomegalovirus infection in children born to HIV-1-infected mothers in the era of highly active antiretroviral therapy. Clin Infect Dis. 2009;48:1516–25. doi: 10.1086/598934. [DOI] [PubMed] [Google Scholar]