We analyzed gene expression profiles of an unprecedented number of 137 IBC samples and identified a 107-gene signature associated with pathological complete response to neoadjuvant chemotherapy. This signature was biologically relevant and validated in three independent sets including two IBC sets and one non-IBC set, with independent significant predictive value in both validation sets. No robust signature related to metastasis-free survival was found.
Keywords: gene expression, inflammatory breast cancer, profiling, prognosis, response to chemotherapy
Abstract
Background
Inflammatory breast cancer (IBC) is an aggressive disease. To date, no molecular feature reliably predicts either the response to chemotherapy (CT) or the survival. Using DNA microarrays, we searched for multigene predictors.
Patients and methods
The World IBC Consortium generated whole-genome expression profiles of 137 IBC and 252 non-IBC (nIBC) samples. We searched for transcriptional profiles associated with pathological complete response (pCR) to neoadjuvant anthracycline-based CT and distant metastasis-free survival (DMFS) in respective subsets of 87 and 106 informative IBC samples. Correlations were investigated with predictive and prognostic gene expression signatures published in nIBC (nIBC-GES). Supervised analyses tested genes and activation signatures of 19 biological pathways and 234 transcription factors.
Results
Three of five tested prognostic nIBC-GES and the two tested predictive nIBC-GES discriminated between IBC with and without pCR, as well as two interferon activation signatures. We identified a 107-gene signature enriched for immunity-related genes that distinguished between responders and nonresponders in IBC. Its robustness was demonstrated by external validation in three independent sets including two IBC sets and one nIBC set, with independent significant predictive value in IBC and nIBC validation sets in multivariate analysis. We found no robust signature associated with DMFS in patients with IBC, and neither of the tested prognostic GES, nor the molecular subtypes were informative, whereas they were in our nIBC series (220 stage I–III informative samples).
Conclusion
Despite the relatively small sample size, we show that response to neoadjuvant CT in IBC is, as in nIBC, associated with immunity-related processes, suggesting that similar mechanisms responsible for pCR exist. Analysis of a larger IBC series is warranted regarding the correlation of gene expression profiles and DMFS.
introduction
Inflammatory breast cancer (IBC) is rare (5% of BC) but represents the most aggressive form of disease [1]. Despite multidisciplinary treatment, 5-year overall survival is 40%. The rate of pathological complete response (pCR) after neoadjuvant anthracycline-based chemotherapy (CT) ranges from 15% to 40%. Prognostic features are controversial. No factor reliably predicts either the response to CT or survival.
Because IBC is rare and the pretreatment biopsy samples are small, past molecular studies have not been conclusive [2]. No specific molecular therapeutic target has been identified to date. A few years ago, genome-wide approaches, essentially gene expression profiling, were applied to clinical samples. Most studies showed that a molecular signature of IBC can be established [3–10], but they suffered from limitations [11], including a nonuniform definition of IBC and small series. Two issues—prognosis and prediction of response to primary CT—were addressed only by two groups [5, 6] but in small series and without any independent validation. In contrast with non-IBC (nIBC), no robust molecular signature predictive of therapeutic response or survival has been identified for IBC.
Here, we report the results of the second project spearheaded by the World IBC Consortium, which aims at identifying molecular determinants of survival and response to CT in IBC. We generated and pooled Affymetrix expression profiles of 389 BC samples including an unprecedented number of 137 IBC samples combined with 252 nIBC samples [12]. We searched for gene expression signatures (GES) associated with pCR to neoadjuvant CT and distant metastasis-free survival (DMFS) in IBC.
materials and methods
patients and samples
Tumor samples were obtained from patients treated for invasive breast adenocarcinoma at the Institut Paoli-Calmettes (IPC, Marseille), the MD Anderson Cancer Center (MDACC, Houston), or the General Hospital Sint-Augustinus (TCRU, Antwerp). All samples were taken before any systemic treatment: diagnostic biopsy (IBC and advanced-stage nIBC), and operative specimen (early-stage nIBC). Other selection criteria included available histoclinical annotations, notably the TNM classification, and available good-quality extracted tumor RNA. All IBC samples were from consecutively treated patients. The definition of IBC strictly adhered to the international consensus diagnostic criteria [13]: rapid onset (<6 months) of breast erythema, edema, and/or peau d'orange, and/or warm breast, with or without an underlying palpable mass. Additional information is available in the supplementary File, available at Annals of Oncology online.
IBC patients were treated with primary anthracycline-based polychemotherapy often including sequential taxane, and coupled with trastuzumab in more than 50% of cases with ERBB2 amplification. CT was followed by surgery (mastectomy and axiilary lymph node dissection) for clinically nonprogressive and consenting patients, then radiotherapy. Surgical specimens were examined to determine the pathological response to CT, which was scored on both the primary tumor and the lymph nodes using Chevallier grading [14]. Grades 1 and 2 were considered as pCR, and grades 3 and 4 as residual disease (RD). From the 137 IBC samples, 87 were available for pCR analysis (Table 1). After radiotherapy, adjuvant hormone therapy was given to patients with ER-positive IBC, as well as adjuvant trastuzumab in cases with ERBB2 amplification. A total of 106 patients with nonmetastatic IBC were assessable for DMFS analysis, as well as 220 patients with nonmetastatic nIBC; these two groups showed histoclinical features coherent with literature (supplementary Table S2, available at Annals of Oncology online).
Table 1.
Histoclinical data of the 87 IBC samples included in the pCR analysis
| Characteristic | IBC (N = 87) |
|---|---|
| Median age, years (range) | 49 (24–82) |
| AJCC stage | |
| I | 0 (0%) |
| II | 0 (0%) |
| III | 76 (87%) |
| IV | 11 (13%) |
| Histological type | |
| Ductal | 75 (88%) |
| Lobular | 6 (7%) |
| Other | 4 (5%) |
| ESR1 status (mRNA level) | |
| Negative | 45 (52%) |
| Positive | 42 (48%) |
| ERBB2 status (mRNA level) | |
| Negative | 61 (70%) |
| Positive | 26 (30%) |
| Genomic grade index | |
| High | 59 (68%) |
| Low | 28 (32%) |
| Molecular subtypes | |
| Basal-like | 19 (22%) |
| Claudin-low | 20 (23%) |
| ERBB2-enriched | 18 (21%) |
| Luminal A | 14 (16%) |
| Luminal B | 10 (11%) |
| Normal-like | 6 (7%) |
| Neoadjuvant systemic therapy | |
| Anthracycline-based CT | 36 (41%) |
| Anthracycline–taxane-based CT | 51 (59%) |
| Hormone therapy | 0 (0%) |
| Trastuzumab | 16 (18%) |
| Pathological complete response | |
| No | 59 (68%) |
| Yes | 28 (32%) |
CT, chemotherapy.
expression data analysis
RNA hybridization of 389 samples on to Affymetrix GeneChips (HGU133-series) has been described [12]. More information on data processing, normalization, and analyses is available in the supplementary File, available at Annals of Oncology online. Each of the 389 samples was classified according to GES identified in nIBC patients: the molecular subtypes defined using the PAM50 predictor [15], the nine-cell line claudin-low predictor [16], and seven GES: five prognostic for DMFS (Recurrence Score [17], 70-gene GES [18], Wound-response GES [19], Invasiveness GES [20], and Risk of Relapse score GES based on subtype and proliferation ROR-P [15,21]), and two predictive for pathological response to anthracycline/taxane-based CT (stromal GES [22] and FAC/T response GES [23]).
Supervised analysis addressed two comparisons of IBC samples: pathological response and metastatic relapse. For both analyses, we divided our dataset into learning and validation sets. P-values, corrected for multiple comparisons, were considered significant only if the false discovery rate (FDR) was smaller than 0.25, unless explicitly stated. Regarding the response analysis, our classifier was built in the learning set and its robustness was assessed in IBC by leave-one-out cross-validation in the learning set and by external validation in the independent validation set (43 samples). Then, we tested its predictive performances in a publicly available pooled set of 675 nonredundant nIBC samples defined as non-T4d and treated with neoadjuvant anthracycline-based CT followed by surgery (supplementary Table S3, available at Annals of Oncology online). To further explore functional differences, those two supervised analyses (pathological response, metastatic relapse) were repeated at the pathway and transcription factor levels (supplementary File, available at Annals of Oncology online, supplementary Table S4, available at Annals of Oncology online).
statistical analysis
Analyses are described in the supplementary File, available at Annals of Oncology online.
results
pathological complete response to primary chemotherapy in IBC and correlations with histoclinical and molecular variables
A total of 87 IBC patients from our series (IPC: 44; MDACC: 21; TCRU: 22) treated with neoadjuvant anthracycline-based CT were eligible for analysis: 59 patients had RD (no pCR) and 28 (32%) had a pCR. We compared the pCR and RD groups with respect to histoclinical features, molecular subtypes, and several GES (Table 2).
Table 2.
Histoclinical and molecular correlations with pCR to chemotherapy in IBC
| Characteristics | N | IBC |
|||
|---|---|---|---|---|---|
| RD (N = 59) | pCR (N = 28) | P-value* | |||
| Age, years (range) | 85 | 50.5 (27–82) | 46 (24–71) | 0.18 | |
| Histological type | 0.561 | ||||
| Ductal | 75 | 51 (68%) | 24 (32%) | ||
| Lobular | 6 | 5 (83%) | 1 (17%) | ||
| Other | 4 | 2 (50%) | 2 (50%) | ||
| ESR1 status (mRNA level) | 0.262 | ||||
| Negative | 45 | 28 (62%) | 17 (38%) | ||
| Positive | 42 | 31 (74%) | 11 (26%) | ||
| ERBB2 status (mRNA level) | 1 | ||||
| Negative | 61 | 41 (67%) | 20 (33%) | ||
| Positive | 26 | 18 (69%) | 8 (31%) | ||
| Genomic grade index | 0.462 | ||||
| High | 59 | 38 (64%) | 21 (36%) | ||
| Low | 28 | 21 (75%) | 7 (25%) | ||
| Molecular subtypes | 0.0725 | ||||
| Basal | 19 | 12 (63%) | 7 (37%) | ||
| Claudin-low | 20 | 11 (55%) | 9 (45%) | ||
| ERBB2 | 18 | 10 (56%) | 8 (44%) | ||
| Luminal A | 14 | 13 (93%) | 1 (7%) | ||
| Luminal B | 10 | 7 (70%) | 3 (30%) | ||
| Normal-like | 6 | 6 (100%) | 0 (0%) | ||
| Recurrence score | 0.0295 | ||||
| Low | 16 | 15 (94%) | 1 (6%) | ||
| Intermediate | 16 | 11 (69%) | 5 (31%) | ||
| High | 55 | 33 (60%) | 22 (40%) | ||
| 70-gene GES | 0.0457 | ||||
| Good | 18 | 16 (89%) | 2 (11%) | ||
| Poor | 69 | 43 (62%) | 26 (38%) | ||
| Invasiveness GES | 0.00526 | ||||
| Good | 38 | 32 (84%) | 6 (16%) | ||
| Poor | 49 | 27 (55%) | 22 (45%) | ||
| Wound-response GES | 0.0848 | ||||
| Good | 27 | 22 (81%) | 5 (19%) | ||
| Poor | 60 | 37 (62%) | 23 (38%) | ||
| Risk of Relapse score GES (ROR-P) | 0.175 | ||||
| Low | 29 | 23 (79%) | 6 (21%) | ||
| Median | 12 | 9 (75%) | 3 (25%) | ||
| High | 46 | 27 (59%) | 19 (41%) | ||
| FAC/T-response GES | 0.0409 | ||||
| RD | 39 | 31 (79%) | 8 (21%) | ||
| pCR | 48 | 28 (58%) | 20 (42%) | ||
| Stromal GES | 0.0108 | ||||
| RD | 49 | 39 (80%) | 10 (20%) | ||
| pCR | 38 | 20 (53%) | 18 (47%) | ||
| AKT activity pathwaya | 87 | 0.04 | −0.13 | 0.498 | |
| Catenin activity pathwaya | 87 | 0.14 | 0.5 | 0.0606 | |
| E2F1 activity pathwaya | 87 | −0.2 | 0.11 | 0.262 | |
| EGFR activity pathwaya | 87 | 0.11 | −0.42 | 0.0151 | |
| ER activity pathwaya | 87 | 1.04 | 0.66 | 0.254 | |
| HER2 activity pathwaya | 87 | 0.23 | 0.17 | 0.522 | |
| MYC activity pathwaya | 87 | 0.18 | 0.62 | 0.0742 | |
| P53 activity pathwaya | 87 | −0.46 | −0.8 | 0.0159 | |
| PI3K activity pathwaya | 87 | 0.01 | 0.04 | 0.867 | |
| PR activity pathwaya | 87 | −0.55 | −2.56 | 0.218 | |
| RAS activity pathwaya | 87 | 0.13 | 0.39 | 0.208 | |
| SRC activity pathwaya | 87 | −0.02 | 0.16 | 0.817 | |
| STAT3 activity pathwaya | 87 | 0.36 | −0.07 | 0.0582 | |
| TGFb activity pathwaya | 87 | −0.11 | −0.64 | 0.0044 | |
| TNFa activity pathwaya | 87 | 0.11 | 0.9 | 0.0658 | |
| IFNa activity pathwaya | 87 | −0.01 | 0.81 | 0.0159 | |
| IFNg activity pathwaya | 87 | −0.05 | 1.03 | 0.00186 | |
| P63 activity pathwaya | 87 | 0.3 | 0.31 | 0.199 | |
| VEGF activity pathwaya | 87 | 0.13 | 0.35 | 0.673 | |
aMean activation scores.
*Fisher's exact test.
Higher pCR rate was observed in ESR1-negative samples, in GGI-high samples, in non-luminal A and non-normal-like samples, in Wound-response GES-based poor-prognosis samples and in ROR-P high-risk samples; however, differences were not significant, likely due to the series size. For example the OR for pCR was 8.6 between non-normal-like samples and luminal A samples. Significant differences were observed for three prognostic GES, with more patients with pCR in the poor-prognosis groups (Recurrence Score, P = 0.029; 70-gene GES, P = 0.045; Invasiveness GES, P = 5.3E−03). The nIBC pCR-GES carried out well in IBC to predict the response: stromal GES (P = 0.010) and FAC/T response GES (P = 0.040).
We identified two immune pathways (IFNα- and INFγ-pathways) as hyperactivated in samples from patients with pCR (P = 0.015 and P = 0.001 respectively, Mann–Whitney test), as well as three pathways (EGFR, P53, TGBβ) as hypoactivated in patients with pCR (P = 0.015, P = 0.015, P = 0.004, respectively). TF analysis revealed three transcription factors (STAT5: P = 3.6E-05, GLI: P = 8.7E−04, SMAD: P = 9.6E−04, Mann–Whitney test) as hypoactivated in samples from patients with pCR after correction with FDR inferior to 20% (data not shown).
signature predictive for pCR to primary chemotherapy in IBC
The 87-sample population was divided into a learning set (N = 44) and a validation set (N = 43), which displayed similar pCR rates (36% and 28%, respectively, P = 0.5, Fisher's exact test).
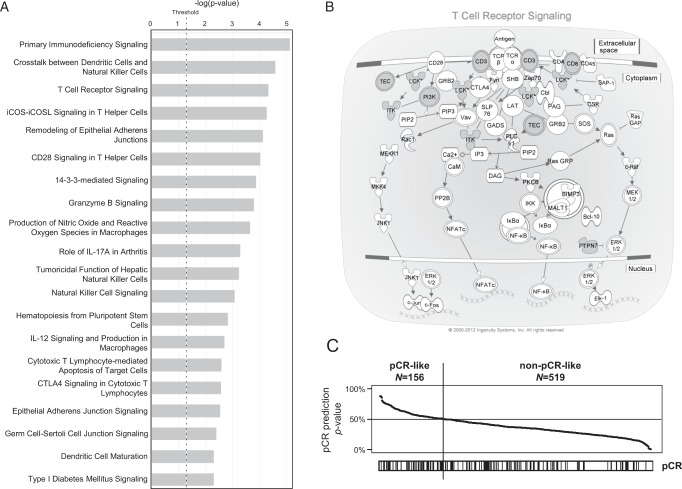
In the learning set, SAM (FDR inferior to 25%) identified 124 differentially expressed probe sets (107 unique genes), between responders and nonresponders, all of which were overexpressed in samples with pCR (supplementary Table S5, available at Annals of Oncology online). Several processes related to T-cell-dependent immunology were strongly overrepresented in this gene list (Figure 1A; supplementary Table S6, available at Annals of Oncology online), including the ‘T-cell receptor signaling’ canonical pathway (Figure 1B). We derived a predictive SVM model from the 124-probe set list in the learning set. Its validity was internally tested by LOOCV (75% of samples correctly assigned), and by external validation in the independent set of 43 samples, in which the rate of accurate classification was 81% with an odds ratio for pCR equal to 14.2 in the ‘predicted-pCR’ subgroup compared with the ‘predicted-RD’ subgroup (P = 5E−04, Fisher's exact test). We further tested our model in an additional validation set including 21 pretreatment IBC samples (supplementary Table S7, available at Annals of Oncology online): the accuracy of classification was 95% and the odds ratio for pCR was equal to infinite (P = 0.014). Combining the two validation sets (N = 64) gave a classification accuracy of 86% and an odds ratio of 24.6 (Table 3; P = 4.34E−06). We then compared the predictive value of our model in this combined validation set (N = 64) with that of other pretreatment variables significant in univariate analysis (prognostic and predictive GES, IFNα, INFγ, EGFR, P53, and TGBβ activation profiles) using multivariate analysis (Table 4). Our signature remained the strongest predictive variable with an odds ratio equal to 50.8 and showed significance despite the relatively small number of samples (P = 0.0096), suggesting additional predictive value beyond all other signatures.
Figure 1.
Analysis of pathological response to primary chemotherapy in patients with IBC. (A) Ingenuity (IPA) canonical pathways associated with the 107-gene pCR signature. The barplot indicates the –log(P-value) (x-axis) of the top 20 biological pathways (y-axis) that are enriched for differentially expressed genes between samples from patients with and without pCR. The P-value threshold is indicated by the dashed black vertical line. (B) IPA representation of the ‘T-cell receptor signaling’ pathway. Six genes (gray circles) are included in the signature (CD3, CD8, ITK, LCK, PIK3CD, and PTPN7). (C) Application of the pCR-model on a gene expression series of 675 nIBC samples from patients with and without pCR: Top, probability (from 0% to 100%) for each sample to be predicted as pCR by the SVM model based on the signature. The black vertical line indicates the threshold of 50% (equiprobability according to SVM model) that separates the two model-predicted classes of samples. Bottom: observed pCR status (black, pCR; white no pCR).
Table 3.
Performance of the pCR signature in the pooled IBC validation set
| Pathological response | Observed |
|
|---|---|---|
| Predicted | RD | pCR |
| RD | 44 | 3 |
| pCR | 6 | 11 |
Fisher's exact test, P = 4.34E−06 (OR = 24.65 [4.84–178.7]).
Accuracy = 86%; sensitivity for RD prediction = 88%; specificity = 78%.
Table 4.
Multivariate analysis for pCR in the pooled IBC validation set
| Variable |
Multivariate |
|||
|---|---|---|---|---|
| N | Odds ratio (95 CI) | P-value* | ||
| Recurrence score | Intermediate versus Low | 64 | 2.34 (0.08–104) | 0.687 |
| High versus Low | 0.62 (0.01–38.2) | 0.846 | ||
| 70-gene GES | Poor versus Good | 64 | Inf. (0–Inf.) | 0.995 |
| Invasiveness GES | Poor versus Good | 64 | 11.1 (0.8–3570) | 0.186 |
| FAC/T-response GES | pCR versus RD | 64 | 0.93 (0.13–7.44) | 0.949 |
| Stromal GES | pCR versus RD | 64 | 0.37 (0.02–2.86) | 0.469 |
| EGFR activity pathway | 64 | 2.11 (0.52–9.89) | 0.386 | |
| P53 activity pathway | 64 | 1.45 (0.45–5.09) | 0.608 | |
| TGFb activity pathway | 64 | 1.43 (0.48–4.79) | 0.601 | |
| IFNa activity pathway | 64 | 0.60 (0.07–5.24) | 0.697 | |
| IFNg activity pathway | 64 | 3.49 (0.42–36.0) | 0.346 | |
| pCR IBC signature | pCR versus RD | 64 | 50.8 (6.2–1117) | 9.58E-03 |
*Logistic regression.
We then applied the SVM modelon to gene expression data of 675 nIBC treated with neoadjuvant anthracycline-based CT. A significant correlation (P = 1.89E−03, Fisher's exact test) was observed between the predicted and observed pCR phenotypes, with an odds ratio for pCR equal to 1.94 in the ‘predicted-pCR’ subgroup compared with the ‘predicted-RD’ subgroup (Figure 1C). Interestingly, when we incorporated into multivariate analysis the variables significant in univariate analysis (molecular subtypes, ESR1, ERBB2, GGI, and FAC/T-response GES) and our SVM model, the latter remained significant (supplementary Table S8, available at Annals of Oncology online), suggesting an independent predictive value.
survival analysis in IBC
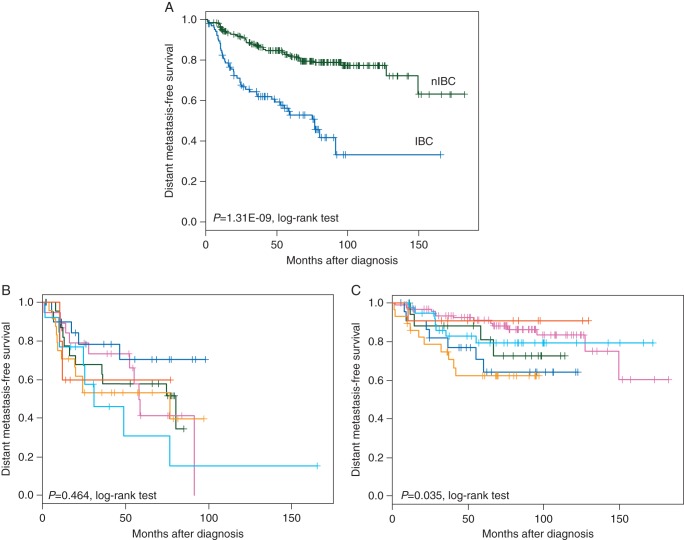
Follow-up data of patients with non-metastatic disease were available for 106 IBC and 220 nIBC patients with respective 5-year DMFS equal to 53% and 82% (Figure 2A). Univariate analysis tested each variable listed in supplementary Table S2, available at Annals of Oncology online for its association with DMFS (supplementary Table S9, available at Annals of Oncology online): none of them was associated with DMFS in IBC. Similarly, no TF activation profile was associated with DMFS (data not shown). Patients with luminal A/B subtypes showed poor DMFS, but the difference with the other subtypes was not significant (Figure 2B). By contrast, in our series of nIBC samples and after adjustment for the delivery of systemic adjuvant or neoadjuvant CT, several variables were significant in univariate analysis (Figure 2C; supplementary Table S9, available at Annals of Oncology online). As expected, patients with luminal A nIBC showed better outcome than patients with basal-like or ERBB2-enriched nIBC.
Figure 2.
Distant metastasis-free survival (DMFS) analysis. (A) Kaplan–Meier DMFS curves in IBC versus nIBC. The difference between IBC and nIBC is highly significant. (B and C) DMFS curves according to the molecular subtypes in IBC (B) and nIBC (C). The basal-like, claudin-low, ERBB2-enriched, luminal A, luminal B, and normal-like subtypes are indicated in red, orange, pink, blue, light blue, and green, respectively.
Finally, we searched for a GES associated with DMFS in our 106 IBC samples. In the learning set (79 samples), Cox regression analysis did not identify any significant probe set. We thus pooled the learning and validation sets and repeated the Cox analysis. The same result was observed, suggesting that no robust prognostic signature could be established.
discussion
This study was conducted thanks to collaborations through the World IBC Consortium. First, supervised analysis identified a 107-gene signature associated with pathological response to neoadjuvant CT in IBC. Despite a FDR with relatively low stringency, the signature was very robust, as demonstrated by both internal and external validation in IBC samples, biological relevance, and validation in a large nIBC series. Very few data are available in the literature regarding the pathological response to CT and the identification of predictive factors in IBC specifically. Our data suggest that similar molecular mechanisms may be involved in IBC and nIBC. First, the two prognostic nIBC-GES previously shown to be associated with pCR [24,25] and the two predictive nIBC-GES [22,23] effectively discriminated between IBC patients with versus without pCR. Although not significant, likely due to the relatively small series size, the ESR1 status, GGI and the molecular subtypes were associated with pathological response as described in nIBC [16]. The pCR rate was 7% for the luminal A subtype and from 30% to 45% for the other subtypes, in agreement with the nIBC literature, except for the luminal B subtype that displayed high pCR rate (30%). Second, our signature was also significantly predictive for pCR in nIBC. Third, as in nIBC [26], the response to CT seems enhanced by a pre-existing immune response. Our signature was strongly enriched for immunity-related genes involved in CD8+ T-cell lymphocyte activation processes (Th1-response), suggesting a prominent role for adaptive immunity in determining response to CT in IBC. Innate immunity was also represented by the presence of ontologies such as ‘Crosstalk between dendritic cells and natural killer cells’, and ‘communication between innate and adaptive immune cells’. Fourth, in multivariate analysis, both in the IBC validation set and in nIBC, our predictor showed predictive value independent from that of multigene GES, with a strong odds ratio (55.4) in IBC and significance despite the small series size. Of note, our predictor carried out well in the 22 ERBB2-positive IBC samples of the validation set independently of trastuzumab treatment (data not shown). In the future, our signature could have clinical implications. First, better predicting the pathological response to primary anthracycline-based CT could help tailoring systemic treatment: patients of the ‘predicted-RD group’ might be enrolled in clinical trials testing nonanthracycline-based regimens. Second, the functional validation of relevant genes, such as those involved in immunity, could lead to the development of new therapies combined or not with CT.
Second, we analyzed the correlation of expression profiles with DMFS in IBC and compared with that observed in nIBC. Although not significant likely due to the limited sample size (76 informative cases), the pathological response was the histoclinical factor displaying the largest hazard ratio (HR = 0.56; P = 0.249). We acknowledge that our analyses in IBC are underpowered because of the size limitation. Even if it seems that differences exist between IBC and nIBC regarding the metastatic phenotype and the associated transcriptional changes, no definitive conclusion may be drawn for IBC and further analysis in larger series is warranted. The difference in DMFS of subtypes in IBC and nIBC, particularly the luminal A/B subtypes, which is not due to the shorter follow-up in IBC when compared with nIBC, needs confirmation. It likely explains in part the absence of prognostic value of ESR1 expression, GGI, molecular subtypes, and tested prognostic GES in IBC when compared with nIBC. If confirmed, a speculative explanation might be the fact that, within ER-negative subtypes (i.e. basal-like and ERBB2-enriched), IBC and nIBC tumors benefit similarly from CT, whereas within the luminal A/B ER-positive subtypes, IBC tumors benefit from endocrine therapy much less than nIBC. Finally, we could not identify any robust prognostic GES in IBCs, suggesting the need for analysis of a larger series of samples to define IBC-specific prognostic signatures.
Our study presents several strengths: originality (the first multicentric study of this type in IBC, the largest series profiled so far using high-throughput molecular analyses, the first one addressing clinical issues), uniform consensual IBC definition, consistent technological platform, and identification of a response signature biologically relevant, validated in three independent sets including two IBC sets and one nIBC set, with independent significant predictive value in IBC and nIBC validation sets. The first limitation concerns the relatively small number of IBC samples, inherent to IBC research. This limitation may explain the relatively high pCR rate observed for luminal B IBC samples when compared with the public 675 nIBC series (18%). Further comparison of pCR rates in larger series of IBC and nIBC is warranted before eliminating the hypothesis that IBCs respond better to CT. The second limitation is the retrospective nature of the study. Efforts are ongoing to gather more samples inside the Word IBC Consortium.
In conclusion, our results provide novel insights into the molecular mechanisms of response to neoadjuvant CT. Response to CT in IBC, as in nIBC, is similarly associated with immunity-related processes. Regarding DMFS, analysis of larger IBC series is warranted before concluding that different mechanisms for metastasis exist in IBC and in nIBC.
funding
This work is supported in part by the Institut National du Cancer (INCa) Translational Research grant 2007 (FB) and Translational Research Grant 2009 (DB), the Ligue Nationale Contre le Cancer (DB), Roche (PV), the National Institute of Health R01CA138239-01 and R01 CA138239 ARRA supplement (WAW, JMR); a State of Texas Grant for Rare and Aggressive Cancers (NU, JMR, AL, WAW, SK, FMR); the American Airlines Komen Foundation Promise grant KGO81287 (WAW, JMR, AL), and Komen Foundation Grant KG101478 (WAW).
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Robertson FM, Bondy M, Yang W, et al. Inflammatory breast cancer: the disease, the biology, the treatment. CA Cancer J Clin. 2010;60:351–375. doi: 10.3322/caac.20082. doi:10.3322/caac.20082. [DOI] [PubMed] [Google Scholar]
- 2.Charafe-Jauffret E, Tarpin C, Viens P, et al. Defining the molecular biology of inflammatory breast cancer. Semin Oncol. 2008;35:41–50. doi: 10.1053/j.seminoncol.2007.11.015. doi:10.1053/j.seminoncol.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Bekhouche I, Finetti P, Adelaide J, et al. High-resolution comparative genomic hybridization of inflammatory breast cancer and identification of candidate genes. PLoS One. 2011;6:e16950. doi: 10.1371/journal.pone.0016950. doi:10.1371/journal.pone.0016950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertucci F, Finetti P, Rougemont J, et al. Gene expression profiling for molecular characterization of inflammatory breast cancer and prediction of response to chemotherapy. Cancer Res. 2004;64:8558–8565. doi: 10.1158/0008-5472.CAN-04-2696. doi:10.1158/0008-5472.CAN-04-2696. [DOI] [PubMed] [Google Scholar]
- 5.Bertucci F, Finetti P, Rougemont J, et al. Gene expression profiling identifies molecular subtypes of inflammatory breast cancer. Cancer Res. 2005;65:2170–2178. doi: 10.1158/0008-5472.CAN-04-4115. doi:10.1158/0008-5472.CAN-04-4115. [DOI] [PubMed] [Google Scholar]
- 6.Bieche I, Lerebours F, Tozlu S, et al. Molecular profiling of inflammatory breast cancer: identification of a poor-prognosis gene expression signature. Clin Cancer Res. 2004;10:6789–6795. doi: 10.1158/1078-0432.CCR-04-0306. doi:10.1158/1078-0432.CCR-04-0306. [DOI] [PubMed] [Google Scholar]
- 7.Boersma BJ, Reimers M, Yi M, et al. A stromal gene signature associated with inflammatory breast cancer. Int J Cancer. 2008;122:1324–1332. doi: 10.1002/ijc.23237. doi:10.1002/ijc.23237. [DOI] [PubMed] [Google Scholar]
- 8.Dressman HK, Hans C, Bild A, et al. Gene expression profiles of multiple breast cancer phenotypes and response to neoadjuvant chemotherapy. Clin Cancer Res. 2006;12:819–826. doi: 10.1158/1078-0432.CCR-05-1447. doi:10.1158/1078-0432.CCR-05-1447. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen DM, Sam K, Tsimelzon A, et al. Molecular heterogeneity of inflammatory breast cancer: a hyperproliferative phenotype. Clin Cancer Res. 2006;12:5047–5054. doi: 10.1158/1078-0432.CCR-05-2248. doi:10.1158/1078-0432.CCR-05-2248. [DOI] [PubMed] [Google Scholar]
- 10.Van Laere S, Van der Auwera I, Van den Eynden GG, et al. Distinct molecular signature of inflammatory breast cancer by cDNA microarray analysis. Breast Cancer Res Treat. 2005;93:237–246. doi: 10.1007/s10549-005-5157-z. doi:10.1007/s10549-005-5157-z. [DOI] [PubMed] [Google Scholar]
- 11.Bertucci F, Finetti P, Birnbaum D, et al. Gene expression profiling of inflammatory breast cancer. Cancer. 2010;116:2783–2793. doi: 10.1002/cncr.25165. doi:10.1002/cncr.25165. [DOI] [PubMed] [Google Scholar]
- 12.Van Laere S, Ueno NT, Finetti P, et al. Uncovering the molecular secrets of inflammatory breast cancer biology: an integrated analysis of three distinct affymetrix gene expression data sets. Clin Cancer Res. 2013;19:4685–4696. doi: 10.1158/1078-0432.CCR-12-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawood S, Merajver SD, Viens P, et al. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol. 2011;22:515–523. doi: 10.1093/annonc/mdq345. doi:10.1093/annonc/mdq345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chevallier B, Chollet P, Merrouche Y, et al. Lenograstim prevents morbidity from intensive induction chemotherapy in the treatment of inflammatory breast cancer. J Clin Oncol. 1995;13:1564–1571. doi: 10.1200/JCO.1995.13.7.1564. [DOI] [PubMed] [Google Scholar]
- 15.Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. doi:10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prat A, Parker JS, Karginova O, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. doi:10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. doi:10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 18.van de Vijver MJ, He YD, van't Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. doi:10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 19.Chang HY, Sneddon JB, Alizadeh AA, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. doi:10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu R, Wang X, Chen GY, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356:217–226. doi: 10.1056/NEJMoa063994. doi:10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen TO, Parker JS, Leung S, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16:5222–5232. doi: 10.1158/1078-0432.CCR-10-1282. doi:10.1158/1078-0432.CCR-10-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farmer P, Bonnefoi H, Anderle P, et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med. 2009;15:68–74. doi: 10.1038/nm.1908. doi:10.1038/nm.1908. [DOI] [PubMed] [Google Scholar]
- 23.Hess KR, Anderson K, Symmans WF, et al. Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide in breast cancer. J Clin Oncol. 2006;24:4236–4244. doi: 10.1200/JCO.2006.05.6861. doi:10.1200/JCO.2006.05.6861. [DOI] [PubMed] [Google Scholar]
- 24.Gianni L, Zambetti M, Clark K, et al. Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J Clin Oncol. 2005;23:7265–7277. doi: 10.1200/JCO.2005.02.0818. doi:10.1200/JCO.2005.02.0818. [DOI] [PubMed] [Google Scholar]
- 25.Straver ME, Glas AM, Hannemann J, et al. The 70-gene signature as a response predictor for neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat. 2010;119:551–558. doi: 10.1007/s10549-009-0333-1. doi:10.1007/s10549-009-0333-1. [DOI] [PubMed] [Google Scholar]
- 26.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. doi:10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.