Abstract
Genkwanin, a flavonoid which has anti-oxidant and anti-tumor activities, was isolated and purified from flowers of Daphne genkwa Sieb. et Zucc. in a large-scale by normal-phase flash chromatography (NPFC). Dried flower buds were extracted with methanol at room temperature and concentrated. The residues were suspended in water and first extracted with petroleum ether, and then chloroform. Genkwanin was concentrated in the chloroform and insoluble fractions. Under the target-guidance of thin layer chromatography (TLC) as well as solubility, a solvent system composed of cyclohexane-acetone (22:3, v/v) was selected. At a flow rate of 30 mL/min,the insoluble and chloroform fractions were separated to yield 1.5 g and 1.35 g of genkwanin with high purities of 98.3% and 98.6% by HPLC analysis, respectively. The chemical structure of the compound was identified by ESI-MS and NMR. Results of the present study indicated that NPFC was a large preparative-scale, speedy and simple process separation technology and it was feasible to find the appropriate proportion of solvent system by transformation from TLC condition.
Keywords: Daphne genkwa Sieb. et Zucc., Genkwanin, Normal-phase flash chromatography, thin layer chromatography
INTRODUCTION
Daphne genkwa Sieb. et Zucc., a well-known traditional Chinese medicinal herb, has been used in the treatment for edema, asthma and cancer.[1-5] It contains several types of compounds, including flavonoids, biscoumarin, lignans, volatile oils, diterpene esters, chlorogenic acids, phenolic glycosides, among which flavonoids and diterpene esters are the main efficacy components.[6-9] As the major secondary metabolites in the herb, flavonoids have been reported to be responsible for anti-inflammatory, analgesia, anti-leukemia anti-tumor and immunomodulatory activities.[10-12] Genkwanin is one of the main flavonoids in D. genkwa.[13,14] Due to its role in quality control of D. genkwa,[15] and biological activities such as anti-oxidant and anti-tumor,[16,17] a large amount of pure compound is needed for further studies on pharmacology, mechanism and new drug exploration.
Several techniques have been reported for the separation of genkwanin from various plant materials including silica gel, preparative HPLC, Sephadex LH-20 and countercurrent chromatography.[18-21] However, there are some problems in these methods, such as tedious, time consuming, and multiple step developments and high capital requirement. Since genkwanin is a low polarity flavonoid and has poor solubility in the solvent system of water-methanol, HPLC has a problem of long retention and low sample recovery. Considering these limitations, development of an economically effective method for separation and purification of genkwanin is justified.
Normal-phase flash chromatography (NPFC) is an effective method for separation and purification of low polarity compounds from herbal medicines. It was used for the purification of polymethoxyflavones from citrus in 50 minutes, yielding up to a gram level with high purities.[22] NPFC has the advantages of simple sample pretreatment, large handling capacity and easy operation. Compared with silica gel column chromatography, it has a better separation effect by using smaller particle size of silica gel as stationary phase and saves labor while the solvent is transported by pump. Although it requires large volumes of organic solvents, they could be recovered and the polarities also can be measured with a density comparison, which could reduce the production cost enormously.
The solvent system for the separation using NPFC can be established easily by the guidance of TLC and solubility experiments. Once a TLC condition is developed, the polar solvent in the solvent system should be reduced to one third for NPFC. Generally, there would be several suitable solvent systems to this step. Next we need to test their solubilities to the sample, and the one which has the best solubility should be selected for the separation.
In this paper, a simple and efficient method using NPFC for large-scale purification of genkwanin from D. genkwa was described and its chemical structure was identified by ESI-MS and NMR. To the best of our knowledge, this is the first report on separation of genkwanin from D. genkwa by flash chromatography.
EXPERIMENTAL
Apparatus
The preparative NPFC apparatus (EZ purifier ) Shanghai Lisui E-Tech Co., Shanghai, China) fitted with several parameters of quick hands twist solid sample loading columns. It supports solid and liquid sample loading. The flow rate can be regulated from 5 to 100 mL/min. The system was also equipped with a valveless metering pump, a Model 2048-UV detector and a Version 1.36 recorder.
Samples were analyzed by a Shimadzu LC-20AT Multisolvent Delivery System equipped with a Shimadzu SPD-M20A UV detector, an injection valve (Model 7725i) with a 20 μL loop, an SCL-20AVP system controller. Chromatography data were collected using an LC solution workstation (Shimadzu, Japan). An Elite ODS C18 column (250 mm × 4.6 mm I.D., 5 μm, Dalian, China) was used for the analytic process. The nuclear magnetic resonance (NMR) spectrometer was a Mercury Plus 400 NMR system (Varian Inc., USA). The electro-spray mass spectrometer was an LCQ Deca XP Max system (Finnigan, USA).
Reagents
All organic solvents used for active fraction preparation and NPFC separation including methanol, petroleum ether, chloroform, cyclohexane and acetone were of industrial grade (Guangzhou Chemical Reagent Naxin., Guangzhou, China). Methanol used for HPLC was of chromatographic grade (Dikma Co., Ltd., Beijing, China), and water was distilled. Silica gel (particle size 35-50 and 50-75μm) was purchased from Qingdao Puke Parting Materials Co. (Qingdao, China).
D. genkwa was purchased from Qing Ping Market of Chinese Tradition Medicine (Guangzhou, China) and indentified by assistant Professor Kang Li (Medical College of Guangdong, China).
Preparation of Crude Sample
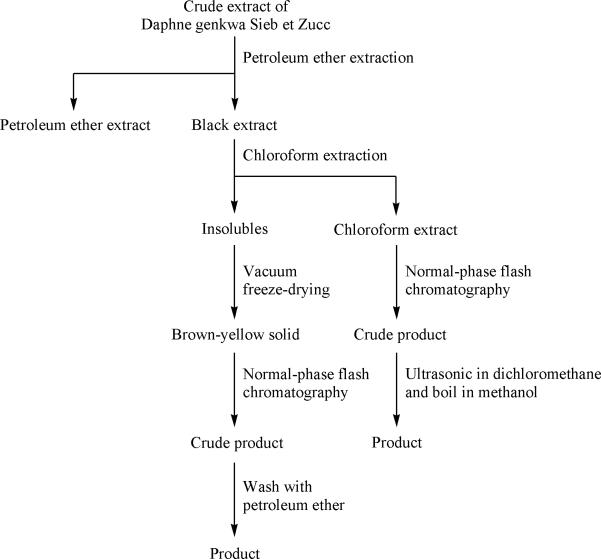
Because of the large quantity of medicinal herb, cool leach method was applied in the experiment, which could eliminate an excessive amount of impurities such as starch, polysaccharide and protein. Due to the complexity of the crude extract, petroleum ether was used to remove the volatile oil and chloroform was used to remove the lignans. Air-dried D. genkwa (10 kg) was extracted twice with decuple 95% aqueous methanol for 72 h at room temperature. The filtrate was concentrated under reduced pressure to yield 1700 g of dried residues which were then suspended in water, and extracted with petroleum ether and chloroform in sequence. The chloroform solutions were combined and evaporated to dryness under reduced pressure at 40°C, yielding 280 g of a crude sample, while 226 g brown-yellow solid was obtained from the insoluble fraction after vacuum freeze-drying. These two parts were stored in a refrigerator at 4°C for further use.
HPLC Analysis Crude Samples and Genkwanin
The brown-yellow solid and chloroform extract from D. genkwa, as well as genkwanin were analyzed with an Elite ODS C18 column (250 mm × 4.6 mm I.D., 5 μm, Dalian, China) at room temperature with a binary mobile phase consisting of methanol (solvent B) and water (solvent A) at a flow rate of 1.0 mL/min. The isocratic elution program was as follows: 0-30 min, 70% B. All HPLC analysis was done with a DAD detector at 332 nm.
Selection of Solvent System
There were three steps for selecting the solvent system. Firstly, the TLC condition should be determined. Three conventional organic solvent systems composed of petroleum ether-ethyl acetate, cyclohexane-acetone and dichloromethane-acetone were tested. The second step was to determine the solvent system for NPFC. This could be conveniently done by reducing the proportion of polar solvents used in TLC by several folds. The third step was the solubility experiment. Equivalent brown-yellow solid were put in three test tubes, to which ten times amount of the above three solvent systems for the NPFC were added to test the solubility at room temperature by shaking the test tubes repeatedly followed by standing for enough time. The results were recorded.
NPFC Separation Procedure and Purification Crude Products
The brown-yellow solid and chloroform extract were separated by NPFC as follows:
Firstly, 100 g of brown-yellow solid was dissolved in 800 ml of an equal volume mixture of methanol and tetrahydrofuran, to which an equivalent amount of silica gel (particle size 50-75μm) was added. The supernatant was concentrated under reduced pressure to yield dried residues. Then, the residues were put into the 1500 g quick hands twist solid sample loading column, which had been filled with proper silica gel (particle size 35-50μm). The column was equilibrated with cyclohexane for three column volumes prior to separations. The following preparation conditions were used: 0-400 min, cyclohexane-acetone (22:3, v/v); 400-600 min, 100% methanol. The flow rate was maintained at 30 mL/min and the effluent was detected at 339 nm. Individual fractions were collected per 500 mL and confirmed by TLC further. The fractions which contained the target compound were pooled together and dried. Afterwards, the crude product was washed by petroleum ether thoroughly at room temperature.
The separation procedure of 280 g chloroform extract was the same with the brown-yellow solid except that was diluted with a proper amount of chloroform. The crude product was treated by ultrasound for 10 minutes in dichloromethane with the power of 200 W at 20°C, then boiled for ten minutes in methanol at 50°C and filtered immediately.
The process of the above preparation was summarized in Figure 2.
Figure 2.
The preparation process of the target compound from flower buds of Daphne genkwa.
HPLC Analysis and Structural Identification
The purities of the two parts were determined by HPLC. Identification of the product was carried out by ESI-MS and NMR.
RESULTS AND DISCUSSION
HPLC Analysis Crude Samples
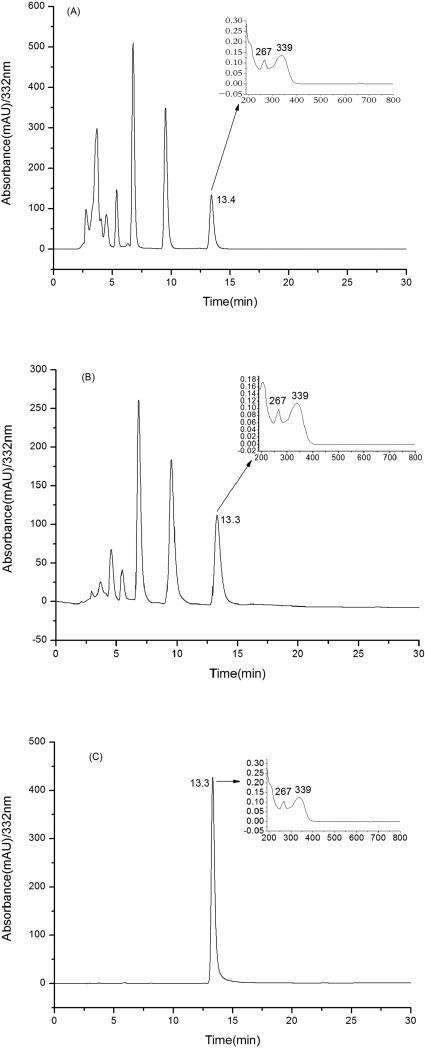
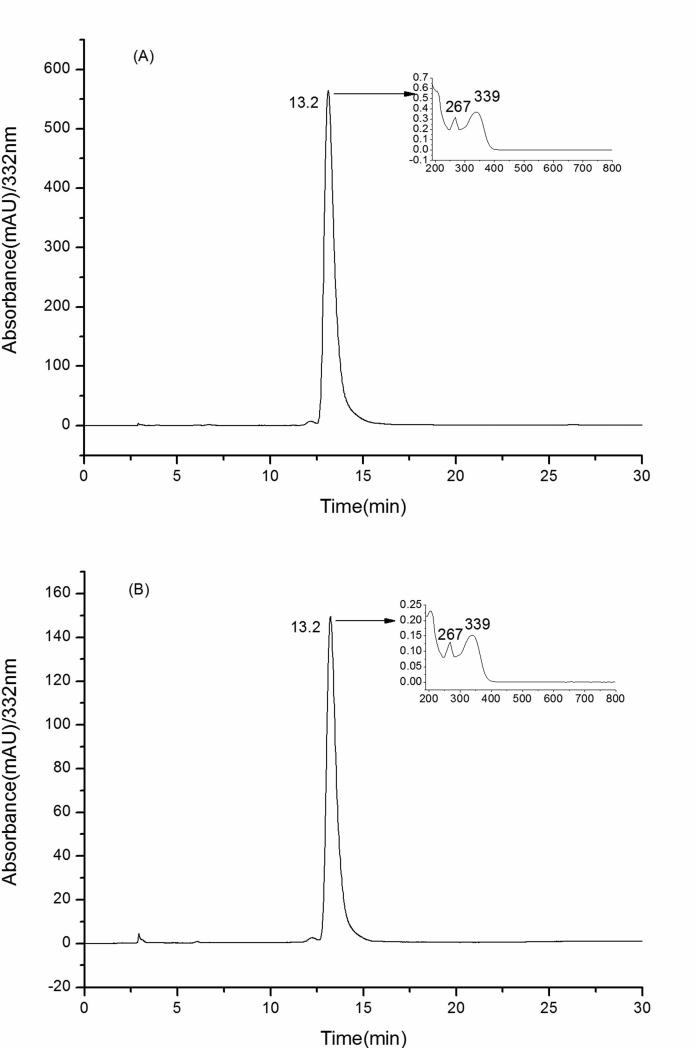
Since our aim is to purify genkwanin, we first had to confirm whether it existed in the crude extracts. The figures A, B, C clearly demonstrate that genkwanin is present in the brown-yellow solid and chloroform extract with the peak areas of 9.6% and 20.8% (Figure 3), respectively. However, the real content of genkwanin in the two parts were below the peak areas while there were some impurities which gave no response on the wavelength of 332 nm. Especially the chloroform extract contained a large amount of lignans. These two parts were each used to prepare genkwanin in the next step.
Figure 3.
HPLC chromatograms of A, B and C along with UV spectra (A): brown-yellow solid; (B): chloroform extract; (C): genkwanin.
Selection of Solvent System
Although NPFC is an effective method for separation and purification of low polarity compounds, the problem is how to select the solvent system, since an appropriate solvent system is essential for a successful NPFC separation. It is supposed to take the following aspects into consideration: a high resolution to the target compound and a good solubility to the extract. Therefore, it was necessary to take TLC and solubility experiments.
In the TLC experiment, petroleum ether-ethyl acetate (2:1, v/v), cyclohexane-acetone (2:1, v/v) and dichloromethane-acetone (40:1, v/v) were determined with Rf values of 0.31, 0.32 and 0.25, respectively. Compared with TLC, the migration of sample in flash column was given an opposite direction from up to down, a three-dimensional mode instead of plane and a promotion with the pressure. Based on these differences, the polar solvent's proportion should be decreased to about one third, which implied that the resolution would be guaranteed in NPFC with petroleum ether-ethyl acetate (22:3, v/v), cyclohexane-acetone (22:3, v/v) or dichloromethane-acetone (99:1, v/v). Finally, cyclohexane-acetone (22:3, v/v) was chosen as the solvent system due to its best solubility to the brown-yellow solid.
NPFC Separation Procedure and Purification Crude Products
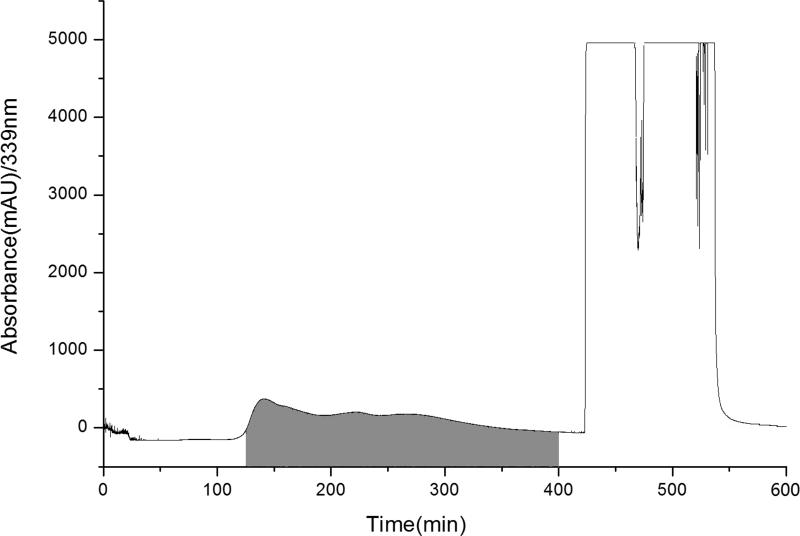
It took 600 min for the separation of 100 g of the brown-yellow solid. In order to save time, the column was washed with 100% methanol instead of cyclohexane-acetone (22:3, v/v) after 400 min while there was no target product in the eluent with TLC detection. The fractions between 125-400 min were pooled together, dried, then washed off the oil impurities yielding to 1.5 g (shaded) of the target product (Figure 4).
Figure 4.
Preparative flash chromatogram of brown-yellow solid (100 g) of D.genkwa; Experimental conditions: EZ purifier middle pressure preparative system and 1500 g of quick hands twist solid sample loading column; Solvent system: 0-400 min, cyclohexane-acetone (22:3, v/v); 400-600 min, 100% methanol; elution mode: isocratic elution; flow rate: 30 mL/min; detection wavelength: 339 nm.
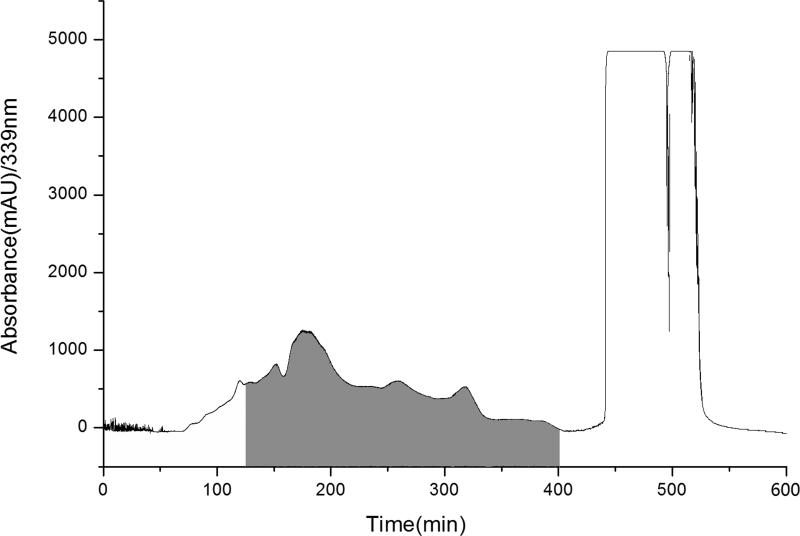
Similarly, 280 g of the chloroform extract was separated in 600 min with a target compound in 125-403 min, while the solvent system was replaced by 100% methanol after 403 min. After removing the impurities, 1.35 g of product (shaded) were obtained (Figure 5). Compared with the first preparation, the crude product was dark green viscous material instead of light yellow solid. Some impurities had strong absorption at the wavelength of 339 nm, which lead to a larger area of object region.
Figure 5.
Preparative flash chromatograms of chloroform extract (280 g) of D.genkwa; Experimental conditions: EZ purifier middle pressure preparative system and 1500 g of quick hands twist solid sample loading column; Solvent system: 0-403 min, cyclohexane-acetone (22:3, v/v); 403-600 min, 100% methanol; elution mode: isocratic elution; flow rate: 30 mL/min; detection wavelength: 339 nm.
As shown in Figure 6, the products from the two extracts had high purities of 98.3% from the brown-yellow sold and 98.6% (based on the HPLC analysis). They were put together for the ESI-MS and NMR identification.
Figure 6.
HPLC chromatograms of A, B along with UV spectra (A): the product prepared from brown-yellow solid; (B): the product prepared from chloroform extract.
Chemical Structure Identification
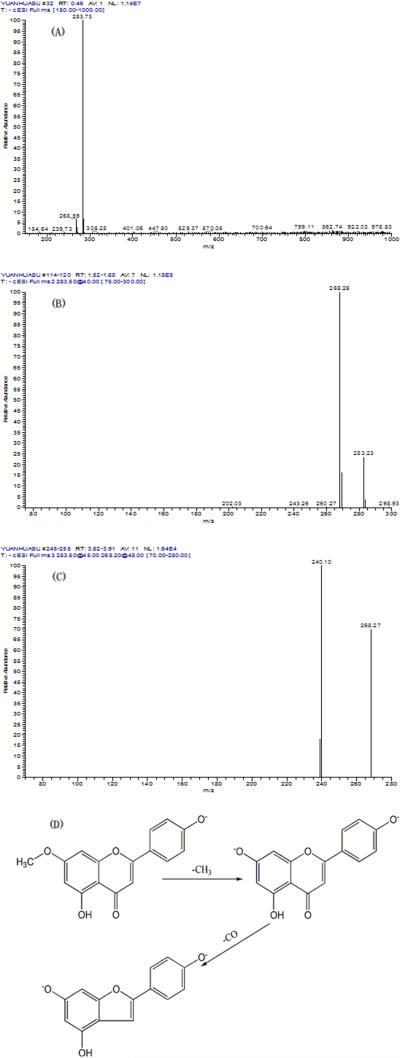
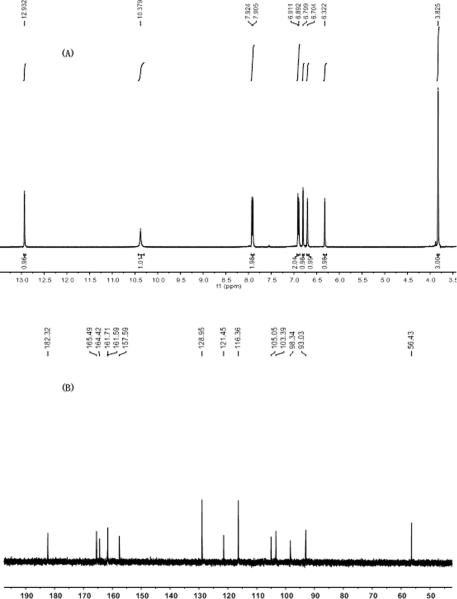
The chemical structure of product was identified from ESI-MSn and NMR data (Figures 7 and 8). Initially, the ESI-MS experiments were performed in the negative mode for determining molecular weights. Then the ESI-MSn spectra were obtained in the negative mode for further structural elucidation. The detailed data are given below: ESI-MS1 m / z 284, ESI-MS2 m / z 268, ESI-MS3 m / z 240 [M - H]-. 1H-NMR (DMSO-d6 ) δ: 3.83 (3H, s, 7-OCH3), 6.32 (1H, d, J=2.0Hz, 6-H), 6.70 (1H, d, J=2.0Hz, 8-H), 6.80 (1H, s, 3-H), 6.89 ( 2H, d, J=8.8Hz, 3′, 5′-H), 7.91 (2H, d, J=8.8Hz, 2′, 6′-H), 10.38 (1H, s, 4′-H), 12.93 (1H, s, 5-OH); 13C-NMR (DMSO-d6 ) δ: 182.3 (C-4), 165.5 (C-7), 164.4 (C-2), 161.7 (C-4′), 161.6, (C-5), 157.6 (C-9), 129.0 (C-2′, 6′), 121.5 (C-1′), 116.4 (C-3′, 5′), 105.1 (C-10), 103.4 (C-3), 98.3 (C-6), 93.0 (C-8), 56.4 (C7-OCH3). Compared the data with the literatures,[9,23] it was identified as genkwanin.
Figure 7.
ESI-MSn mass spectra of the product (A): ESI-MS spectrum of the [M-H]- ion of the product; (B): MS2 on product ion m/z 268; (C): MS3 on product ion m/z 240; (D): Scheme proposed fragmentation mechanisms of [M-H]- ion of the product.
Figure 8.
NMR spectra of the product (A): 1H-NMR; (B): 13C-NMR.
CONCLUSIONS
In this study, a simple and efficient method was developed for preparation of genkwanin from D. genkwa using flash chromatography. Because chloroform had excellent dissolving capacity for the lignans, it was chosen for the extraction, which led to a good solubility of the brown-yellow solid in polar solvents. The condition for the preparation was established easily at the guidance of TLC and solubility experiments. Using the present method, genkwanin was successfully separated with high purity in a gram level quantity. Therefore, this method is capable for simple and large-scale separations of genkwanin. Moreover, the developed NPFC method has a broad applicability for the preparation of low polarity compounds from crude plant extracts.
Figure 1.
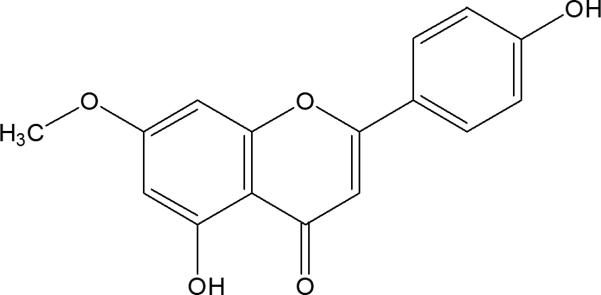
The chemical structure of genkwanin.
REFERENCES
- 1.Kai H, Koine T, Baba M, Okuyama T. Pharmacological Effects of Daphne genkwa and Chinese Medical Prescription, “Jyu-So-To”. Yakugaku Zasshi. 2004;124:349–354. doi: 10.1248/yakushi.124.349. [DOI] [PubMed] [Google Scholar]
- 2.Zhan ZJ, Fan CQ, Jian D, Yue JM. Novel Diterpenoids with Potent Inhibitory Activity Against Endothelium Cell HMEC and Cytotoxic Activities from a Well-known TCM Plant Daphne genkwa. Bioorg. Med. Chem. 2005;13:645–655. doi: 10.1016/j.bmc.2004.10.054. [DOI] [PubMed] [Google Scholar]
- 3.Lee MY, Park BY, Kwon OK, Yuk JE, Oh SR, Kim HS, Lee HK, Ahn KS. Anti-inflammatory Activity of (-)-Aptosimon Isolated from Daphne genkwa in RAW264.7 Cells. Int. Immunopharmacol. 2009;9:878–885. doi: 10.1016/j.intimp.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Zheng WF, Shi F, Wang L. Analgesic Activity of Ethanol Extracts from Root of Daphne genkwa. Chin. Tradit. Herbal Drugs. 2006;37:398–402. [Google Scholar]
- 5.Park BY, Min BS, Ahn KS, Kwon OK, Joung H, Bae KH, Lee HK, Oh SR. Daphnane Diterpene Esters Isolated from Flower Buds of Daphne genkwa Induce Apoptosis in Human Myelocytic HL-60 Cells and Suppress Tumor Growth in Lewis Lung Carcinoma (LLC)-inoculated Mouse Model. J. Ethnopharmacol. 2007;111:496–503. doi: 10.1016/j.jep.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Li LZ, Song SJ, Gao PY. Research Progress in the Chemical Constituents and Pharmacological Activities of Daphne genkwa Sieb. et Zucc. J. Shenyang Pharm. Univ. 2007;24:587–592. [Google Scholar]
- 7.Park BY, Min BS, Oh SR, Kim JH, Bae KH, Lee HK. Isolation of Flavonoids, a Biscoumarin and an Amide from the Flower Buds of Daphne genkwa and the Evaluation of their Anti-complement Activity. Phytother. Res. 2006;20:610–613. doi: 10.1002/ptr.1915. [DOI] [PubMed] [Google Scholar]
- 8.Li LZ, Gao PY, Li FF, Huang XX, Peng Y, Song SJ. Isolation and Identification of Chemical Constituents of Buds of Daphne genkwa Sieb. et Zucc. J. Shenyang Pharm. Univ. 2010;27:699–703. [Google Scholar]
- 9.Wang CF, Li RR, Huang LL, Zhong LQ, Yuan ST. Studies on Chemical Constituents of Daphne genkwa. J. Chin. Med. Mater. 2009;32:508–511. [PubMed] [Google Scholar]
- 10.Carlo GD, Mascolo N, Izzo AA, Capasso F. Flavonoids: Old and New Aspects of a Class of Natural Therapeutic Drugs. Life Sci. 1999;65:337–353. doi: 10.1016/s0024-3205(99)00120-4. [DOI] [PubMed] [Google Scholar]
- 11.Martini ND, Katerere DRP, Eloff JN. Biological Activity of Five Antibacterial Flavonoids from Combretum Erythrophyllum (Combretaceae). J. Ethnopharmcol. 2004;93:207–212. doi: 10.1016/j.jep.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 12.Jeong HJ, Shin YG, Kim IH, Pezzuto JM. Inhibition of Aromatase Activity by Flavonoids. Arch. Pharm. Res. 1999;22:309–312. doi: 10.1007/BF02976369. [DOI] [PubMed] [Google Scholar]
- 13.Yuan ST, Zhang BX, Wang ZJ, Xia K. HPLC Analysis on Influence of Genkwanin in Flos Genkwa. China J. Chin. Mater. Med. 1996;21:728–729. [PubMed] [Google Scholar]
- 14.Lei PL, Li RR, Huang LL, Yuan ST. Study on Quality Specifications of Flos Genkwa. Chin. J. Pharm. Anal. 2008;28:834–837. [Google Scholar]
- 15.China Pharmacopoeia Committee . Pharmacopoeia of the People's Republic of China. Beijing, China: 2010. p. 148. [Google Scholar]
- 16.Cuvelier ME, Richard H, Berset C. Antioxidative Activity and Phenolic Composition of Pilot-Plant and Commercial Extracts of Sage and Rosemary. J. Am. Oil Chem. Soc. 1996;73:645–652. [Google Scholar]
- 17.Androutsopoulos VP, Ruparelia K, Arroo RRJ, Tsatsakis AM, Spandidos DA. CYP1-mediated Antiproliferative Activity of Dietary Flavonoids in MDA-MB-468 Breast Cancer Cells. Toxicology. 2009;264:162–170. doi: 10.1016/j.tox.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Isaev IM, Agzamova MA, Isaev MI. Genkwanin and Iridoid Glycoside from Leonurus turkestanicus. Chem. Nat. Compd. 2011;47:132–134. [Google Scholar]
- 19.O'Rourke C, Byres M, Delazar A, Kumarasamy Y, Nahar L, Stewart F, Sarker SD. Hirsutanonol, Oregonin and Genkwanin from the Seeds of Alnus Glutinosa (Betulaceae). Biochem. Syst. Ecol. 2005;33:749–752. [Google Scholar]
- 20.Deiana M, Rosa A, Casu V, Cottiglia F, Bonsignore L, Dessi MA. Chemical Composition and Antioxidant Activity of Extracts from Daphne gnidium I. J. Am. Oil Chem. Soc. 2003;80:65–70. [Google Scholar]
- 21.Li YN, Yin LH, Xu LN, Peng JY. A Simple and Efficient Protocol for Large-scale Preparation of Three Flavonoids from the Flower of Daphne Genkwa by Combination of Macroporous Resin and Counter-Current Chromatography. J. Sep. Sci. 2010;33:2168–2175. doi: 10.1002/jssc.201000054. [DOI] [PubMed] [Google Scholar]
- 22.Uckoo RM, Jayaprakasha GK, Patil BS. Rapid Separation Method of Polymethoxyflavones from Citrus using Flash Chromatography. J. Sep. Sci. 2010;33:2168–2175. [Google Scholar]
- 23.Zeng YM, Xiao J, Li X, Wang JH. Isolation and Identification of Flavonoids from Buds of Daphne Genkwa Sieb. et Zucc Processed by Rice Vinegar. J. Shenyang Pharm. Univ. 2009;26:353–356. [Google Scholar]