Abstract
Heat shock proteins (HSP) are a subset of the molecular chaperones, best known for their rapid and abundant induction by stress. HSP genes are activated at the transcriptional level by heat shock transcription factor 1 (HSF1). During the progression of many types of cancer, this heat shock transcriptional regulon becomes co-opted by mechanisms that are currently unclear, although evidently triggered in the emerging tumor cell. Concerted activation of HSF1 and the accumulation of HSPs then participates in many of the traits that permit the malignant phenotype. Thus cancers of many histologies exhibit activated HSF1 and increased HSP levels that may help to deter tumor suppression and evade therapy in the clinic. We review here the extensive work that has been carried out and is still in progress aimed at: (1) understanding the oncogenic mechanisms by which HSP genes are switched on, (2) determining the roles of HSF1 / HSP in malignant transformation and, (3) discovering approaches to therapy based on disrupting the influence of the HSF1 controlled transcriptome in cancer.
Keywords: Heat shock proteins, heat shock factor, cancer, carcinogenesis, drug resistance, apoptosis, metastasis, prognosis
Introduction
Heat shock protein (HSP) genes were discovered approximately sixty years ago in experiments on the fruit fly drosophila Melanogaster (Ritossa 1962; Ritossa 1964). They were discovered as genes that became activated on exposure of drosophila tissues to stresses such as heat shock and were subsequently found in all cellular organisms (Lindquist and Craig 1988). The primary functions of HSP genes then emerged, and it was found that HSPs belong to the molecular chaperone family of proteins (Ellis 2006). Molecular chaperones participate in the folding of proteins in normal metabolism and the induction of the HSP subset of chaperones in stress permits amplified levels of repair and refolding of damaged polypeptides (Craig 1985). The ability of cells to respond to stress by increasing their HSP levels depends on the activity of a unique transcription factor-heat shock factor 1 (HSF1) that can bind to the 5′promoter regions of all HSP genes and trigger instantaneous and massive transcription of these stress protein genes (Wu 1995; Calderwood et al. 2010). The mechanisms of activation of HSP genes are still under investigation but are known to involve stress-induced formation of a HSF1 homotrimer and a number of posttranslational modifications (PTM) that convert the factor into an active form that moves toward a nuclear localization and binds the promoter of HSP genes in a productive manner (Sorger and Pelham 1988; Westwood and Wu 1993). HSF1 is thought to be repressed by the molecular chaperone heat shock protein 90 (Hsp90) under growth conditions (Zou et al. 1998). Stress reverses the repression and permits HSF1 activation. There are five main HSP families that can be induced by stress, each encoding a structurally dissimilar group of HSPs. These include the HSPA (Hsp70), HSPB (small HSP family), HSPC (Hsp90), HSPD (Hsp60) and HSPH (large HSP) families (reviewed: (Kampinga et al. 2009)). Members of the HSPA, HSPB and HSPC families are thought to play key roles in cancer (Ciocca and Calderwood 2005; Calderwood et al. 2006).
(1) Induction of elevated HSP expression in cancer
The pathways of induction of HSPs in cancer are still under intense investigation and no clear consensus has yet emerged. Such mechanisms may include:
(a) Transcription and translation of HSPs due to coupling of HSF1 expression to malignant cell signal transduction
As mentioned the primary factor in HSP transcription is HSF1. Although the mechanisms for HSF1 regulation in stress are not fully understood key roles for a number of PTMs are thought to be essential. HSF1 is hyperphosphorylated in stressed cells and this phosphorylation pattern is thought to be essential in transcription (Sarge et al. 1993; Chu et al. 1996). HSF1 becomes dephosphorylated on serine 303 and phosphorylated on serine 326 when cells are subjected to pro-malignant signaling and activation in mammary cancer involves the receptor tyrosine kinases HER2 and HER3 and the cytoplasmic serine kinase phosphatidyl-inositol3 kinase (PI-3kinase) (Khaleque et al. 2005). An additional activation step is the phosphorylation of HSF1 on serine 320 by protein kinase A (PKA) permitting transcriptional elongation (Murshid et al. 2010; Zhang et al. 2011). Both the HER2>Pi-3 kinase and PKA signaling pathways are triggered in malignant cell signaling in mammary cancer (Ciocca and Calderwood 2005; Murshid et al. 2010). HSF1 activation is also accompanied by sumoylation (Hietakangas et al. 2003). Sumoylation is a PTM observed frequently in transcription factors that are associated with PML bodies, important sites of PTM in malignant cells. HSF1 has also been shown to be activated by deacetylation though the deacetylase sirtuin-1, a factor associated with cancer (Westerheide et al. 2009).
(b) Epigenetic mechanisms for HSP expression in cancer
Although activation of HSF1 in stress is an entirely posttranslational phenomenon, and HSF1 is neither produced nor consumed, activation in cancer is associated with increases in its levels (Santagata et al. 2011). The mechanisms behind this are not clear and may involve increased transcription and translation. Another possibility is epigenetic regulation. The HSF1 gene contains a number of CpG dinucleotides that could lead to its silencing under some conditions (Singh et al. 2009). In many cancers, CpG islands become demethylated during tumor progression and pro-oncogenic genes are “reawoken” as the epigenentic repression is overturned (Jones and Baylin 2002). Currently this hypothesis has not been tested for HSF1.
(c) Regulation of translation
Study of gene expression in cancer has shown that the coupling between transcription and translation is often interrupted. Indeed in a study of HSP gene expression in human prostate carcinoma, it was shown that HSP mRNA expression and protein expression varied considerably and that the levels of some HSPs were increased while HSP mRNA levels were not markedly altered (Tang et al. 2005). Recent studies have shown that translation of many genes in cancer is altered due to changes in microRNA (miRNA) levels that accompany malignant transformation and progression (Spizzo et al. 2009; Visone and Croce 2009). MiRNA expression is switched from a pattern that tends to oppose transformation towards a pro-malignant repertoire of miRNAs. While little is known of miRNA involved in HSP expression in cancer, recent studies have shown that miRNA-1 and miRNA-206 activate Hsp60 expression in cardiomyocytes and that miRNA-1, miRNA-21 and miRNA-24 activate HSF1 and Hsp70 levels in mouse cardiac tissues expose to ischemia (Yin et al. 2009; Shan et al. 2010). Future studies of miRNA in HSP regulation in cancer are anticipated.
(d) The “addiction to chaperones” hypothesis
A popular rationale for HSP increases in cancer is the “addiction to chaperones” hypothesis. According to this hypothesis, HSP increases are fueled in cancer through the proliferation of mutated proteins due to the “mutator phenotype ” associated with cancer, to increases in total levels of mRNA translation that accompany transformation and increased protein expression due to the polyploidy of many malignant cells (Fishel et al. 1993; Kamal et al. 2003; Dai et al. 2007; Trepel et al. 2010). Addiction is thus caused by the requirement for HSPs to chaperone the increased protein load that accompanies transformation and the inherent instability of many mutant proteins. As elevated protein expression and gene mutation are thought to be key drivers of tumor progression, an expanding supply of molecular chaperones is required to maintain the addiction. This is an attractive hypothesis as it couples the findings in oncology with what is suspected to be the driving force for HSP induction during stress. Although this mechanism is inherently difficult to test, there is considerable indirect evidence to suggest its merit, coming from studies of HSP inhibitors in cancer. An overwhelming amount of evidence obtained by testing inhibitors of Hsp90 in in vitro studies, in animal experiments and in clinical trial suggests that inhibiting Hsp90 using small molecule inhibitors causes the depletion of a wide spectrum of oncogenes (presumably due to unfolding and proteolysis) and inhibition of tumor growth (reviewed: (Workman et al. 2007; Trepel et al. 2010). This drug family includes two compounds in clinical trial: 17-AAG (or tanespimycin) and 17-DMAG (Chiosis and Tao 2006; Eccles et al. 2008). Although inhibitors of HSF1 and the other HSPs have not currently been tested in cancer, such an approach is anticipated (Powers et al. 2008; Whitesell and Lindquist 2009; Powers et al. 2011).
Each of these mechanisms may be involved in the amplification of the HSP response in cancer cells and tissues.
(2) The HSF1>HSP system as an engine of tumorogenesis
In a recent review, it has been suggested that there are eight key traits, described as “the hallmarks of cancer,” required for the emergence of the complete malignant phenotype (Hanahan and Weinberg 2011). HSF1 and HSPs have been implicated in seven of these hallmarks including; (1) maintenance of sustained proliferative signaling, (2) resisting cell death, (3) inhibition of replicative senescence, (4) induction of tumor angiogenesis, (5) activation of invasion and metastasis (6) reprogramming of energy metabolism and (7) evasion of immune destruction (Fig. 1). (For the eighth hallmark-evasion of growth suppressors, there is currently no conclusive information regarding the participation of HSPs). We will briefly summarize the results below:
Abundant evidence indicates that Hsp90 and co-chaperones such as Cdc37 are required to stabilize a wide range of oncogenic proteins and sustain cancer growth. Inhibiting Hsp90 leads to destruction of associated oncoproteins and reduces the growth rate of a wide range of cancers (Gray et al. 2008; Trepel et al. 2010). Similar results might be predicted for Hsp27 and Hsp70, whose levels have been shown to increase during tumorigenesis.
When increased in concentration, as observed the development of cancer, HSF1, Hsp70 and Hsp27 inhibit programmed cell death (Khaleque et al. 2005; Garrido et al. 2006; Dudeja et al. 2011). Indeed, high levels of Hsp27 and Hsp70 are thought to block programmed cell death by directly sequestering intermediates in the caspase-dependent apoptosis pathway (Gabai and Sherman 2005; Garrido et al. 2006). The elevation in Hsp70 and Hsp27 observed in many cancers is thus involved in evading programmed cell death, increasing tumor growth rate and activating tumor progression.
Likewise Hsp70 family members and Hsp27 inhibit replicative senescence when increased in concentration in cancer (O’Callaghan-Sunol et al. 2007; Gabai et al. 2009). One pathway by which elevated levels of HSPs immortalize cancer cells may involve antagonizing the pro-senescence activity of the wild-type p53 protein (Sherman et al. 2010).
Little is known regarding the role of the HSF-1>HSP axis in angiogenesis. However, elevated molecular chaperones may favor angiogenesis as the key angiogenesis factor hypoxia inducible factor 1-a is dependent on chaperoning by both Hsp70 and Hsp90 (Neckers and Ivy 2003). In addition other factors associated with angiogenesis including vascular endothelial growth factor and nitric oxide synthase require Hsp90 for their synthesis (Sun and Liao 2004; Pfosser et al. 2005)
Although this area has not been thoroughly studied, HSF1 could be involved in promoting cancer cell invasion and metastasis through its ability to associate with metastasis associated factor 1 in mammary carcinoma cells activated by the transforming cytokine heregulin (MTA1) (Khaleque et al. 2008). MTA1 is a gene co-repressor and may function to decrease expression of genes that inhibit invasion and metastasis (Mazumdar et al. 2001). In addition clinical studies have suggested a correlation between expression of Hsp27 and Hsp70 and metastatic potential (reviewed in: (Ciocca and Calderwood 2005; Calderwood et al. 2006)).
Cancer cells undergo profound changes in metabolism involving a switch from oxidative phosphorylation to glycolysis as first observed in the classical studies of Otto Warburg (Warburg 1956). This leads to elevated glucose consumption and an increased rate of metabolism in malignant cells (Gullino 1966). A role for HSF1 in this switch in metabolism was suggested by the findings that glucose uptake in cancer cells is decreased by hsf1 knockout in mice and that HSF1 is required for expression of the glycolytic intermediate lactate dehydrogenase in cells expressing the oncogene HER2 (Dai et al. 2007; Zhao et al. 2009). Recent studies have also shown that HSF1 can regulate lipid metabolism in hepatocellular carcinoma (Jin et al. 2011).
Most tumors appear to be immunosuppressive in nature due to a range of mechanisms including release of the immunosuppressive cytokine interleukin-10 and activation of regulatory T lymphocytes and myeloid-derived suppressor cells (MDSC) (Pekarek et al. 1995; Mantovani et al. 2008; Marigo et al. 2008). Extracellular HSPs play a complex role in tumor immunity, dependant on context. For instance, HSP-antigen complexes, when extracted from tumors can be used as powerful anti-cancer vaccines, and lead to tumor regression (see recent review (Murshid et al. 2011). However HSPs can be released from tumors in situ and secretion of Hsp70 appears in this context to be immunosuppressive due to the Hsp70-dependant attraction of MDSC (Mambula and Calderwood 2006; Chalmin et al. 2010). This appears to be a key issue and future studies are encouraged to determine the role of HSF1 and extracellular HSPs released from tumors in immunity and tumor progression.
Fig.1.
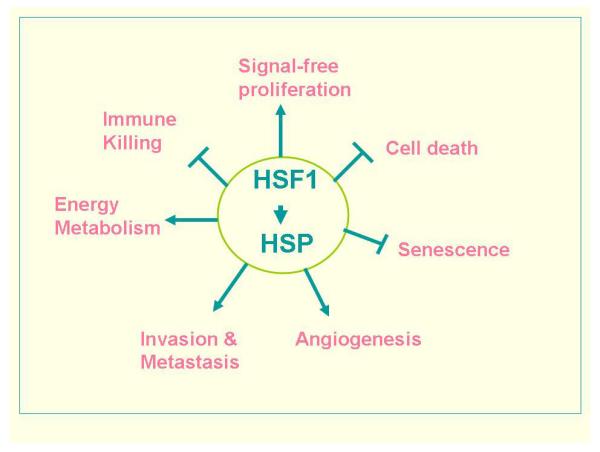
The HSF1 / HSP transcriptome regulates multiple traits associated with malignant transformation and tumorigenesis.
(3) The role of individual HSPs in cancer
a) Hsp90
Four members characterize the HSP90 gene family, including two Hsp90 isoforms (stress-induced Hsp90α and constitutively expressed Hsp90β), the mitochondrial chaperone TRAP1 (TNF receptor-associated protein 1) and GRP94 (glucose-regulated protein 94), a chaperone localized in endoplasmic reticulum (Felts et al. 2000). Both Hsp90α and Hsp90β form homodimers that bear essential ATP-dependent chaperone activity. Indeed, their complete knock-out is lethal in all eukaryotes examined to date. Despite their apparent different posttranslational modifications and sensitivity to radicicol, little is known about functional differences between these isoforms. Both are constitutively expressed under normal conditions and account for up to 2% of total cellular protein content in all cell types that have been investigated. This high level of expression may reflect an excess buffering chaperone activity that could be used to maintain homeostasis in the face of proteotoxic stresses (Sangster et al. 2004), which, when they occur, nevertheless up-relate Hsp90α expression. Apart from its role in stressed cells where it deters protein denaturation and aggregation and enhances refolding, Hsp90 plays a prominent role in the non-stressed cell by assisting the assembly/disassembly of multi-protein complexes, the conformational maturation of signaling molecules and their translocation across membranes (Buchner 1999). To perform such a diversity of functions, Hsp90 does not have pleiotropic enzymatic activities, but instead associates, in a dynamic and complex way with co-factors known as co-chaperones (for example Aha1, Hop Cdc37, p23, PP5, CHIP, and the immunophilins FKBP51 and FKBP52) (Taipale et al. 2010). As HSPs are not enzymes, their interacting partners in the proteome are known as clients. Hsp90 clients were originally defined as proteins whose dissociation from Hsp90 results in their degradation. Hsp90 usually stabilizes its client proteins, particularly when they are mutated. Hsp90 can restore the native folding of some mutated clients and therefore attenuate an array of morphological phenotypes that depend on underlying genetic variation (Queitsch et al. 2002; Rutherford and Lindquist 1998; Sangster et al. 2004). Hsp90, co-chaperones and clients form complexes that cycle through a variety of conformational states and their interactions occur through rounds of ATP hydrolysis (Hessling et al. 2009; Mickler et al. 2009). The ATP-binding motif present in the N-terminal domain of Hsp90 belongs to the GHKL (bacterial Gyrase, Hsp90, histidine Kinase, MutL) super-family and has no similarity to the ATPase motifs of Hsp70 or protein kinases (Dutta and Inouye 2000).
Of great interest, the ratio between Hsp90α and Hsp90β dimers, which is changed in stress conditions in favor of Hsp90α, is modified as a consequence of malignant transformation. Further analysis revealed that Hsp90 is involved in many key processes in oncogenesis such as self-sufficiency in growth signals, stabilization of mutant proteins, angiogenesis, and metastasis. These effects are essentially due to Hsp90 ability to target client proteins that are pro-cancerous. For example, Hsp90 stabilizes growth factor receptors, survival-signaling kinases (such as Akt and PI3K), oncogenes, (such as v-Src, Bcr/Abl, Raf-1, ErB-2 and mutant forms of p53) and less stable proteins produced by DNA mutations (Blagosklonny 2002; Taipale et al. 2010). Inhibition of apoptosis by Hsp90 occurs through multiple cytosolic pathways, such as the inhibition of Apaf-1 activation by cytochrome c, which prevents the assembly of the apoptosome consequently of the formation of an Hsp90-Apaf-1 complex (Pandey et al. 2000) (Fig. 2). Another inhibitory event concerns the pro-apoptotic kinase ASK1 (apoptosis signal-regulating kinase 1) whose activity is inhibited through formation of a complex with Hsp90-Akt (Zhang et al. 2005). Hsp90 also exerts it anti-apoptotic activity inside mitochondria. Indeed, in cancer cells, mitochondria unlike their normal counterparts, contain a large fraction of the intracellular pool of Hsp90 (Fulda et al. 2010). Within this organelle, Hsp90 interacts with TRAP1 (tumor necrosis factor receptor-associated protein 1), CYPD (cyclophilin D) and survivin, thereby exerting anti-apoptotic functions. It also prevents AIF (apoptosis-inducing factor) mitochondrial-cytosolic translocation and inhibits the nucleolytic activities of both AIF and endonuclease G (Fulda et al. 2010). Hsp90 can also promote angiogenesis and metastasis through the chaperoning of specific client targets, such as VEGF (vascular endothelial growth factor), NOS (nitric oxide synthase) and MMP2 (matrix metalloprotease). Consequently, inhibition of Hsp90 expression triggers apoptosis through a general knock-down of pro-cancerous signaling pathways (Whitesell and Lindquist 2005). In spite of these observations, our understanding of Hsp90 function and how it is altered in various cancers is far from been complete. For example, understanding of potential Hsp90 activity in cellular processes such as energy metabolism, protein trafficking, epigenetics, and DNA quality control is still lacking (Trepel et al. 2010). Hence, Hsp90 plays a two-faced Janus-like role in being essential for both normal and cancer cells. However, it was nonetheless proposed as a molecular target for anticancer therapeutics (Whitesell et al. 1994). More than a decade later, a large number of publications have identified over 200 client polypeptides interacting with Hsp90 (for an updated list see: http://www.picard.ch/downl-loads). The still growing list of clients points to the fact that Hsp90 is a global regulator of cell systems (McClellan et al. 2007; Moulick et al. 2011). Moreover, its recently discovered role, together with Hsc70, in the assembly of RISC (RNA-induced silencing complex) (Iwasaki et al. 2010) confirms its ability to regulate global networks (Salmena et al. 2011).
Fig. 2.
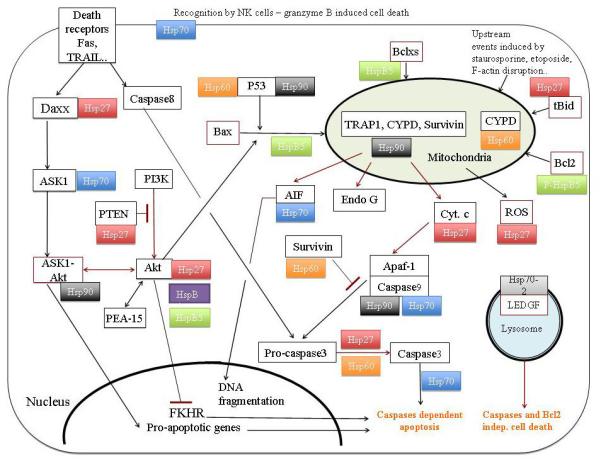
A circuit diagram of the programmed cell death pathways observed in cancer indicating the involvement of HSPs at multiple stages.
Based on the ATP-dependence of the Hsp90 chaperone machine, drugs that interact with ATP binding sites of kinases have been tested for their ability to inactivate its chaperone activity. Geldanamycin, which has originally been thought to function solely as a kinase inhibitor, was reported in 1994 as the first inhibitor of Hsp90 chaperone activity that could have clinical benefit (Neckers et al. 1999). In preclinical testing, geldanamycin proved to be too poorly soluble and too toxic and it was rapidly replaced by less toxic inhibitors, such as 17AAG (17-allylaminogeldanamycin). In vivo, these drugs caused the destabilization and eventual degradation of virtually all proteins chaperoned by Hsp90, albeit with vastly differing kinetics, and showed promising antitumor activity in preclinical model systems (Neckers 2002). Amongst the client proteins that were degraded in response to an Hsp90 inhibitor one can cite oncogenic fusion proteins, mutated and activated serine/threonine protein kinases, tyrosine kinases, as well as transcription factors with oncogenic activity (Georgakis and Younes 2005). Moreover, Hsp90 inhibitors attenuated several of the compensatory mechanisms used by tumors to overcome targeted and cytotoxic therapy (Trepel et al. 2010). The first generation of inhibitors showed elevated levels of toxicity in cancer patients. The second-generation of inhibitors, that still required intravenous administrations weekly, had greater bioavailability and were less toxic towards possible liver damage. The third-generation of drugs were taken orally (such as retaspimycin hydrochloride) and were more stable, less toxic, and had greater anticancer properties. To date, 17 distinct Hsp90 inhibitors have been tested in clinical trials that enrolled over a thousand patients but, unfortunately, without achieving FDA approval (Trepel et al. 2010). The reasons for this lack of success may be multiple and essentially linked to toxicity problems, limited drug resistance and anti-tumor efficacy, phenomena that resulted, at least in part, of an heat shock response induction by these drugs. In that respect an interesting example of unsuccessful drug is Tanespimycin (Erlichman 2009). Moreover, during these clinical trials, it has not been possible to detect an Hsp90 client that was specifically degraded in response to drug administration. The most reliable indicator of the efficiency of the inhibitors has been the induction of Hsp70, consequently of the release of HSF1 from its interaction with Hsp90 (Zou et al. 1998).
Today, investigators are trying to understand why Hsp90 inhibitors were not more rapidly successful in the clinic. Firstly, it is not surprising to note that a better care in patient selection based on tumor dependence on Hsp90 inhibition is already proving fruitful. New drugs that are now in clinical development are expected to have a higher specificity since they are based on the fact that the ATPase domain of Hsp90 has little similarity to the ATPase domains found in protein kinases or Hsp70. Another point to consider concerns our understanding of the highly complex Hsp90 machinery and advances in this area can help in the development of Hsp90 based therapeutic drugs. For example, currently used clinical Hsp90 inhibitors do not enter mitochondria and thus do not alter TRAP1/Hsp90 and consequently mitochondrial clients like survivin, a polypeptide of high interest since it inhibits apoptosis and promotes tumorigenesis (Dohi et al. 2004; Fortugno et al. 2003; Matassa et al. 2012). Survivin is sharply overexpressed in cancer versus normal tissues and frequently linked to unfavorable disease outcome (Altieri 2003). Hence, chances are high that mitochondrially-targeted Hsp90 inhibitors, like shepherdin, could have tumor-selective cytotoxic properties. Shepherdin is a cell-permeable peptide modeled on the binding interface between Hsp90 and survivin (Plescia et al. 2005) that destabilizes Hsp90 client proteins and results in the breakdown of multiple cell survival pathways, such as those depending on survivin, Akt, CDK-4, and CDK-6 (Fortugno et al. 2003). Survivin inhibitors recently entered clinical trials. However, reservations remain regarding this approach as recent observations suggest that survivin plays a role in normal adult cells (Kanwar et al. 2010). Hsp90 inhibitors administrated together with other chemotherapeutics drugs can also be promising, as for example, in multiple myeloma (Mimnaugh et al. 2004; Richardson et al. 2010). The combination of different Hsp90 inhibitors could also appear efficient such as in the case of HER2-positive breast cancer patients (Arteaga 2011). A broad view taking into account all the physiological effects appears therefore essential in planning how best to combine Hsp90 inhibitors with other drugs. Indeed, to generate an efficient therapeutic effect, an inhibitor should act as a “perturbagen” that modulates not just its immediate target, but the entire cellular machinery (Lamb et al. 2 006).
b) Hsp70
At least eight homologous chaperones belong to the human Hsp70 family. Six family members reside in the cytosol and nucleus whereas the remaining two members reside in the mitochondria and endoplasmic reticulum. Recent studies revealed that several members of the family perform distinct and non-overlapping tasks that are essential for growth and survival of cells. The most studied member of this family is the major stress-inducible Hsp70 (Hsp70.1 or haspa1a) (Bukau and Horwich 1998; Young et al. 2004). Structural analysis of Hsp70 chaperones reveals that they share a β-sheet cavity covered by a helical lid, to interact with a wide spectrum of substrates ranging from oligopeptides, unfolded polypeptides and aggregated proteins (Schlecht et al. 2011). It is now believed that Hsp70 chaperones have flexible binding mechanism that provides a basis for the broad spectrum of substrate conformers of Hsp70 molecules. In fact, Hsp70 molecules can not only bind to extended peptide stretches of protein substrates but can also accommodate regions with substantial tertiary structure. This should enable Hsp70 chaperones to have differential interaction with many client proteins and consequently multiple roles in normal, stressed and pathological cells. In that respect, recent data have suggested that individual members of the Hsp70 family can bring about non-overlapping functions essential for growth and survival of cancer cells (Daugaard et al. 2007b). Of interest, constitutively elevated levels of Hsp70.1 are known to be a characteristic of many tumor cells and experimental and clinicopathological data have implicated this chaperone in cancer cell survival and tumorogenicity (Aghdassi et al. 2007; Gurbuxani et al. 2001; Jäättelä 1995; Jaattela et al. 1992). The molecular functions of Hsp70.1 in cancer cells are far from being completely unraveled but at least one aspect relies on apoptosis inhibition through binding to specific client proteins (Fig. 2). Hsp70.1 was described to act in programmed cell death downstream of the execution protease caspase-3-like (Jaattela et al. 1998) and at the level of apoptotic initiation by procaspase-9 recruitment to the apaf-1 apoptosome (Beere et al. 2000; Saleh et al. 2000) (Fig. 2). In addition to Apaf-1, another well characterized binding target of Hsp70.1 was reported to be AIF (apoptosis inducing factor), a mitochondrial intermembrane flavoprotein that induces chromatin condensation (Ravagnan et al. 2001). The mechanism of action towards these two client proteins is probably different since the ATP-binding domain of Hsp70.1 is only required for binding to Apaf-1. In recent years, many other activities of Hsp70 molecules have been discovered that regulate apoptotic pathway: for example, Hsp70.1 can interact with mutant forms of the tumor suppressor p53, a property shared by Hsc70 (Hsp70-8), the major constitutively expressed member of the Hsp70 family (Fourie et al. 1997). Hsp70 also interacts with ASK1 (apoptosis signal-regulating kinase1) (Park et al. 2002) and, together with co-chaperone CHIP, it can inhibits TNFα induced apoptosis by promoting degradation of ASK1 through formation the complex of Hsp70/CHIP/ASK1 (Gao et al. 2010). Hsp70-1 can efficiently block Bax translocation to mitochondria (Yang et al. 2012) and a relation exists between Hsp70 and the survival kinase Akt (Koren et al. 2010) but the molecular mechanisms that are involved in these phenomena are still not well characterized. Hsp70 has also the intriguing ability, in addition to its ability to protect against cell death by directly interfering with mitochondrial apoptosis pathways, to sensitize cells to certain apoptotic stimuli like the one induced by tumor necrosis factor alpha (TNFα) (Ran et al. 2004). For example, Hsp70 over-expression in human colon cancer cells results in the inhibition TNFα-induced NF-κB activation but in the stimulation of TNFα-induced activation of c-Jun N-terminal kinase (JNK) through interaction with TNF receptor-associated factor 2 (TRAF2). Consequently, in response to TNFα stimuli, Hsp70-TRAF2 interaction promotes TNFα-induced JNK activation but, through reduced recruitment of receptor-interacting protein (RIP1) and IκBα kinase (IKK) signalosome to the TNFR1-TRADD complex, it inhibits the activation of the transcription factor NF-κB. These events may contribute to the pro-apoptotic roles of Hsp70 in TNFα-induced apoptosis of human colon cancer cells (Dai et al. 2010). Another intriguing observation made in cancer cells as well as in normal cells exposed to stress concerns a small portion of Hsp70-1 that translocates to the lysosomal compartment where it promotes cell survival by inhibiting lysosomal membrane permeabilization, a hallmark of stress and cancer. The lysosomal binding target of Hsp70.1 is an endolysosomal anionic phospholipid bis(monoacylglycero)phosphate (BMP), an essential co-factor for lysosomal sphingomyelin metabolism (Kirkegaard et al. 2010; Petersen et al. 2010). This particular activity of Hsp70 may be of some use to counteract lysosomal storage disorders, as for example in patients with Niemann-Pick disease, a genetic disorder associated with reduced acid sphingomyelinase activity.
Hsp70.2, a chaperone essential for the growth of spermatocytes, was also shown to be required for cancer cell growth and survival (Daugaard et al. 2005; Rohde et al. 2005). Concomitant depletion of Hsp70.1 and Hsp70.2 has a synergistic antiproliferative effect suggesting that both proteins are specifically required for cancer cells. In contrast, Hsc70 (Hsp70-8) has a more broad survival effect towards cells (normal or tumorigenic). Remarkably, despite its high level of homology, Hsp70.2 enhances cancer cell growth and survival by using a molecular mechanism distinct from that of Hsp70.1. For example, cancer cells depleted for either Hsp70.1 or Hsp70.2 displayed strikingly different morphologies, cell cycle distributions (G2/M versus G1 arrest) and gene expression profiles. Further analysis revealed that Hsp70.2 depletion triggers lysosomal membrane permeabilization and cathepsin-dependent death in human cancer cells (Daugaard et al. 2007a). LEDGF (lens epithelium-derived growth factor) was then identified as an Hsp70.2-regulated guardian of lysosomal stability. Indeed, depletion of LEDGF destabilizes lysosomal membranes of cancer cells and induces caspase-independent and Bcl-2-resistant cell death (Daugaard et al. 2007a). Hence, LEDGF appears to be a specific Hsp70.2 client protein that has as an oncogenic role that controls a caspase-independent lysosomal cell death pathway. Indeed, over-expression of this protein stabilizes lysosomes and protects cancer cells against cytotoxicity induced by anticancer agents that trigger the lysosomal cell death pathway. High levels of LEDGF have been detected in many human tumors such as human breast and invasive bladder carcinomas where its expression correlates with that of Hsp70.2. The pathological effect linked to LEDGF was then demonstrated by analyzing the tumorigenic potential of LEDGF over-expressing human cancer cells in immunodeficient mice.
Another intriguing property of Hsp70 concerns its presence in the plasma membrane of solid tumors, metastases and leukemic blasts; a phenomenon not observed in the corresponding normal tissues (Multhoff 2007; Stangl et al. 2011). A fraction of the intracellular Hsp70 is transported to, and anchored on the plasma membrane (Gehrmann et al. 2008; Schilling et al. 2009) via an active mechanism that may require the translocation of phosphatidylserines from the inner to the outer membrane leaflet (Arispe et al. 2004) and putative Hsp70 interaction with specific client proteins. Tumor cells expressing Hsp70 at the plasma membrane are usually highly resistant to chemo- and/or radiotherapy. On the other hand, these cells can be recognized and killed by pre-activated natural killer (NK) cells through the release of the apoptosis-inducing enzyme granzyme B that penetrates in the cancer cell via a perforin-independent, but Hsp70 dependent manner (Gross et al. 2003). Moreover, membrane Hsp70 can be secreted (Pockley et al. 2003) in exosome-like vesicles (Gastpar et al. 2005). Consequently, high levels of Hsp70 have been reported in the circulation of cancer patients, such as those with colorectal (Kocsis et al. 2010) and prostate (Wang et al. 2004) cancer. Plasma Hsp70 is also considered as a potential marker for predicting diseases progression, such as in patients with chronic myeloid leukemia (CML) (Yeh et al. 2009) or acute leukemia (Yeh et al. 2010). Hsp70 exosome-like vesicles induce the migratory and cytolytic capacity of NK cells (Gastpar et al. 2005). However, tumor exosomes can also exert immunosuppressive functions (Zitvogel et al. 2008) and may trigger regulatory T cells and myeloid-derived suppressor cells (MDSC) (Viaud et al. 2008).
The inhibition of Hsp70 could be of potential value in cancer gene therapy since many studies dealing with the use of RNA interference (RNAi) approaches have confirmed the important role played by at least Hsp70.11 and Hsp70.2 in the growth and survival of many cancer cells (Cai et al. 2011; Daugaard et al. 2005; Rohde et al. 2005). However, no clinically available RNAi-based inhibitors have been tested in clinical trials. Other approaches, targeting inhibition of intracellular or membrane Hsp70 isoforms may be indicated. Targeting membrane Hsp70 by specific antibodies can be a successful approach as it selectively induces antibody-dependent toxicity of the tumor cell in contact to un-stimulated mouse spleen cells (Stangl et al. 2011). Only a few studies have been successful in identifying drugs that have inhibitory activity towards Hsp70 deleterious pro-cancerous activity. One example is the adenosine-derived inhibitor VER-155008 that targets the ATPase binding domain of Hsp70 (Massey et al. 2010). This drug successfully inhibits the proliferation of human breast and colon cancer cell lines and induces Hsp90 client protein degradation in both HCT116 and BT474 cells. It induces caspase-3/7 dependent apoptosis in BT474 cells and non-caspase dependent cell death in HCT116 cells. Moreover, VER-155008 potentiates the apoptotic potential of Hsp90 inhibitor in HCT116 consequently of the stimulated disruption of Hsp70-Hsp90-client proteins complexes. Unfortunately, in vivo, VER-155008 is rapidly eliminated and its level in tumor never reaches the predicted pharmacologically active level. Another study recently identified the small molecule 2-phenylethyenesulfonamide (PES) as a novel Hsp70 inhibitor (Leu et al. 2011). In tumor cells this drug disrupts the Hsp70/Hsp90 chaperone machines and impairs the autophagy-lysosome system and the proteasome pathway. Consequently, many cellular proteins, including Hsp70 and Hsp90 client protein substrates, are functionally inactivated, aggregated or degraded and most cancer survival pathways are inhibited. Another approach is based on peptide aptamers that specifically target, bind and inactivate Hsp70. Peptide aptamers that target the peptide binding and ATP-binding domains specifically inhibit Hsp70 chaperone activity. An increase in the sensitivity to apoptosis induced by anticancer drugs has been noted as well as a regression of subcutaneous tumors after local or systemic injection that occurred concomitantly with an important recruitment of macrophages and T lymphocytes (Rerole et al. 2011). These studies clearly support the idea that, in addition to targeting Hsp90, the inhibition of Hsp70 is a new interesting therapeutic approach. However, the consequences of these inhibitory approaches towards extracellular and circulating Hsp70 are not known and future studies will have to clarify this point.
c) Hsp70 co-chaperones Hsp40 and Hop
Human Hsp40 comprises a family composed of forty-one polypeptides that are highly conserved through evolution. They are characterized by a J-domain (DNAJ in prokaryotic world) allowing their interaction with Hsp70 chaperones (Michels et al. 1997). They act as co-chaperone and cooperate with Hsp70 polypeptides to regulate protein folding, transport, translational initiation and gene expression. In addition, together with Hsp70 this co-chaperone also plays essential roles in the Hsp90 chaperone pathway (Cintron and Toft 2006). However, compared to the large number of studies dealing with the other HSPs, the Hsp40 polypeptides have been understudied. For example, it is known that they are expressed in several tissues and reside at distinct intracellular locations but until recently little was known about their physiologic roles. Of interest, it has now been reported that some members of the Hsp40 family are involved in various aspects of cancer biology suggesting multi-faceted role of Hsp40 in cancer (Mitra et al. 2009). Oka et al. (Oka et al. 2001) were the first to report an elevated level of Hsp40 in a human tumor tissue (lung cancer). Moreover, autoantibodies to this protein were detected in the serum of patients suggesting that Hsp40 in lung tumor cells may be recognized as a self-antigen. High levels of Hsp40 and Hsp70 were then discovered in colorectal cancer independently of clinicopathological parameters (Kanazawa et al. 2003). In addition, one member of the Hsp40 family, JDP1 (DNAJC12/Hsp40), is highly expressed in breast cancer cells in association to their estrogen receptor-positive status. Expression of this protein is a result of the presence of estrogen response elements in the promoter region of JDP1 gene (De Bessa et al. 2006). Thus JDP1 could be used as a marker of the estrogen receptor transactivation activity and may have a predictive value for response to hormonal therapy. A recent study dealing with the effect of 5-fluorouracil and carboplatin on the killing of hepatoma cells has revealed that these drugs induce the expression of Hsp40 in addition to HspB1 in Hep3B and HepG2 cells. The protective effect of these chaperones was then demonstrated by siRNA-mediated knockdown (Sharma et al. 2009). Collectively, these novel findings highlight the strategic implications of Hsp40 family members in cancer biology as well as their protective role towards anti-cancer drugs. Their mode of action is unknown but it can be speculated that they do interact with specific targets and promote their folding/stabilization and/or degradation through Hsp70-CHIP machinery.
Hop is a co-chaperone that binds to both Hsp70 and Hsp90 (Hernandez et al. 2002). In addition to its association with Hsp70-Hsp90 refolding machines in response to stress that alter protein conformation, Hop plays several role in client protein stabilization involving Hsp90- and Hsp70-dependent as well as Hsp70-Hop-Hsp90 complexes. In the cancer field, Hop is often overexpressed such as in invasive pancreatic cancer (Walsh et al. 2011). Indeed, siRNA mediated Hop knockdown can decrease the invasion of pancreatic cancer cells, concomitantly with a reduced expression of matrix metalloproteinases-2 (MMP-2) and several client proteins of Hsp90 such as HER2, Bcr-Abl, v-Src and c-MET. The current theory is that Hop modulates key signal transduction proteins that decrease the invasiveness of pancreatic cancer cells through the possible modulation of Hsp90 activity (Walsh et al. 2011).
d) Hsp110
In eukaryotic cells, the hsp110 gene is a major member of the hsp70 family and its expression confers cellular resistance to stress through ATP-dependent chaperone function (Oh et al. 1999). The basal level of expression of Hsp110 is often modified in cancer cells. For example, a poor prognosis of esophageal cancer is often associated with reduced levels of Hsp110 expression (Nakajima et al. 2011). The reverse situation is observed during colorectal cancer progression (Slaby et al. 2009). In that respect, a recent study has identified a mutant of Hsp110 (HSP110DeltaE9) that is specifically expressed in a particular form of colorectal cancer (20% of total cases) characterized by microsatellite instability (MSI CRC) (Dorard et al. 2011). This dominant negative mutation alters Hsp110 substrate-binding domain. Hence, it inhibits wild-type Hsp110 chaperone activity and antiapoptotic function and sensitizes cells to the anticancer agents that are routinely prescribed to patients suffering of CRC. This mutation is a major determinant for both prognosis and treatment of CRC pathology.
e) Hsp60
Hsp60 is a mitochondrial matrix-associated protein that is considered an essential chaperone for the transport of proteins from the cytoplasm into the mitochondrial matrix. Hsp60 functions in association with another chaperone Hsp10 (which also resides in the mitochondria) (Bukau and Horwich 1998). HSPs are usually known for their pro-survival functions although Hsp60 is controversial in this regard as both pro-apoptotic and pro-survival functions have been reported (Cappello et al. 2008). Investigation of the role of Hsp60 in cells exposed to apoptotic challenges revealed that it is released from mitochondria and accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis (Samali et al. 1999). It was shown that, in growing cells, some pro-caspase-3 is present in mitochondria in a complex with Hsp60 and Hsp10. Upon exposure to apoptotic inducers, mitochondrial pro-caspase-3 is activated and dissociates from Hsp60 and Hsp10 which are then released from mitochondria. In the cytosol, these proteins accelerate the activation of cytoplasmic pro-caspase-3 by cytochrome c in an ATP-dependent manner, a phenomenon consistent with their function as pro-apoptotic chaperones (Samali et al. 1999). The pro-survival functions of Hsp60 are a more recently described activity that is particularly efficient in primary human tumors, in contrast to normal cells where Hsp60 depletion is well tolerated and does not cause apoptosis. The first report of an abnormal level of expression of Hs60 in a pathological human tissue was detected during colorectal carcinogenesis (Cappello et al. 2003). This pioneer study indicated that coordinate Hsp60 and Hsp10 over-expression is an early event in carcinogenesis and that these chaperones should play a different role in colorectal carcinogenesis compared to in normal cells. Further studies showed distinctive patterns of expression in different malignancies: high levels in colorectal, exocervical and prostate carcinogenesis, and colorectal cancer progression (Cappello et al. 2007; Hwang et al. 2009). Increased expression of Hsp60 was also significantly correlated with recurrence after radical prostatectomy (Glaessgen et al. 2008) and with the prognosis of lung adenocarcinoma (Xu et al. 2011). However, down-regulated levels of Hsp60 were observed during bronchial carcinogenesis (Cappello et al. 2007) and in the invasion phenotype of head and neck cancer (Chiu et al. 2011). In these pathologies, the loss of the tumor suppression function of Hsp60 (probably through its pro-apoptotic activity) contributes to aggressive cancers. Hence, Hsp60 is becoming an attractive therapeutic target (Khalil et al. 2011) and its level of expression a tool of diagnosis (Cappello et al. 2011).
Regarding the question of how cancer cells modulate Hsp60 levels, a relation between c-Myc and Hsp60 has been discovered. Myc directly activates Hsp60 transcription through a regulatory E-box site located in the proximal promoter of the Hsp60 gene. Of interest, siRNA mediated repression of Hsp60 reduced transformation caused by c-Myc overexpression, suggesting that c-Myc may promote transformation, at least in part, through the induction of Hsp60 expression.
The molecular mechanism for the tumor-related pro-survival function of Hsp60 is nowadays better understood in view of recent reports describing that, in addition of being a mitochondrial protein, Hsp60 is also present in the cytosol, the cell surface, the extracellular space and in the peripheral blood, different locations where this chaperone has non-mitochondrial specific functions (Cappello et al. 2008). For example, in cells committed to BMD188-induced apoptosis, cytosolic Hsp60 level increases concomitantly with a significant mitochondrial release. In contrast, in response to multiple other inducers, Hsp60 accumulates in the cytosol in absence of an apparent mitochondrial release. In BMD188-induced apoptosis, Hsp60 has a pro-death role that involves caspase-3 maturation and activation in the cytosol. In contrast, Hsp60 has a pro-survival role in apoptotic conditions when there is no apparent mitochondrial release as its RNAi-mediated knockdown promotes cell death (Chandra et al. 2007). In these latter conditions, Hsp60 does not associate with caspase-3 but with pro-survival client proteins that orchestrate a broad cell survival program. One client is the cell cycle regulator and apoptosis inhibitor survivin (Ghosh et al. 2008). Indeed, it has been shown that depletion of Hsp60 by siRNA destabilizes survivin pool and activates apoptosis. Hence, surviving appears stabilized by at least two chaperones, Hsp60 and Hsp90 (see above). A second client is p53 since Hsp60 depletion disrupts Hsp60-p53 complex and induces p53 stabilization that subsequently stimulates Bax-dependent apoptosis (Ghosh et al. 2008). Recently, a third Hsp60 client has been discovered: cyclophilin D (CypD), a component of the mitochondrial permeability transition pore (Ghosh et al. 2010). Moreover, a multi-chaperone complex comprising Hsp60, Hsp90 and TRAP1 has been characterized in tumor-derived but not in normal mitochondria. Depletion of Hsp60 triggers a CypD-dependent mitochondrial permeability transition and caspase-dependent apoptosis, Therefore, the pro-survival ability of Hsp60 selectively exploited in cancer cells results in at least three events: stabilization of survivin, restrained p53 pro-apoptotic function and CypD-dependent mitochondrial permeability transition. These observations favor Hsp60 as being able to modulate key intracellular pathways of tumor cells through intracellular and extracellular interactions with specific client proteins. Hence, future studies of the Hsp60 interactome are desirable.
Another notable observation relates to the active secretion of Hsp60 by human tumor cells (Merendino et al. 2010). This observation is particularly relevant for cancer biology since it is now believed that extracellular chaperones, like Hsp70, may play an active role in tumor growth and dissemination. However, more studies are needed to confirm that this is a general physiological phenomenon and to determine the mechanisms involved.
f) Hsp27 (HspB1) and other small HSPs
The 10 members of the family of human small Heat shock proteins (sHSP) are characterized by several parameters, such as molecular mass in the 20 to 40 kDa range, ability to oligomerize, an α-crystalline domain located in the C-terminal part of the polypeptides (Ingolia and Craig 1982), a less conserved N-terminal domain decorated with an hydrophobic WD/PF motif and phospho-serine sites (Theriault et al. 2004) and a flexible C-terminal tail (Takemoto et al. 1993) containing a IXI/V motif (Pasta et al. 2004). HspB1, αB-crystalline (HspB5) and HspB8 plus the less conserved Hsp16.2 (HspB11) polypeptide (Bellyei et al. 2007) are stress inducible HSPs. Four of them display ATP-independent chaperone activities: HspB1, αA-crystalline (HspB4), HspB5, HspB8 and HspB11. In stress conditions, these HSPs act as holdase that trap mis-folded proteins and avoid their aggregation and then cooperate with the Hsp70-Hsp90 ATP refoldase machine in their refolding or elimination (Barbash et al. 2007; Bellyei et al. 2007; Boelens et al. 2001; Carra 2009; Carra et al. 2008; Carra et al. 2005; den Engelsman et al. 2003; Ganea 2001; Horwitz et al. 1992; Jakob et al. 1993; Markossian et al. 2009; Parcellier et al. 2006). Expression of these sHSP induces cellular protection against stress (heat shock, oxidative) that damage proteins (Aoyama et al. 1993; Arrigo 2001; Landry et al. 1989; Mehlen et al. 1995; Preville et al. 1999; Rogalla et al. 1999) (Fig. 2). Their expression also correlates with up-modulation of reduced glutathione and stimulation of detoxicant enzymes, such G6PDH (glucose 6 phospho dehydrogenase) (Melhen et al. 1995, 1996a, Preville et al. 1999, Yan et al. 2002). In addition, HspB1, HspB4 and HspB5 regulate the cellular resistance to apoptotic conditions. Concerning HspB1 (Bruey et al. 2000a; Garrido et al. 1999; Mehlen et al. 1996b; Paul et al. 2002), the resistance originates from its interaction with important regulators of the apoptotic pathways, such as: procaspase-3 (Gibert et al. 2012; Pandey et al. 2000), cytochrome c (Bruey et al. 2000a; Paul et al. 2002), Daxx (Charette et al. 2000), Stat3 (Rocchi et al. 2005), eIF4E (Andrieu et al. 2010), F-actin (Paul et al. 2002), HDAC6 (Gibert et al. 2012), Stat2 (Gibert et al. 2012), PTEN (Cayado-Gutiérrez et al. 2012) and the general cell survival kinase Akt (Rane et al. 2001; Rane et al. 2003; Wu et al. 2007). For example, up modulation of Akt activity by HspB1 induces the phosphorylation inhibition of Forkhead transcription factors (Jomary et al. 2006), antagonizes Bax-mediated mitochondrial damages (Havasi et al. 2008) and PEA-15 dependent Fas-induced apoptosis (Hayashi et al. 2012). HspB5 protects from apoptosis by inhibiting the maturation of caspase-3 (Kamradt et al. 2001; Kamradt et al. 2002; Kamradt et al. 2005) and by inhibiting Bax and Bcl-xs translocation to the mitochondria (Mao et al. 2004). In addition, HspB5 can block the activation of the proto-oncogene RAS (Li et al. 2005). HspB5 and HspB4 are also described to modulate Akt, PKCα and Raf/MEK/ERK pathways (Liu et al. 2004). However, it is intriguing to note that HspB5 can also be pro-apoptotic since its serine59 phosphorylation prevents Bcl-2 translocation to mitochondria (Launay et al. 2010).
An important characteristics of sHSP is their constitutive expression in mammalian tissues (Bhat and Nagineni 1989; Srinivasan et al. 1992), particularly when they differentiate (Arrigo 2005) or in pathological conditions, such as in tumor cells where they accumulate (Calderwood et al. 2006; Ciocca and Calderwood 2005). Indeed, through their anti-apoptotic activities, particularly towards death receptors, such as TRAIL, TNFα and Fas (Hayashi et al. 2012; Kamradt et al. 2005; Mehlen et al. 1995; Mehlen et al. 1996a; Mehlen et al. 1996b; Zhuang et al. 2009), they can generate a deleterious survival effect when they are expressed to high levels. In that respect, HspB1 is tumorigenic (Garrido et al. 1998) and stimulates metastasis formation and dissemination (Bausero et al. 2006; Bausero et al. 2004; Blackburn et al. 1997; Katoh et al. 2000; Lemieux et al. 1997; Gibert et al, 2012). It also provides cancer cells with resistance to many anti-cancer drugs, which in turn, stimulate HspB1 expression (Arrigo 2000; Garrido et al. 2001; Kamada et al. 2007; Kang et al. 2008; Kase et al. 2009; Mehlen et al. 1996b; Richards et al. 1996; Rocchi et al. 2006). Down-regulation of HspB1 in cancer cells induces multi-nucleation (Gibert et al. 2012) and causes senescence through the activation of the p53 pathway and induction of p21waf (Gabai et al. 2009; O’Callaghan-Sunol et al. 2007). Short-term silencing of HspB1 also increases proteasome activity as well as CD8+ T-cell-mediated tumor killing and memory responses that stimulate established breast tumors regression (Nagaraja et al. 2012). Consequently, high levels of HspB1 predict a poor clinical outcome of breast, prostate, gastric, uterine, ovarian, head and neck tumors and those from the nervous and urinary systems. HspB1, H4 and B5 also promote cell migration and invasion. One interesting example is HspB5, this protein is highly expressed in human breast basal-like tumors, and induces EGF- and anchorage-independent growth, increases cell migration and invasion, and constitutively activates the MAPK kinase/ERK (MEK/ERK) pathway. Moreover, HspB5 expression has also the intriguing property to transform immortalized human mammary epithelial cells that can subsequently form invasive mammary carcinomas in nude mice that have the same aspect as basal-like breast tumors. Hence, HspB5 is a novel oncoprotein linked to short patient survival (Gruvberger-Saal and Parsons 2006; Moyano et al. 2006). In contrast, HspB4 has been described, at least in pancreatic cancer, as a negative regulator of carcinogenesis (Deng et al. 2010). This protein modulates the activity of ERK MAP kinase and regulates AP-1 expression and activity to counteract cell transformation. In addition, HspB4 retards cell migration and prevents tumor growth.
Tumor progression, invasion of tumor cells into surrounding tissues and dissemination to form metastatic colonies is an other important aspect where at least HspB1 plays an important role (Bausero et al. 2006; Bausero et al. 2004; Blackburn et al. 1997; Katoh et al. 2000; Lemieux et al. 1997). A recent striking example is the promoting effect of HspB1 in breast cancer cells metastasis and bone tumor development (Gibert et al, 2012). In spite of these observations, the molecular mechanism that drives Hsp27 to promote tumor progression and metastasis is still not well understood. One hypothesis could refer to the property of HspB1 to modulate cytoskeleton integrity and indirectly extracellular matrix organization (Dalle-Donne et al. 2001; Mounier and Arrigo 2002; Perng et al. 1999). Two important observations support this hypothesis: the first refers to the interaction of cytoplasmic β-catenin with HspB1 that results in the subsequent modulation of cadherin-catenin cell adhesion proteins (Fanelli et al. 2008). The second one concerns phosphorylated HspB1 which mediates the activation of matrix metalloproteinase type 2 (MMP-2), an enzyme that digests components of the extracellular matrix surrounding tumor masses and subsequently stimulates tumor cells invasion (Xu et al. 2006).
The multiple roles of sHSP, particularly HspB1, raise an immediate question: how these proteins can achieve such a huge endeavor? Are they bearing multiple enzymatic activities in addition to the chaperone one? Or could their apparent pleotropic activities be a consequence of indirect effects resulting of their ability to bind and modulate client protein targets? Recent observations are in favor of the second hypothesis. This is based on the recently described interaction of HspB1 with many crucial cell regulators (see Table 1) that are subsequently modulated in their activity and/or half-life. For example, HspB1 can modulate client proteins that are essential to apoptotic cell death as well as tumorigenic and metastatic processes, such as pro-caspase3 (Gibert et al. 2012), cytochrome c (Bruey et al. 2000a), Daxx (Charette et al. 2000), Stat3 (Rocchi et al. 2005), HDM2 (O’Callaghan-Sunol et al. 2007), eIF4E (Andrieu et al. 2010), HDAC6 (Gibert et al. 2012), Stat2 (Gibert et al. 2012), Akt (Rane et al. 2001; Rane et al. 2003; Wu et al. 2007), Her2 (Kang et al. 2008), PTEN (Cayado-Gutiérrez et al. 2012), β-catenin (Fanelli et al. 2008) and several others (see Table 1). Modulation of the half-life of the client polypeptides is frequent but this is not a general rule since HspB1 can also regulate their enzymatic activities (Charette and Landry 2000; Rane et al. 2003) or modifications (Brunet Simioni et al. 2009). Such a behavior resembles that of Hsp90 and its interacting partners (Georgakis and Younes 2005; Neckers et al. 1999). However, how sHSP could specifically recognize so many protein targets and modulate their half-life or activities? In case of HspB1, one possibility could be that the complex changes in its oligomerization and phosphorylation status are key parameters that modulate its ability to interact with client substrates (Paul et al. 2010). Indeed, in response to transient changes in the cellular environment, HspB1 native size and phosphorylation are deeply changed. Of importance, these changes are reversible and specific to the modifications of the cellular environment (or stress) sensed by the cell (Arrigo 2011; Paul et al. 2010). The same conclusion was reached by structural analysis. It was found that an astonishing 300 different stoichiometries are possible to interact with putative client proteins (Stengel et al. 2010). The structural heterogeneity of sHSP not only makes the system quite distinct in behavior to ATP-dependent chaperones, but also renders it difficult to analyze by conventional structural biology approaches. Taken together, these observations suggest that fluctuations in HspB1 quaternary structures could act as a sensor of the cellular environment. The dynamic plasticity in HspB1 structure would then favor the recognition of the more appropriated client proteins hence allowing the cell to respond and/or adapt to the challenge. Moreover, such a model could explain the multiple unrelated cellular effects associated to HspB1 over- or under-expression that are described in the literature. In that respect, it should be noted that complex oligomeric properties also characterize Hsp90, the well-know chaperone prone to interact with client proteins (Lee et al. 2011). Some information is already available concerning the structural organization of HspB1 that recognizes some client polypeptide (see Table 1). For example, the active form of HspB1 that interacts with Daxx is the small oligomer form (Charette and Landry 2000) while HspB1 interaction with either pro-caspase3, Stat2 or HDAC6 results in the formation of larger complexes (Gibert et al. 2012). Phosphorylation also appears important for interaction with GATA-1 (de Thonel et al. 2010) or AUF1, an AU-rich element (ARE)-binding protein (Knapinska et al. 2011). Moreover, the small phosphorylated oligomers appear to be the active form that has F-actin capping activity, negatively modulates F-actin fibers growth and protects F-actin integrity in stress conditions (Mounier and Arrigo 2002). Unfortunately, in most of the studies reported in the literature the phosphorylated status of HspB1 has not been determined, particularly in relation to the oligomeric status of the protein. This is a crucial point since it has recently been demonstrated in cells exposed to different apoptotic inducers, that HspB1 has multiple possibilities of forming differentially phosphorylated oligomers; a phenomenon which probably reflects the different and inducer-specific anti-apoptotic strategies set up by this protein (Paul et al. 2010). Hence, future studies will have to characterize the structures formed by HspB1 when it interacts with the most crucial client targets.
Table 1.
Hsp27(B1) client proteins
| Interacting Clients | Functional Effect | HspBl structure | References |
|---|---|---|---|
| HspB5 | HspB5 stabilization | Mosaic oligomers | (Fu and Liang 2003) |
| Her2 | Her2 stabilization | nd | (Kang et al. 2008) |
| Stat-2 | Stat-2 stabilization | 200-600 kDa | (Gibert et al. 2012) |
| Stat-3 | Stat-3 stabilization | nd | (Rocchi et al. 2005) |
| Androgen Receptor | AR stabilization | nd | (Zoubeidi et al. 2007) |
| Caspase-3 | Pro-caspase-3 stabilization | 150-200 kDa | (Pandey et al. 2000) (Gibert et al. 2012) |
| HDAC6 | HDAC6 stabilization | 500-700 kDa | (Gibert et al. 2012) |
| Cytochrome c | Inhibition APAF | nd | (Bruey et al. 2000a) |
| Daxx | Inhibition Daxx activity | Small oligomers | (Charette and Landry 2000) |
| PEA-15 | Inhibition Fas apoptosis | nd | (Hayashi et al. 2012) |
| PTEN | Increase PTEN level | nd | (Cayado-Gutierrez et al. 2012) |
| Akt, P38, MK2 | Akt activation | nd | (Wu et al. 2007) |
| GATA-1 | GATA-1 degradation | Phospho-HspB1 | (de Thonel et al. 2010) |
| AUF1 | AUF1 degradation | Phospho-HspB1 | (Knapinska et al. 2011) |
| p27kip1 | p27kip1 degradation | nd | (Parcellier et al. 2006) |
| Smad Smurf2 | HspB1 degradation | nd | (Sun et al. 2011) |
| Ubiquitin | Protein degradation | nd | (Parcellier et al. 2003) |
| GranzymeA | Stimulation activity | Monomers/dimers | (Beresford et al. 1998) |
| F-actin | Chaperone F-actin | Small P-oligomers | (Mounier and Arrigo 2002) |
| GFAP | Inhibition IF interaction | nd | (Perng et al. 1999) |
| Factor XIII | Platelet FXIII regulation | Phospho-HspB1 | (Zhu et al. 1994) |
| eIF4G | Inhibition translation | nd | (Cuesta et al. 2000) |
| eIF4E | Tumor cell survival | nd | (Andrieu et al. 2010) |
| β-catenin | Tumor invasion, Metastasis | nd | (Fanelli et al. 2008) |
| TRAF6 | TRAF6 ubiquitination | Phospho-HspB1 | (Wu et al. 2009) |
| HSF-1 | HSF sumoylation | Large oligomers | (Brunet Simioni et al. 2009) |
| Ubc9 | HSF sumoylation | Large oligomers | (Brunet Simioni et al. 2009) |
| ERβ | Estrogen signaling | Phospho-HspB1 | (Al-Madhoun et al. 2007) |
| β-amyloid | Inhibition aggregation | nd | (Wilhelmus et al. 2006) |
| α-synuclein | Inhibition aggregation | nd | (Bruinsma et al. 2011) |
| RhoA, PKCα | Muscle contraction | Phospho-HspB1 | (Patil et al. 2004) |
| PKCΔ | Cell sensitization | nd | (Lee and Lee 2010) |
| XPORT | Transport of TRP and Rh1 | nd | (Rosenbaum et al. 2011) |
| p90Rsk | HspB1 phosphorylation | nd | (Zoubeidi et al. 2010) |
| Phk | nd | Small oligomers | (Chebotareva et al. 2010) |
| CD10 | nd | nd | (Dall’Era et al. 2007) |
| Tubulin | nd | nd | (Hino et al. 2000) |
nd: not determined; CD10: 100 kDa transmembrane metallo-endopeptidase; p90rsk: p90 ribosomal S6 kinase; IF: intermediate filaments; GATA-1: globin transcription factor 1; HSF-1: heat shock factor 1; GFAP: glial fibrillary acidic protein; DAXX: death domain-associated protein 6; STAT2, 3: signal transducer and activator of transcription 2, 3; Fbx4: Fbox only protein 4; eIF4E, eukaryotic translation initiation factor 4E; eIF4G: eukaryotic translation initiation factor 4G; Smad-Smurf2: Smad ubiquitination regulatory factor 2; Factor XIII: transglutaminase, platelet Factor XIII; PhK: rabbit skeletal muscle phosphorylase kinase; XPORT: exit protein of TRP and Rh1; TRP: transient receptor potential channels; Rh1: rhodopsin; MK2: MAPK-activated protein kinase-2; P38: P38 MAP Kinase; TRAF6: tumor necrosis factor receptor-associated factor 6; AR: androgen receptor; ERβ: estrogen receptor β. PKCΔ: protein kinase C Δ. Akt: also known as protein kinase B (PKB); Her2: Human Epidermal Growth Factor Receptor-2; HDAC6: histone deacetylase 6; p27kip1: cyclin-dependent kinase inhibitor p27kip1; PEA-15: astrocytic phosphoprotein PEA-15; PTEN: phosphatase and TENsin homolog.
Two particular properties should be noted as they increase the complexity of the sHSP world: i) the relationships between structural organization, holdase activity and changes in response to modifications of the cellular environment are not well conserved between the different members of the family (Ahmad et al. 2008; Aquilina et al. 2004; Horwitz et al. 2004; Koteiche and McHaourab 2003; Liang and Akhtar 2000; Paul et al. 2010; Rogalla et al. 1999). ii) sHSP can form mosaic oligomeric structures with the other members of the family. Thus, multiple combinatorial phosphorylated mosaic structures could bear specific protein target recognition abilities (Michiel et al. 2009; Saha and Das 2004; Simon et al. 2007; Sun and Liang 1998; Sun et al. 2006; Zantema et al. 1992). Another consequence is the dominant effect of a mutated small HSP towards the other members of the family (Bruey et al. 2000a; Diaz-Latoud et al. 2005). Unfortunately, only few of these mosaic structures have been characterized yet. Hence, it is reasonable to declare that today we are far from having a clear understanding of sHSP dynamic interactome.
In cancer, HspB1 is now well established as being a therapeutic target. This was demonstrated by approaches aimed at decreasing HspB1 expression by anti-sense DNA vectors (Aloy et al. 2007; Paul et al. 2002) and RNAis, such as OGX 437 RNAi molecules (Oncogenex Inc). These approaches sensitized cancer cells to apoptotic inducers, anticancer drugs and radiations. Decreased tumorigenic and metastatic potentials of several cancer cells have been observed (Bausero et al. 2006; Kamada et al. 2007; Kaur et al. 2011; Kim et al. 2007; Lee and Lee 2010; Rocchi et al. 2006; Straume et al. 2012)(Gibert et al, 2012). RNAi targeting is believed to destabilize HspB1 interactome leading to the inactivation, destabilization and/or degradation of inappropriate, tumorigenic and/or metastatic client proteins. In that respect, it has recently been proposed that HspB1 plays a key role in the balance between tumor dormancy and tumor progression, mediated by tumor-vascular interactions (Straume et al. 2012).
The search for drugs that inactivate HspB1 deleterious activity without interfering with its level of expression is now beginning. This approach has been considered feasible since, for example, exogenous dominant-negative mutant can knock out the activity of endogenous wild-type HspB1 through formation of inactivated mosaic complexes (Bruey et al. 2000a; Diaz-Latoud et al. 2005). Hence, molecules that can mimic dominant mutant and destabilize HspB1 structure and its associated network of interacting polypeptides are actively searched for. The targets can be the oligomeric status or crucial modifications, such as the argpyrimidine modification which modulates HspB1 ability to bind cytochrome c (Padival et al. 2003). The peptide sequence that characterizes the binding domain of a client protein is another possible way to tackle the problem, one example is the seven amino acids of the PKC delta V5 region which inhibit HspB1-induced resistance against DNA damaging agents and cisplatin (Kim et al. 2007; Lee and Lee 2010). Testing existing drugs can sometime be successful, as for example the anti-viral drug RP101 (Bromovinyldeoxyuridine, Brivudine) which has recently been reported to improve the efficiency of human pancreatic cancer chemotherapy through a specific interaction with HspB1 (Heinrich et al. 2011). RP101. This drug, which recognizes two phenylalanine residues (Phe29 and Phe33) in the N-terminal domain, inhibits HspB1 interaction with pro-cancerous binding partners and stimulates caspases activation. Peptide aptamers (Colas et al. 1996; de Chassey et al. 2006) that specifically recognize HspB1 is another possibility. In that respect, two peptide aptamers that perturb the oligomerization of HspB1 have found to down-regulate its anti-apoptotic activity and ability to stimulate cellular radioresistance (Aloy et al. 2007; Gibert et al. 2011). Of interest, in vivo, these peptide aptamers are more efficient to reduce tumor growth (oral squamous carcinoma non-metastatic SQ20B cells) than HspB1 depletion (Gibert et al. 2011). This suggests that HspB1 poisoning is probably more efficient than its depletion that could be overcome through activation of complementary protective mechanisms. Based on their particular sequences, peptide aptamers could represent a first step towards the engineering of functional peptido-mimetic drugs. However, due to HspB1 dynamic plasticity, particularly in tumor cells (Bruey et al. 2000b), and ill-defined structure of its oligomers, challenging studies will be needed until theoretical designed active molecules could specifically destroy HspB1 interaction with a particular pathological client. The use of technologies resulting in a broad inhibition of HspB1-client interaction may indeed prove to be disappointing as it is now observed for the inhibitors of Hsp90 chaperone activity which induce only modest effects in most of the cancer clinical trials that have been reported to date.
Fig. 2 summarizes the main apoptotic pathways that are under the control of HSPs.
(4) Clinical implication of the HSF1>HSP system in cancer
a) From tumor cell initiation to clinical behavior
The clinical applications of HSPs in cancer have expanded considerably in recent years; here we will mention examples of the articles that have been published in this field (Table 2).
Table 2.
Examples of recent clinical studies evaluating HSPs
| Cancer type | Main observations | Author |
|---|---|---|
| Bladder cancer (n=68) |
Hsp60 (and IL-13) as urinary marker (immuno- assay) for diagnosis and staging |
Margel et al. 2011 |
| Bladder cancer (n=54) |
Hsp60 expression prior to neoadjuvant chemo- radiotherapy predicts good pathological response |
Urushibara et al. 2007 |
| Breast cancer (n=1,841) |
>HSF1 nuclear levels in estrogen receptor-positive tumors is associated with poor survival |
Santagata et al. 2011 |
| Breast cancer (n=107) |
Autoantibodies against Hsp60 more frequently found in DCIS (higher grade) and early breast cancer. In tissues: >Hsp60 with increasing stages of carcinogenesis. |
Desmetz et al. 2008 |
| Cholangiocarcinoma | >Hsp27 and Hsp70 bile levels (immunoassay) in intraepithelial neoplasia and in cancer patients (but not in the serum) |
Sato et al. 2012 |
| Colon carcinomas of all stages (n=355) |
>Hsp27 and Hsp70 expression associated with worse clinical outcome |
Bauer et al. 2012 |
| Colon cancer | >Hsp27 (mRNA/protein) in right sided colon cancer (molecular genetic basis for onco- biological difference in left sided colon cancer and right sided colon cancer?) |
Pei et al. 2011 |
| Colorectal cancer (n=112) |
> Hsp60 serum levels (immunoassay) in cancer patients |
Hamelin et al. 2011 |
| Colorectal cancer (n=175) |
>Hsp27 correlated with poor prognosis | Wang et al. 2011 |
| Colorectal cancer (n=182) |
>Hsp27 correlated with poor survival | Yu et al. 2010 |
| Colorectal cancer (n=404 and n=315) |
>Hsp27 correlated with poor survival in rectal cancer BUT not in colon cancer |
Tweedle et al. 2010 |
| Colorectal cancer (n=50) |
Hsp110 up-regulation associated with lymph node involvement. Negative trend of Hsp110 mRNA levels with overall survival |
Slaby et al. 2009 |
| Esophageal cancer (n=124) |
HSP110 correlated inversely with depth of invasion LN metastasis, pathological stage, lymphatic invasion, blood vessel invasion, infiltrative growth pattern and correlated positively with CD4+ T lymphocyte infiltration. <Hsp110 expression correlated with poor prognosis. |
Nakajima et al. 2011 |
| Esophageal cancer (n=125) |
Hsp70 correlated inversely with depth of invasion, pathological stage and blood vessel invasion |
Nakajima et al. 2009 |
| Esophageal adeno- carcinoma (n=34) |
<Hsp27 predicted response to neoadjuvant chemotherapy (platin/5-fluorouracil-based) |
Langer et al. 2008 |
| Head and neck carcinoma |
>Gp96 or Grp78, and <Hsp60, associated with advanced cancer and poor survival |
Chiu et al. 2011 |
| Head and neck SCC (meta-analysis) |
>Hsp27 in cancer, may be a useful biomarker | Norton et al. 2010 |
| Hepatocellular | >miR-17-5p in cancer >p38MAPK/Hsp27 >migration/metastasis |
Yang et al. 2010 |
| Leukemia | >Hsp27, Hsp60, Hsp90α, and HspBP1 (not Hsp70) in the peripheral blood of leukemia patients |
Sedlackova et al. 2011 |
| Lung cancer (non- small cell) (n=109) |
>Hsp27 and Hsp70 serum levels (immunoassay) in cancer patients |
Zimmermann et al. 2012 |
| Lung adenocarcinoma (n=103) |
>Hsp60 expression independent prognostic factor (disease-free survival) |
Xu et al. 2011 |
| Lung adenocarcinoma (n=103) |
Hsp27 did not correlated with clinicopathological parameters and disease prognosis |
Wang et al. 2011b |
| Lung (NSCLC) (n=583) |
The functional HSPB1 promoter -1271G>C variant may affect lung cancer susceptibility and survival by modulating endogenous Hsp27 synthesis levels |
Guo et al. 2010 |
| Melanoma (n=18) | >Hsp105 in melanomas (more than Hsp70) | Park et al. 2009 |
| Melanomas (n=62) | >Hsp105 expression in melanomas, especially with advanced and metastatic lesions |
Muchemwa et al. 2008 |
| Neuroblastomas | >Hsp27 correlated with favorable prognosis (BUT not in Ewing’s sarcoma) |
Zanini et al. 2008 |
| Osteosarcoma (n=73) | Hsp27 and Hsp70 differential markers to distinguish conventional and low grade central osteosarcoma. Hsp27 possible prognostic marker in conventional osteosarcoma. |
Moon et al. 2010 |
| Pancreatic cancer | RP101 (Bromovinyldeoxyuridine, BVDU, Brivudine) binds in vitro to Hsp27 and inhibits interaction with its binding partners, enhancing chemosensitivity |
Heinrich et al 2011 |
| Prostate cancer | >Hsp72 in the serum of cancer patients treated with radiation therapy |
Hurwitz et al. 2010 |
| Uterine cervix SSC and CIN3 |
>Hsp27 expression in CIN3 (92%) and in SCC (100%) |
Tozawa-Ono et al. 2012 |
| Uterine cervix (n≅90) |
Hsp27 related with tumor development and progression |
Ono et al. 2009 |
Notes: CIN: cervical intraepithelial neoplasia; DCIS: ductal carcinoma in situ; SSC: squamous cell carcinoma; NSCLC: non-small cell lung carcinoma
The recent publications affirm and expand the main concepts elucidated in previous review of this area (Ciocca and Calderwood 2005), namely that the HSPs have diagnostic, prognostic, predictive, and treatment implications in cancer depending on the type of malignancy, the HSP analyzed, and the molecular context. Ciocca et al. (2010) reviewed the clinical correlations of HSPs in prostate cancer. Some HSPs appear as interesting serum markers. For example Cappello et al. (2011) have reviewed the implications of Hsp60 in colorectal cancer.
Here we present the molecular contexts by which the HSPs participate in the clinical behavior of cancers.
Carcinogenesis to invasion
HSPs can participate in the initiation and promotion of cancer. Chromium can cause hepatocarcinogenesis and Hsp27 and Hsp70 are among the indicators of the carcinogenic process (Lee and Lim 2012). In this study the authors reported that SJSZ glycoprotein attenuated the activity of Hsp27, Hsp70, PKC, MAPKs, and PCNA in hepatocytes and liver tissue suggesting that SJSZ glycoprotein might be a preventive agent against hepatocarcinogenesis induced by oxidative stress. Epstein-Barr virus infection has been involved at an early stage of gastric carcinogenesis and this viral infection upregulates the phosphorylation of Hsp27 via the PI3K/Akt pathway (Fukagawa et al. 2008). Another oncogenic virus, HPV18, has also been associated with overexpression of Hsp27 (Lo et al. 2007). In a skin cancer experimental model, Hsp27 was identified as one of the proteins implicated during tumor promotion induced by the synthetic pyrethroid insecticide cypermethrin (George et al. 2011).
Alterations in the phosphorylation pathways are among the key molecular lesions that influence the behavior of cancer cells. For example Hsp27 phosphorylation followed by p38 MAPK activation was required for TGF beta1-induced migration of mouse dental papilla-derived MDPC-23 cells (in addition, Hsp27 itself contributed to cell migration) (Kwon et al. 2010). SPARC (secreted protein, acidic and rich in cysteine) modulates cell-matrix interactions influencing cell adhesion and migration. In colorectal cancer stromal SPARC expression inversely correlated with VEGF-A expression and positively correlated with HSP27 expression (Yoshimura et al. 2011). In gliomas, SPARC promotes invasion up-regulating the p38 MAPK/MAPKAPK2/Hsp27 signaling pathway which promotes cancer cell survival by up-regulating pAKT (Hsp27 and AKT interact to regulate the activity of each other) (Golembieski et al. 2008, Schultz et al. 2012, McClung et al. 2012). And there is evidence that down regulation of Hsp27 can cause up-regulation of phosphatase and tensin homologue (PTEN) which has tumor suppressor activity as protein tyrosine phosphatase and as phosphatidylinositol phosphate (PIP) phosphatase (negatively regulating the PI3K/Akt signaling pathway) (Cayado-Gutierrez et al. 2012). These authors have suggested an interaction between Hsp27 and PTEN.
Another example is the influence of the HSPs on the relations of cancer cells and the normal cells that form the niche (Nagaraja et al. 2012). These authors showed that the overexpression of Hsp25/Hsp27 caused repression of normal proteasome function, induced poor antigen presentation, and resulted in increased breast tumor burden. When Hsp27 was decreased, the proteasome activity increased as well as the CD8+ T-cell-mediated tumor killing and the memory responses. In addition the cytokine IFN-gamma suppresses Hsp27 basal transcription and promoter activity enhancing hyperthermia-induced tumor cell death in vitro and tumor suppression in vivo (Oba et al. 2008).
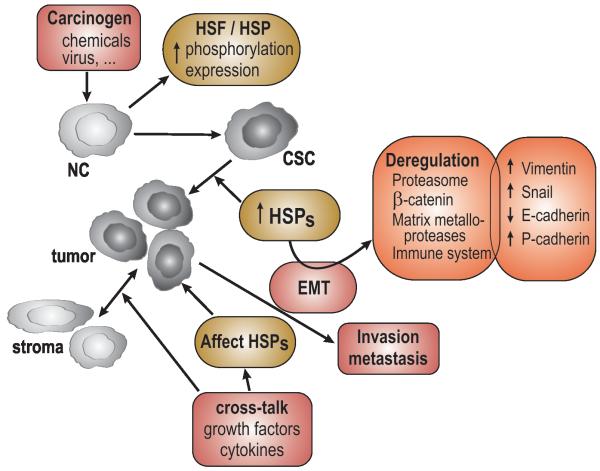
Stem cells
Cancer stem cells (tumor-initiating cells) are responsible to maintain the tumor cell population, are a source of metastasis, and are difficult to kill by anticancer therapies. Increased activation of p38MAPK, MAPKAPK2, and Hsp27 was observed in lung cancer stem cells compared with non-stem control cells and the stem cells exhibited significantly decreased apoptotic response to treatment with superoxide, cisplatin, gemcitabine, or a combination of cisplatin and gemcitabine (Hsu et al. 2011). In breast, high intracellular aldehyde dehydrogenase activity and positivity for CD24-CD44+ helped to identify cancer stem cells, and Hsp27 seems to contribute to the maintenance of these cells (Wei et al. 2011). These authors have shown that knockdown of Hsp27 suppressed epithelial-mesenchymal transition signatures (decreasing the expression of snail and vimentin and increasing the expression of E-cadherin), and the nuclear translocation as well as the activity of NF-κB. The cancer stem cells interact (cross-talk) with the extracellular matrix harboring fibroblasts, endothelial cells and inflammatory cells which provide the adequate niche resulting in the stem cell behaviors: proliferation, differentiation, migration and dormancy (Lander et al. 2012). The cross-talk between the stroma and tumor cells has been shown in a recent study, the presence of caveolin-1 in the tumor microenvironment modulated tumor development, suggesting that stromal caveolin-1 expression may be a potential therapeutic target and a valuable prognostic indicator of breast cancer progression (Sloan et al. 2009). The onset of mammary tumors driven by Her-2/neu expression was accelerated in mice lacking caveolin-1, concomitant with a significant reduction in the extent of apoptosis (Ciocca et al. 2012). The authors reported that Bcl-2, Bax, and survivin levels in the tumors were not different but that the amount of Hsp70 was almost double in the caveolin-1 −/−tumors. In contrast, Hsp27/Hsp25 levels were significantly lower in the caveolin-1 −/− tumors. These results demonstrate that the disruption of the caveolin-1 gene can cause alterations of specific HSPs as well as tumor development.
Figure 3 shows a summary of the implications of the HSF1 / HSP system in the behavior of the cancer cells.
Fig. 3.
Role of HSF1 and HSPs in cancer cell biology. Activation of HSF1 and elevated expression of HSPs may mediate multiple stages in malignant cell development, including normal cell transformation, stemness, EMT, angiogenesis and metastasis. (NC: normal cell; CSC: cancer stem cell; EMT: epithelial mesenchymal transition)
b) Resistance to anticancer therapies
Since HSP members play essential roles in cell survival and preventing misfolding and aggregation of proteins, it may not be surprising that they are involved in the resistance to anticancer therapy.
Chemotherapy
Cancer cells have the potential to acquire resistance to chemotherapy through acquisition of multidrug resistance (MDR). One of the main MDR mechanisms involves the cellular efflux of chemotherapeutic agents mediated by the overexpression of the ATP-Binding Cassette (ABC) transporters: ABCB1/P-glycoprotein (MDR1), the ABCC/multidrug resistance protein (MRP) family, and the ABCG2/breast cancer resistance protein (BCRP) (Takara et al. 2012). One of the first questions here is whether the proteins/genes of the ABC transporter are linked to the HSP response. Indeed, HSF1 is an inducer of the MDR response in cell culture (Tchénio et al. 2006). The induced MDR phenotype occurs in the absence of heat shock or cellular stress, independently from the canonical heat shock-activated mechanism involved in HSP induction, suggesting a novel HSF1 action at the posttranscriptional level. In addition, yeast HSF can activate the expression of PDR3, encoding an MDR transcription factor that also directly activates RPN4 gene expression, which in term encodes a transcription factor that activate the expression of a number of genes encoding proteasome subunits (Hahn et al. 2006). These authors suggest a close linkage between stress responses and pleiotropic drug resistance, at least in yeast due to the overlapping transcriptional regulatory networks involving HSF, Yap1 and Pdr3.
Another link between MDR and HSPs has been reported in doxorubicin-resistant MCF-7 cells (Kanagasabai et al. 2011). In these cells the transcriptional activation HSF1 and expression of HSPs (including Hsp27 which is normally known to augment proteasomal p53 degradation) are inhibited, resulting in the accumulation of transcriptionally active mutant p53. A consequence of mutant p53 accumulation is the induction of the MDR1 gene (directly or NF-κB-dependent). This pathway is abrogated by forced Hsp27 expression.
These mechanisms of drug resistance are independent of the mechanisms implicating HSP overexpression directly with drug resistance (Ciocca et al. 1992). A more recent article has associated Hsp27 with inhibition of cisplatin-induced apoptosis in hepatocellular carcinoma cell death through autophagy activation (Chen et al. 2011). Both Hsp27 and Hsp70 (and other HSPs) can block apoptosis therefore it is complicated to maintain the fine tuning in HSP expression levels to allow the drugs to effectively kill cancer cells. In line with this, Fujita et al (2011) reported in MCF-7 cells that survived to doxorubicin that the POU4F2/Brn-3b transcription factor is increased with concomitant increases in Hsp27 expression. The POU4F2/Brn-3b transcription factor is required to maximally trans-activate Hsp27 and is elevated in >60% of breast cancers altering growth and behavior of cancer cells by regulating distinct subsets of target genes. The authors also reported that the unphosphorylated Hsp27 protein promoted the survival and migration of the breast cancer cells. In a recent study on drug resistance using different cell lines, Skalnikova et al (2011) reported that Rho GDP-dissociation inhibitor 2, Y-box binding protein 1, and the HSP70/90 organizing protein have a role in the resistance to cyclin-dependent kinases inhibitor. Figure 4 shows the main mechanisms implicating the HSP response with resistance to chemotherapy drugs.
Fig. 4.
Potential roles for HSF1 and HSPs in drug resistance of cancer cells. (Left side) HSF1 appears to regulate multiple pathways involved in expression of the multiple drug resistance phenotype (MDR) that leads to escape from chemotherapy mediated cell killing. (Right side) HSPs, most notably HSP27 and Hsp70 can suppress the activity of p53 and reduce apoptosis and senescence associated with transformation as well as therapy.
Many chemotherapeutic drugs are genotoxic through induction of DNA damage. A number of efficient mechanisms exist to correct mutations and prevent the consequences of DNA damage, including: a) direct DNA damage reversal, b) base excision repair, c) mismatch repair, d) nucleotide excision repair and e) homologous and non-homologous recombination. Nadin and Ciocca (2010) have reviewed the participation of HSPs in DNA repair and the implications in cancer therapy and drug sensitivity. Some of the HSPs can enter the nucleus and it is apparent that although the HSPs are not capable of repairing DNA damage independently, they efficiently contribute to DNA repair as part of their molecular chaperone function, interacting positively with DNA repair proteins. Nadin et al. (2007) reported in peripheral blood lymphocytes of cancer patients that the mismatch repair proteins hMLH1 and hMSH2 colocalize with Hsp27 and Hsp72. HSPs accomplished a cytoprotective function in pre-chemotherapy peripheral blood lymphocytes (heat shock before doxorubicin or cisplatin treatment), but not in post-chemotherapy samples. In cisplatin-treated patients the Hsp27 nuclear/cytoplasmic ratio was related to disease free survival and overall survival, and hMSH2 correlated with overall survival. The results point to the utility of these molecules and of the comet assay as possible predictive biomarkers. Table 3 shows recent examples targeting the HSF1-HSP systems for obtaining a more effective anticancer therapy. Recent reviews targeting HSPs and including patents in humans have recently been published (Tutar 2011, Kim and Kim 2011).
Table 3.
Examples of pre-clinical and clinical anticancer therapies targeting the HSF1/HSP system
| Cancer type | Therapy | Effect | Author |
|---|---|---|---|
| Human pancreatic cancer cells, xenograft model |
Triazole nucleoside analog |
⇓ HSF1, Hsp27 Hsp70, Hsp90 |
Xia et al 2012 |
| Melanoma cell lines in vitro and in vivo (vemurafenib-resistant) |
XL888 | Hsp90 inhibitor1 | Paraiso et al. 2012 |
| Human reuroblastoma cell lines and biopsies |
Retinoic acid (⇑ differentiation) |
Nuclear Grp75 binds retinoid receptors |
Shih et al 2012 |
| Glioma cells | Inhibition of HSP27 and pAKT is more effective than temozolomide treatment alone |
⇓ Hsp27 | Schultz et al 2012 |
| Ovarian SK-OV-3 cancer cells |
Melatonin + cisplatin |
Inactivation of ERK/ p90 ribosomal S6 kinase/Hsp27 cascade |
Kim et al. 2012 |
| Colorectal cancer cell/tissues with microsatellite instability carrying mutant Hsp110 |
Oxaliplatin and 5-fluorouracil |
⇓ chaperone activity and antiapoptotic functions of Hsp110 |
Dorard et al. 2011 |
| Phase I (various tumors) |
PF-04929113 | Hsp90 inhibitor2 long-lasting stabilizations |
Rajan et al. 2011 |
| Human bladder cancer KoTCC-1 cells |
siRNA (Hsp70) | gemcitabine plus Hsp70 siRNA: ⇑ cytotoxicity |
Behnsawy et al. 2011 |
| Phase I (recurrent or refractory acute leukemia) |
Tanespimycin + cytarabine |
Hsp90 inhibitor3 | Kaufmann et al. 2011 |
| Human pancreatic HPAF-II carcinoma cells |
Green tea extract | Inhibited Hsp90, Hsp75, Hsp27, Akt activation, mp53 |
Zhang et al. 2011a |
| Human HCT-116 colon cancer cell xenograft |
KRIBB11 (⇓ Hsp70) | ⇓ HSF1 ⇓ tumor growth |
Yoon et al. 2011 |
| Phase I (various tumors) |
Tanespimicin + sorafenib (Raf kinase inhibitor) |
Hsp90 inhibitor Clinical response |
Vaishampayan et al. 2010 |
| Phase I (leukaemia) | Alvespimycin | Hsp90 inhibitor | Lancet et al. 2010 |
| TRAIL-resistant human lung adenocarcinoma A549 cell line |
siRNA (Hsp27) + TRAIL |
TRAIL-induced apoptosis |
Zhuang et al 2010 |
The reversal of the resistance phenotype was associated with the degradation of PDGFRβ, COT, IGFR1, CRAF, ARAF, S6, cyclin D1, and AKT, which in turn led to the nuclear accumulation of FOXO3a, an increase in BIM (Bcl-2 interacting mediator of cell death) expression, and the downregulation of Mcl-1.
Although no clinically significant drug-related ocular toxicity was seen in this study, the development of PF-04929113 has been discontinued because of ocular toxicity seen in animal models and in a separate phase I study.
Because exposure to potentially effective concentrations occurs only for a brief time in vivo, at clinically tolerable doses tanespimycin has little effect on resistance-mediating client proteins in relapsed leukemia and exhibits limited activity in combination with cytarabine.
Radiotherapy
The integrity of p53 is essential for damaged cells to enter apoptosis and senescence and we mentioned earlier that HSPs mediate resistance to these processes. Moreover, Hsp27 can augment proteasomal p53 degradation while Hsp90 is a key molecular chaperone of several pro-oncogenic pathways including Akt, Raf-1, and mutated p53. Thus cancer cells overexpressing Hsp27, Hsp70, Hsp90 and decrease wild type p53 are prone to be resistant to radiotherapy. Indeed, Hunt et al (2004) showed increased levels of spontaneous and radiation-induced chromosomal aberrations in mouse fibroblasts with hsp70.1 and hsp70.2 knockout, suggesting these proteins to be involved in maintaining genome stability. Hsp27 is significantly up-regulated in patients with radioresistant nasopharyngeal carcinomas (Wu et al. 2012). Cancer cells treated with antisense Hsp27 cDNA or with anti Hsp27 peptide had a significantly increased sensitivity to radiation, with concomitant decrease in glutathione basal levels which are associated with detoxification of reactive oxygen species (Teimourian et al. 2006, Aloy et al. 2008, Lee at al. 2011). Hadchity et al. (2009) showed in vivo that targeting Hsp27 (which induced Akt inactivation) can cause radiation sensitization of head and neck squamous cell carcinoma. This is in agreement with the results reported by Choi et al. (2011) in human non-small lung cancer xenografts. Finally, radioresistant head and neck cancer sublines (established by fractionated irradiation) showed up-regulation of Gp96, Grp78, Hsp60 and that Gp96 knockdown enhanced the in vitro and in vivo radiosensitivity (Lin et al. 2010).
On the other hand, a balance in HSP expression levels appears important to obtain effective cancer cell killing. For example, heat shock and ionizing radiation can increase the susceptibility of cancer cells to NK cell-mediated cytotoxicity (Kim et al. 2006). These authors reported that heat shock and ionizing radiation enhanced NKG2D ligands (rendering target cells sensitive to NK cell attack) while Hsp70 was induced by heat shock but not by ionizing radiation. Thus the induced Hsp70 might be helping to present the tumor associated antigens (TAAs) of the damaged cells but that the levels should not be so high to prevent apoptosis or senescence. In addition, heat shock treatment can induce Hsp70 which interacts with the catalytic unit of telomerase (hTERT) extending the life span of the cancer cells. Thus inactivation of telomerase before combined hyperthermia and radiotherapy could improve tumor cell killing (Agarwal et al. 2008). Laszlo et al (2009) reported that Hsc70 modulates the magnitude of heat radiosensitization. Ionizing radiation can kill cancer cells through producing DNA damage and HSPs can counteract this effect improving several DNA repair mechanisms. Fig. 5 shows a summary of the main mechanisms of radiation resistance due to the HSP response.
Fig. 5.
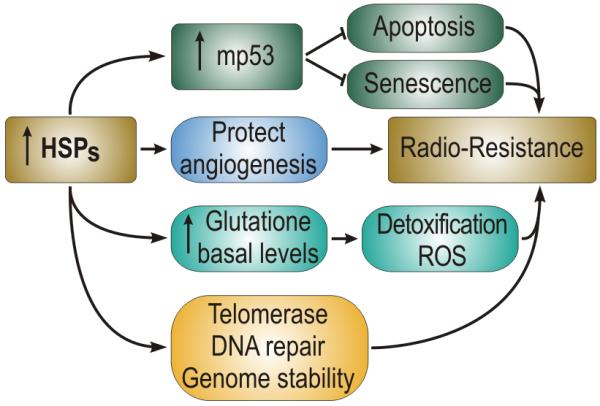
Potential role for HSPs in the resistance to radiation therapy, through: (1) mediation of p53 expression, (2) fostering of angiogenesis and oxygen supply, (3) elevation in glutathione and reduction in oxidative stress and (4) fostering of telomerase and reduction in chromosome damage.
Hormonotherapy
Prostate and breast cancer are among the most frequent malignancies in humans, are often hormone-dependent and thus responsive to anti-hormonal therapies. One might ask therefore, do HSPs have a role in hormone pathways? The answer appears to be yes, as HSPs are involved in the folding, activation, trafficking and transcriptional activity of most steroid receptors (Tao and Zheng 2011). Hsp27 is important for estrogen receptor alpha trafficking and functioning at the plasma membrane (Radanzi et al. 2010). An important question now is therefore whether HSPs are involved in resistance to hormonotherapy? This appears to be the case, at least in prostate cancer (Table 4) (Ciocca et al. 2010).
Table 4.
Examples where the HSPs are implicated in hormonotherapy
| Cancer type | Treatment | Effect/Observation | Author |
|---|---|---|---|
| Human prostate LNCaP/ PC-3 cancer cells |
Modulation Hsp27 expression |
Modulation of androgen receptor |
Stope et al. 2012 |
| Castrate-resistant prostate cancer xenograft |
OGX-011 + PF-04929113 or 17-AAG |
⇓ clusterin/HSPs ⇓ Akt, PSA ⇓ tumor growth |
Lamoureux et al. 2011 |
| Prostate cancer | Androgen ablation | >Hsp60 associated with early onset of hormone refractory disease and reduced survival |
Castilla et al 2010 |
| Prostate cancer cells and tissues |
p90Rsk inhibition (siRNA) |
<IGF-I-induced Hsp27 phospho- rylation |
Zoubeidi et al. 2010 |
| Breast cancer cells | Heregulin | >HSF1/HSPs, HSF1 associates with MTA1 and HDAC repressing estrogen- dependent transcription |
Khaleque et al. 2008 |
Note: OGX-011 is an antisense drug that targets clusterin, while PF-04929113 and 17-AAG are Hsp90 inhibitors; PSA: prostate specific antigen; HDAC: histone deacetylase; MTA1: metastasis-associated protein 1.
Regarding breast cancer, using tamoxifen-resistant cancer cells, Giordano et al. (2011) showed that the activation of the farnesoid X receptor (FXR) by the primary bile acid chenodeoxycholic acid (CDCA) or the synthetic agonist GW4064, inhibited the growth of the tumor cells, through downregulation of Her-2/neu expression. This shows that Her-2/neu is an important mediator of resistance to endocrine therapy with tamoxifen, which has been shown in the clinic (Ciocca et al. 2006). The interesting relations between Her-2/neu with the HSF1/HSP response are beginning to be elucidated. Zhang et al. (2007) reported that the phosphorylation of Ser78 of Hsp27 correlated with Her-2/neu status and lymph node positivity in Her-2/neu positive breast cancers. Moreover, Kang et al. (2008) reported that upregulated Hsp27 in human breast cancer cells reduces trastuzumab (Herceptin) susceptibility by increasing Her-2/neu protein stability. The cell surface tyrosine kinase receptors c-erbB-3 and c-erbB-4 (once activated, for example with heregulin) form dimers with the “ligandless” Her-2/neu. Heregulin has been found to be a potent inducer of HSF1 activity and of HSP synthesis in breast cancer cells, and HSF1 activation plays a role in the tumorigenic changes induced by heregulin (Khaleque et al. 2008). In addition, HSF1 associates with metastasis associated protein 1 (MTA1) on the promoters of genes as well as other molecules involved in gene repression (HDAC1, HDAC2) in a manner that is enhanced by either heregulin or heat shock. Estrogen receptors, although promoting the growth of breast cancer cells are less associated with invasion/metastasis and estrogen receptor-induced gene expression is involve in this effect. Heregulin can overcome the protective effects of estrogen receptors and at least a component of this appears to be due to MTA1 repression of estrogen regulated element dependent transcription, HSF1 and MTA1 cooperate in gene repression. These two proteins are co-expressed in human breast cancer biopsy samples (Khaleque et al. 2008).
Immunotherapy
There is consensus that as part of their molecular chaperone function, HSPs can bind and present tumor associated antigens to professional antigen presenting cells through MHC class I and class II molecules. Therefore, HSPs are being exploited as important anticancer vaccine adjuvants for the activation of anti-tumor CD8+ and CD4+ T cells. This topic has been reviewed recently (Ciocca et al. 2012). On the other hand, HSPs through their chaperone function may increase the stability of proteins that are target of antibodies interfering with the anticancer effect (Kang et al. 2008).
Conclusions
The HSF/HSP system is thus implicated in many crucial steps in tumorogenesis, being associated with a disparate range of tumor initiators and promoters. This system has thus emerged as a source for potential biomarkers of cell transformation and tumorogenesis. HSP expression levels may be useful in characterizing certain cancer cells, and cancer cell types/subtypes. However, the HSP response in cancer is very versatile and can change in different malignant tissues and in individual tumor cell populations (tumor cell heterogeneity). As a consequence, there can be confusion regarding the role(s) that the HSPs play under individual circumstances. HSPs can appear as biomarkers of cell differentiation (good and poor), markers of cancer stem cells, or indicators of disease prognosis (good and poor). In addition, the results obtained so far strongly indicate that the HSF/HSP system is implicated in the response of cancer cells/tissues to anticancer therapy.
The challenges now are multiple. Firstly it remains to be convincingly determined if the HSF/HSP system is useful as a source of molecular markers to predict which cancer type and patient in particular will be most benefit with an individualized anticancer strategy. However, this is not an easy task, and in the studies both of HSPs and of other molecular markers there have been contradictory findings. For example, Hsp27 overexpression in pancreatic cancer cells has been linked with increased sensitivity to gemcitabine (Schäfer et al. 2011) and with increase resistance to the same drug (Mori-Iwamoto et al. 2007), opening the door to the study of the phosphorylation status of Hsp27 to determine its role in gemcitabine-induced growth suppression of pancreatic cancer (Nakashima et al. 2011). Another challenge is to design the most appropriate approach to pharmacologically perturb the HSF/HSP system in order to promote cancer cell killing while minimizing effects on the normal cell. Finally, understanding mechanisms whereby the HSF1 and HSP transcriptional system is activated in cancer may permit rational approaches to therapy based on this target.
Acknowledgments
SKC was supported by NIH research grants RO-1CA047407, R0-1CA119045 and RO-1CA094397. DRC: Agencia Nacional de Promoción Científica y Tecnológica (PICT 1047, 2007, BID), CONICET (PIP 2428), and the Argentina Foundation for Cancer Research.
References
- Agarwal M, Pandita S, Hunt CR, Gupta A, Yue X, Khan S, Pandita RK, Pratt D, Shay JW, Taylor JS, Pandita TK. Inhibition of telomerase activity enhances hyperthermia-mediated radiosensitization. Cancer Res. 68:3370–3378. doi: 10.1158/0008-5472.CAN-07-5831. [DOI] [PubMed] [Google Scholar]
- Aghdassi A, Phillips P, Dudeja V, et al. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res. 2007;67(2):616–625. doi: 10.1158/0008-5472.CAN-06-1567. [DOI] [PubMed] [Google Scholar]
- Ahmad MF, Raman B, Ramakrishna T, Rao Ch M. Effect of phosphorylation on alpha B-crystallin: differences in stability, subunit exchange and chaperone activity of homo and mixed oligomers of alpha B-crystallin and its phosphorylation-mimicking mutant. J Mol Biol. 2008;375(4):1040–1051. doi: 10.1016/j.jmb.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Al-Madhoun AS, Chen YX, Haidari L, et al. The interaction and cellular localization of HSP27 and ERbeta are modulated by 17beta-estradiol and HSP27 phosphorylation. Mol Cell Endocrinol. 2007;270(1-2):33–42. doi: 10.1016/j.mce.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Aloy MT, Hadchity E, Bionda C, Diaz-Latoud C, Claude L, Rousson R, Arrigo AP, Rodriguez-Lafrasse C. Protective role of Hsp27 protein against gamma radiation-induced apoptosis and radiosensitization effects of Hsp27 gene silencing in different human tumor cells. Int J Radiat Oncol Biol Phys. 2008;70:543–553. doi: 10.1016/j.ijrobp.2007.08.061. [DOI] [PubMed] [Google Scholar]
- Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3(1):46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- Arteaga CL. Why is this effective HSP90 inhibitor not being developed in HER2+ breast cancer? Clin Cancer Res. 2011;17(15):4919–4921. doi: 10.1158/1078-0432.CCR-11-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrieu C, Taieb D, Baylot V, et al. Heat shock protein 27 confers resistance to androgen ablation and chemotherapy in prostate cancer cells through eIF4E. Oncogene. 2010;29(13):1883–1896. doi: 10.1038/onc.2009.479. [DOI] [PubMed] [Google Scholar]
- Aoyama A, Frohli E, Schafer R, Klemenz R. Alpha B-crystallin expression in mouse NIH 3T3 fibroblasts: glucocorticoid responsiveness and involvement in thermal protection. Mol Cell Biol. 1993;13(3):1824–1835. doi: 10.1128/mcb.13.3.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquilina JA, Benesch JL, Ding LL, Yaron O, Horwitz J, Robinson CV. Phosphorylation of alphaB-crystallin alters chaperone function through loss of dimeric substructure. J Biol Chem. 2004;279(27):28675–28680. doi: 10.1074/jbc.M403348200. [DOI] [PubMed] [Google Scholar]
- Arispe N, Doh M, Simakova O, Kurganov B, De Maio A. Hsc70 and Hsp70 interact with phosphatidylserine on the surface of PC12 cells resulting in a decrease of viability. Faseb J. 2004;18(14):1636–1645. doi: 10.1096/fj.04-2088com. [DOI] [PubMed] [Google Scholar]
- Arrigo AP. sHsp as novel regulators of programmed cell death and tumorigenicity. Pathol Biol (Paris) 2000;48(3):280–288. [PubMed] [Google Scholar]
- Arrigo AP. Hsp27: novel regulator of intracellular redox state. IUBMB Life. 2001;52(6):303–307. doi: 10.1080/152165401317291156. [DOI] [PubMed] [Google Scholar]
- Arrigo AP. In search of the molecular mechanism by which small stress proteins counteract apoptosis during cellular differentiation. J Cell Biochem. 2005;94(2):241–246. doi: 10.1002/jcb.20349. [DOI] [PubMed] [Google Scholar]
- Arrigo AP. Structure-functions of HspB1 (Hsp27) Methods Mol Biol. 2011;787:105–119. doi: 10.1007/978-1-61779-295-3_9. [DOI] [PubMed] [Google Scholar]
- Barbash O, Lin DI, Diehl JA. SCF Fbx4/alphaB-crystallin cyclin D1 ubiquitin ligase: a license to destroy. Cell Div. 2007;2:2. doi: 10.1186/1747-1028-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer K, Nitsche U, Slotta-Huspenina J, Drecoll E, von Weyhern CH, Rosenberg R, Höfler H, Langer R. High HSP27 and HSP70 expression levels are independent adverse prognostic factors in primary resected colon cancer. Cell Oncol (Dordr) 2012 Apr 26; doi: 10.1007/s13402-012-0079-3. 2012. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Bausero MA, Bharti A, Page DT, et al. Silencing the hsp25 gene eliminates migration capability of the highly metastatic murine 4T1 breast adenocarcinoma cell. Tumour Biol. 2006;27(1):17–26. doi: 10.1159/000090152. Epub 2005 Dec 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausero MA, Page DT, Osinaga E, Asea A. Surface expression of Hsp25 and Hsp72 differentially regulates tumor growth and metastasis. Tumour Biol. 2004;25(5-6):243–251. doi: 10.1159/000081387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beere HM, Wolf BB, Cain K, et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the apaf-1 apoptosome. Nat Cell Biol. 2000;2(8):469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- Behnsawy HM, Miyake H, Kusuda Y, Fujisawa M. Small interfering RNA targeting heat shock protein 70 enhances chemosensitivity in human bladder cancer cells. Urol Oncol. 2011 Sep 1; doi: 10.1016/j.urolonc.2011.07.007. 2011. [Epub ahead of print]. PMID: 21889367. [DOI] [PubMed] [Google Scholar]
- Bellyei S, Szigeti A, Pozsgai E, et al. Preventing apoptotic cell death by a novel small heat shock protein. Eur J Cell Biol. 2007;86(3):161–171. doi: 10.1016/j.ejcb.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Beresford PJ, Jaju M, Friedman RS, Yoon MJ, Lieberman J. A role for heat shock protein 27 in CTL-mediated cell death. J Immunol. 1998;161(1):161–167. [PubMed] [Google Scholar]
- Bhat SP, Nagineni CN. αB subunit of lens-specific protein α-cristallin is present in other ocular and non-ocular tissues. Bioch Biophys Res Commun. 1989;158(1):319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- Blackburn RV, Galoforo SS, Berns CM, et al. comparison of tumor growth between Hsp25- and Hsp27-transfected murine L929 cells in nude mice. Int J Cancer. 1997;72:871–877. doi: 10.1002/(sici)1097-0215(19970904)72:5<871::aid-ijc26>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Hsp-90-associated oncoproteins: multiple targets of geldanamycin and its analogs. Leukemia. 2002;16(4):455–462. doi: 10.1038/sj.leu.2402415. [DOI] [PubMed] [Google Scholar]
- Boelens WC, Croes Y, de Jong WW. Interaction between alphaB-crystallin and the human 20S proteasomal subunit C8/alpha7. Biochim Biophys Acta. 2001;1544(1-2):311–319. doi: 10.1016/s0167-4838(00)00243-0. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, Bonniaud P, et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000a;2(9):645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Paul C, Fromentin A, et al. Differential regulation of HSP27 oligomerization in tumor cells grown in vitro and in vivo. Oncogene. 2000b;19(42):4855–4863. doi: 10.1038/sj.onc.1203850. [DOI] [PubMed] [Google Scholar]
- Bruinsma IB, Bruggink KA, Kinast K, et al. Inhibition of alpha-synuclein aggregation by small heat shock proteins. Proteins. 2011;79(10):2956–2967. doi: 10.1002/prot.23152. [DOI] [PubMed] [Google Scholar]
- Brunet Simioni M, De Thonel A, Hammann A, et al. Heat shock protein 27 is involved in SUMO-2/3 modification of heat shock factor 1 and thereby modulates the transcription factor activity. Oncogene. 2009;28:3332–3344. doi: 10.1038/onc.2009.188. [DOI] [PubMed] [Google Scholar]
- Buchner J. Hsp90 & Co. - a holding for folding. Trends Biochem Sci. 1999;24(4):136–141. doi: 10.1016/s0968-0004(99)01373-0. [DOI] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92(3):351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Cai H, Yin S, Ma F, et al. ShRNA-mediated gene silencing of Heat shock protein 70 inhibits human colon cancer cell growth in vitro and in vivo. Mol Cell Biochem. 2011 Jul 26; doi: 10.1007/s11010-011-0990-3. 2011. [Epub ahead of print] PMID: 21789515. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, et al. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31(3):164–72. doi: 10.1016/j.tibs.2006.01.006. Epub 2006 Feb 17. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, et al. Signal Transduction Pathways Leading to Heat Shock Transcription. Sign Transduct Insights. 2010;2:13–24. doi: 10.4137/STI.S3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello F, Bellafiore M, Palma A, et al. 60KDa chaperonin (HSP60) is over-expressed during colorectal carcinogenesis. Eur J Histochem. 2003;47(2):105–110. doi: 10.4081/814. [DOI] [PubMed] [Google Scholar]
- Cappello F, Conway de Macario E, Marasa L, Zummo G, Macario AJ. Hsp60 expression, new locations, functions and perspectives for cancer diagnosis and therapy. Cancer Biol Ther. 2008;7(6):801–809. doi: 10.4161/cbt.7.6.6281. [DOI] [PubMed] [Google Scholar]
- Cappello F, Czarnecka AM, La Rocca G, Di Stefano A, Zummo G, Macario AJ. Hsp60 and Hspl0 as antitumor molecular agents. Cancer Biol Ther. 2007;6(4):487–489. doi: 10.4161/cbt.6.4.4087. [DOI] [PubMed] [Google Scholar]
- Cappello F, David S, Peri G, Farina F, Conway de Macario E, Macario AJ, Zummo G. Hsp60: molecular anatomy and role in colorectal cancer diagnosis and treatment. Front Biosci (Schol Ed) 2011;3:341–351. doi: 10.2741/s155. [DOI] [PubMed] [Google Scholar]
- Carra S. The stress-inducible HspB8-Bag3 complex induces the eIF2alpha kinase pathway: implications for protein quality control and viral factory degradation? Autophagy. 2009;5(3):428–429. doi: 10.4161/auto.5.3.7894. [DOI] [PubMed] [Google Scholar]
- Carra S, Seguin SJ, Landry J. HspB8 and Bag3: a new chaperone complex targeting misfolded proteins to macroautophagy. Autophagy. 2008;4(2):237–239. doi: 10.4161/auto.5407. [DOI] [PubMed] [Google Scholar]
- Carra S, Sivilotti M, Chavez Zobel AT, Lambert H, Landry J. HspB8, a small heat shock protein mutated in human neuromuscular disorders, has in vivo chaperone activity in cultured cells. Hum Mol Genet. 2005;14(12):1659–269. doi: 10.1093/hmg/ddi174. [DOI] [PubMed] [Google Scholar]
- Castilla C, Congregado B, Conde JM, Medina R, Torrubia FJ, Japón MA, Sáez C. Immunohistochemical expression of Hsp60 correlates with tumor progression and hormone resistance in prostate cancer. Urology. 2010;76:1017.e1–6. doi: 10.1016/j.urology.2010.05.045. [DOI] [PubMed] [Google Scholar]
- Cayado-Gutiérrez N, Moncalero VL, Rosales EM, Berón W, Salvatierra EE, Radrizzani M, Ciocca DR. Down-regulation of Hsp27 (HSPB1) in MCF-7 human breast cancer cells induces up-regulation of PTEN. Cell Stress and Chaperones. 2012 doi: 10.1007/s12192-012-0367-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra D, Choy G, Tang DG. Cytosolic accumulation of HSP60 during apoptosis with or without apparent mitochondrial release: evidence that its pro-apoptotic or pro-survival functions involve differential interactions with caspase-3. J Biol Chem. 2007;282(43):31289–31301. doi: 10.1074/jbc.M702777200. [DOI] [PubMed] [Google Scholar]
- Chalmin F, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charette SJ, Landry J. The interaction of HSP27 with Daxx identifies a potential regulatory role of HSP27 in Fas-induced apoptosis. Ann N Y Acad Sci. 2000;926:126–131. doi: 10.1111/j.1749-6632.2000.tb05606.x. [DOI] [PubMed] [Google Scholar]
- Charette SJ, Lavoie JN, Lambert H, Landry J. Inhibition of daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol. 2000;20(20):7602–7612. doi: 10.1128/mcb.20.20.7602-7612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebotareva NA, Makeeva VF, Bazhina SG, Eronina TB, Gusev NB, Kurganov BI. Interaction of Hsp27 with native phosphorylase kinase under crowding conditions. Macromol Biosci. 2010;10(7):783–789. doi: 10.1002/mabi.200900397. [DOI] [PubMed] [Google Scholar]
- Chen R, Dai RY, Duan CY, Liu YP, Chen SK, Yan DM, Chen CN, Wei M, Li H. Unfolded protein response suppresses cisplatin-induced apoptosis via autophagy regulation in human hepatocellular carcinoma cells. Folia Biol (Praha) 2011;57:87–95. [PubMed] [Google Scholar]
- Chiosis G, Tao H. Purine-scaffold Hsp90 inhibitors. IDrugs. 2006;9:778–782. [PubMed] [Google Scholar]
- Chiu CC, Lin CY, Lee LY, Chen YJ, Lu YC, Wang HM, Liao CT, Chang JT, Cheng AJ. Molecular chaperones as a common set of proteins that regulate the invasion phenotype of head and neck cancer. Clin Cancer Res. 2011;17:4629–4641. doi: 10.1158/1078-0432.CCR-10-2107. [DOI] [PubMed] [Google Scholar]
- Choi SH, Lee YJ, Seo WD, Lee HJ, Nam JW, Lee YJ, Kim J, Seo EK, Lee YS. Altered cross-linking of HSP27 by zerumbone as a novel strategy for overcoming HSP27-mediated radioresistance. Int J Radiat Oncol Biol Phys. 79:1196–1205. doi: 10.1016/j.ijrobp.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Chu B, et al. Sequential phosphorylation by mitogen-activated protein kinase and glycogen synthase kinase 3 represses transcriptional activation by heat shock factor-1. J Biol Chem. 1996;271:30847–30857. doi: 10.1074/jbc.271.48.30847. [DOI] [PubMed] [Google Scholar]
- Cintron NS, Toft D. Defining the requirements for Hsp40 and Hsp70 in the Hsp90 chaperone pathway. J Biol Chem. 2006;281(36):26235–46224. doi: 10.1074/jbc.M605417200. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10(2):86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocca DR, Fuqua SAW, Lock -Lim S, Toft DO, Welch WJ, Mc Guire WL. Response of human breast cancer cells to heat shock and chemotherapeutic drugs. Cancer Res. 1992;52:3648–3654. [PubMed] [Google Scholar]
- Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocca DR, Gago FE, Fanelli MA, Calderwood SK. Co-expression of steroid receptors (estrogen receptor alpha and/or progesterone receptors) and Her-2/neu: Clinical implications. J Steroid Biochem Mol Biol. 2006;102:32–40. doi: 10.1016/j.jsbmb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Fanelli MA, Cuello-Carrion FD, Castro GN. Heat shock proteins in prostate cancer: from tumorigenesis to the clinic. Int J Hyperthermia. 2010;26:737–747. doi: 10.3109/02656731003776968. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Cuello-Carrión FD, Natoli AL, Restall C, Anderson RL. Absence of caveolin-1 alters heat shock protein expression in spontaneous mammary tumors driven by Her-2/neu expression. Histochem Cell Biol. 2012;137:187–194. doi: 10.1007/s00418-011-0879-y. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Cayado-Gutiérrez N, Maccioni M, Cuello-Carrión FD. Heat Shock Proteins (HSPs) based anti-cancer vaccines. Current Mol Med. 2012 doi: 10.2174/156652412803306684. in press. [DOI] [PubMed] [Google Scholar]
- Colas P, Cohen B, Jessen T, Grishina I, McCoy J, Brent R. Genetic selection of peptide aptamers that recognize and inhibit cyclin-dependent kinase 2. Nature. 1996;380:548–550. doi: 10.1038/380548a0. [DOI] [PubMed] [Google Scholar]
- Craig EA. The heat shock response. CRC Crit Rev Biochem. 1985;18:239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Cuesta R, Laroia G, Schneider RJ. Chaperone Hsp27 inhibits translation during heat shock by binding eIF4G and facilitating dissociation of cap-initiation complexes. Genes Dev. 2000;14(12):1460–1470. [PMC free article] [PubMed] [Google Scholar]
- Dai C, et al. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Jiang L, Wang G, et al. HSP70 interacts with TRAF2 and differentially regulates TNFalpha signalling in human colon cancer cells. J Cell Mol Med. 2010;14(3):710–725. doi: 10.1111/j.1582-4934.2009.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Era MA, Oudes A, Martin DB, Liu AY. HSP27 and HSP70 interact with CD10 in C4-2 prostate cancer cells. Prostate. 2007;67(7):714–721. doi: 10.1002/pros.20558. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Milzani A, Di Simplicio P, Colombo R. The actin cytoskeleton response to oxidants: from small heat shock protein phosphorylation to changes in the redox state of actin itself. Free Radic Biol Med. 2001;31(12):1624–1632. doi: 10.1016/s0891-5849(01)00749-3. [DOI] [PubMed] [Google Scholar]
- Daugaard M, Jaattela M, Rohde M. Hsp70-2 is required for tumor cell growth and survival. Cell Cycle. 2005;4(7):877–880. doi: 10.4161/cc.4.7.1838. [DOI] [PubMed] [Google Scholar]
- Daugaard M, Kirkegaard-Sorensen T, Ostenfeld MS, et al. Lens epithelium-derived growth factor is an Hsp70-2 regulated guardian of lysosomal stability in human cancer. Cancer Res. 2007a;67(6):2559–2567. doi: 10.1158/0008-5472.CAN-06-4121. [DOI] [PubMed] [Google Scholar]
- Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007b;581(19):3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- De Bessa SA, Salaorni S, Patrao DF, Neto MM, Brentani MM, Nagai MA. JDP1 (DNAJC12/Hsp40) expression in breast cancer and its association with estrogen receptor status. Int J Mol Med. 2006;17(2):363–367. [PubMed] [Google Scholar]
- De Chassey B, Mikaelian I, Mathieu AL, et al. An antiproliferative genetic screening identifies a peptide aptamer that targets calcineurin and upregulates its activity. Mol Cell Proteomics. 2006;4:4. doi: 10.1074/mcp.M600102-MCP200. [DOI] [PubMed] [Google Scholar]
- De Thonel A, Vandekerckhove J, Lanneau D, et al. HSP27 controls GATA-1 protein level during erythroid cell differentiation. Blood. 2010;116(1):85–96. doi: 10.1182/blood-2009-09-241778. [DOI] [PubMed] [Google Scholar]
- den Engelsman J, Keijsers V, de Jong WW, Boelens WC. The small heat-shock protein alpha B-crystallin promotes FBX4-dependent ubiquitination. J Biol Chem. 2003;278(7):4699–4704. doi: 10.1074/jbc.M211403200. [DOI] [PubMed] [Google Scholar]
- Deng M, Chen PC, Xie S, et al. The small heat shock protein alphaA-crystallin is expressed in pancreas and acts as a negative regulator of carcinogenesis. Biochim Biophys Acta. 2010;1802(7-8):621–631. doi: 10.1016/j.bbadis.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Desmetz C, Bibeau F, Boissière F, Bellet V, Rouanet P, Maudelonde T, Mangé A, Solassol J. Proteomics-based identification of HSP60 as a tumor-associated antigen in early stage breast cancer and ductal carcinoma in situ. J Proteome Res. 2008;7:3830–3837. doi: 10.1021/pr800130d. [DOI] [PubMed] [Google Scholar]
- Diaz-Latoud C, Buache E, Javouhey E, Arrigo AP. Substitution of the unique cysteine residue of murine hsp25 interferes with the protective activity of this stress protein through inhibition of dimer formation. Antioxid Redox Signal. 2005;7(3-4):436–445. doi: 10.1089/ars.2005.7.436. [DOI] [PubMed] [Google Scholar]
- Dohi T, Beltrami E, Wall NR, Plescia J, Altieri DC. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest. 2004;114(8):1117–1127. doi: 10.1172/JCI22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorard C, de Thonel A, Collura A, Marisa L, Svrcek M, Lagrange A, Jego G, Wanherdrick K, Joly AL, Buhard O, Gobbo J, Penard-Lacronique V, Zouali H, Tubacher E, Kirzin S, Selves J, Milano G, Etienne-Grimaldi MC, Bengrine-Lefèvre L, Louvet C, Tournigand C, Lefèvre JH, Parc Y, Tiret E, Fléjou JF, Gaub MP, Garrido C, Duval A. Expression of a mutant HSP110 sensitizes colorectal cancer cells to chemotherapy and improves disease prognosis. Nat Med. 2011;17:1283–1289. doi: 10.1038/nm.2457. [DOI] [PubMed] [Google Scholar]
- Dudeja V, et al. Prosurvival role of heat shock factor 1 in the pathogenesis of pancreatobiliary tumors. Am J Physiol Gastrointest Liver Physiol. 2011;300:G948–955. doi: 10.1152/ajpgi.00346.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R, Inouye M. GHKL, an emergent ATPase/kinase superfamily. Trends Biochem Sci. 2000;25(1):24–28. doi: 10.1016/s0968-0004(99)01503-0. [DOI] [PubMed] [Google Scholar]
- Eccles SA, et al. NVP-AUY922: a novel heat shock protein 90 inhibitor active against xenograft tumor growth, angiogenesis, and metastasis. Cancer Res. 2008;68:2850–2860. doi: 10.1158/0008-5472.CAN-07-5256. [DOI] [PubMed] [Google Scholar]
- Ellis RJ. Molecular chaperones: assisting assembly in addition to folding. Trends Biochem Sci. 2006;31:395–401. doi: 10.1016/j.tibs.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Erlichman C. Tanespimycin: the opportunities and challenges of targeting heat shock protein 90. Expert Opin Investig Drugs. 2009;18(6):861–868. doi: 10.1517/13543780902953699. [DOI] [PubMed] [Google Scholar]
- Fanelli MA, Montt-Guevara M, Diblasi AM, et al. P-cadherin and beta-catenin are useful prognostic markers in breast cancer patients; beta-catenin interacts with heat shock protein Hsp27. Cell Stress Chaperones. 2008;13(2):207–220. doi: 10.1007/s12192-007-0007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felts SJ, Owen BA, Nguyen P, Trepel J, Donner DB, Toft DO. The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties. J Biol Chem. 2000;275(5):3305–3312. doi: 10.1074/jbc.275.5.3305. [DOI] [PubMed] [Google Scholar]
- Fishel R, et al. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993;75:1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- Fourie AM, Hupp TR, Lane DP, et al. HSP70 binding sites in the tumor suppressor protein p53. J Biol Chem. 1997;272(31):19471–19479. doi: 10.1074/jbc.272.31.19471. [DOI] [PubMed] [Google Scholar]
- Fortugno P, Beltrami E, Plescia J, et al. Regulation of survivin function by Hsp90. Proc Natl Acad Sci U S A. 2003;100(24):13791–13796. doi: 10.1073/pnas.2434345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Liang JJ. Enhanced stability of alpha B-crystallin in the presence of small heat shock protein Hsp27. Biochem Biophys Res Commun. 2003;302(4):710–714. doi: 10.1016/s0006-291x(03)00257-2. [DOI] [PubMed] [Google Scholar]
- Fujita R, Ounzain S, Wang AC, Heads RJ, Budhram-Mahadeo VS. Hsp-27 induction requires POU4F2/Brn-3b TF in doxorubicin-treated breast cancer cells, whereas phosphorylation alters its cellular localisation following drug treatment. Cell Stress Chaperones. 2011;16:427–439. doi: 10.1007/s12192-011-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukagawa Y, Nishikawa J, Kuramitsu Y, Iwakiri D, Takada K, Imai S, Satake M, Okamoto T, Fujimoto M, Okita K, Nakamura K, Sakaida I. Epstein-Barr virus upregulates phosphorylated heat shock protein 27 kDa in carcinoma cells using the phosphoinositide 3-kinase/Akt pathway. Electrophoresis. 2008;29:3192–3200. doi: 10.1002/elps.200800086. [DOI] [PubMed] [Google Scholar]
- Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9(6):447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- Gabai VL, Sherman MY. Hsp72 and cell Signaling. Cambdridge Univ. Press; Cambridge: 2005. [Google Scholar]
- Gabai VL, et al. Heat shock protein Hsp72 controls oncogene-induced senescence pathways in cancer cells. Mol Cell Biol. 2009;29:559–569. doi: 10.1128/MCB.01041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganea E. Chaperone-like activity of alpha-crystallin and other small heat shock proteins. Curr Protein Pept Sci. 2001;2(3):205–225. doi: 10.2174/1389203013381107. [DOI] [PubMed] [Google Scholar]
- Gao Y, Han C, Huang H, et al. Heat shock protein 70 together with its co-chaperone CHIP inhibits TNF-alpha induced apoptosis by promoting proteasomal degradation of apoptosis signal-regulating kinase1. Apoptosis. 2010;15(7):822–833. doi: 10.1007/s10495-010-0495-7. [DOI] [PubMed] [Google Scholar]
- Garrido C, et al. Heat Shock Proteins 27 and 70: Anti-Apoptotic Proteins with Tumorigenic Properties. Cell Cycle. 2006;5 doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- Garrido C, Bruey JM, Fromentin A, Hammann A, Arrigo AP, Solary E. HSP27 inhibits cytochrome c-dependent activation of procaspase-9. Faseb J. 1999;13(14):2061–2070. doi: 10.1096/fasebj.13.14.2061. [DOI] [PubMed] [Google Scholar]
- Garrido C, Fromentin A, Bonnotte B, et al. Heat shock protein 27 enhances the tumorigenicity of immunogenic rat colon carcinoma cell clones. Cancer Res. 1998;58(23):5495–5499. [PubMed] [Google Scholar]
- Garrido C, Gurbuxani S, Ravagnan L, Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun. 2001;286(3):433–442. doi: 10.1006/bbrc.2001.5427. [DOI] [PubMed] [Google Scholar]
- Gastpar R, Gehrmann M, Bausero MA, et al. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65(12):5238–5247. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann M, Radons J, Molls M, Multhoff G. The therapeutic implications of clinically applied modifiers of heat shock protein 70 (Hsp70) expression by tumor cells. Cell Stress Chaperones. 2008;13(1):1–10. doi: 10.1007/s12192-007-0006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakis GV, Younes A. Heat-shock protein 90 inhibitors in cancer therapy: 17AAG and beyond. Future Oncol. 2005;1(2):273–281. doi: 10.1517/14796694.1.2.273. [DOI] [PubMed] [Google Scholar]
- George J, Srivastava AK, Singh R, Shukla Y. Cypermethrin exposure leads to regulation of proteins expression involved in neoplastic transformation in mouse skin. Proteomics. 2011;11:4411–4421. doi: 10.1002/pmic.201100233. [DOI] [PubMed] [Google Scholar]
- Ghosh JC, Dohi T, Kang BH, Altieri DC. Hsp60 regulation of tumor cell apoptosis. J Biol Chem. 2008;283(8):5188–5194. doi: 10.1074/jbc.M705904200. [DOI] [PubMed] [Google Scholar]
- Ghosh JC, Siegelin MD, Dohi T, Altieri DC. Heat shock protein 60 regulation of the mitochondrial permeability transition pore in tumor cells. Cancer Res. 2010;70(22):8988–8993. doi: 10.1158/0008-5472.CAN-10-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibert B, Hadchity E, Czekalla A, et al. Inhibition of heat shock protein 27 (HspB1) tumorigenic functions by peptide aptamers. Oncogene. 2011;34:3672–3681. doi: 10.1038/onc.2011.73. [DOI] [PubMed] [Google Scholar]
- Gibert B, Eckel B, Fasquelle L, et al. Knock Down of Heat Shock Protein 27 (HspB1) Induces Degradation of Several Putative Client Proteins. PLoS One. 2012;7(1):e29719. doi: 10.1371/journal.pone.0029719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibert B, Eckel B, Gonin, et al. Targeting heat shock protein 27 (HspB1) interferes with bone metastasis and tumor formation in vivo. Brit. J. of Cancer. 2012 May 24; doi: 10.1038/bjc.2012.188. doi: 10.1038/bjc.2012.188. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano C, Catalano S, Panza S, Vizza D, Barone I, Bonofiglio D, Gelsomino L, Rizza P, Fuqua SA, Andò S. Farnesoid X receptor inhibits tamoxifen-resistant MCF-7 breast cancer cell growth through downregulation of HER2 expression. Oncogene. 2011;30:4129–4140. doi: 10.1038/onc.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaessgen A, Jonmarker S, Lindberg A, et al. Heat shock proteins 27, 60 and 70 as prognostic markers of prostate cancer. Apmis. 2008;116(10):888–95. doi: 10.1111/j.1600-0463.2008.01051.x. [DOI] [PubMed] [Google Scholar]
- Golembieski WA, Thomas SL, Schultz CR, Yunker CK, McClung HM, Lemke N, Cazacu S, Barker T, Sage EH, Brodie C, Rempel SA. HSP27 mediates SPARC-induced changes in glioma morphology, migration, and invasion. Glia. 2008;56:1061–1075. doi: 10.1002/glia.20679. [DOI] [PubMed] [Google Scholar]
- Gray PJ, Jr., et al. Targeting the oncogene and kinome chaperone CDC37. Nat Rev Cancer. 2008;8:491–495. doi: 10.1038/nrc2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Koelch W, DeMaio A, Arispe N, Multhoff G. Cell surface-bound heat shock protein 70 (Hsp70) mediates perforin-independent apoptosis by specific binding and uptake of granzyme B. J Biol Chem. 2003;278(42):41173–41181. doi: 10.1074/jbc.M302644200. [DOI] [PubMed] [Google Scholar]
- Gruvberger-Saal SK, Parsons R. Is the small heat shock protein alphaB-crystallin an oncogene? J Clin Invest. 2006;116(1):30–32. doi: 10.1172/JCI27462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullino PM. The internal milieu of tumors. Prog Exp Tumor Res. 1966;8:1–25. doi: 10.1159/000386002. [DOI] [PubMed] [Google Scholar]
- Guo H, Bai Y, Xu P, Hu Z, Liu L, Wang F, Jin G, Wang F, Deng Q, Tu Y, Feng M, Lu D, Shen H, Wu T. Functional promoter -1271G>C variant of HSPB1 predicts lung cancer risk and survival. J Clin Oncol. 2010;28:1928–1935. doi: 10.1200/JCO.2009.24.4954. [DOI] [PubMed] [Google Scholar]
- Gurbuxani S, Bruey JM, Fromentin A, et al. Selective depletion of inducible HSP70 enhances immunogenicity of rat colon cancer cells. Oncogene. 2001;20(51):7478–7485. doi: 10.1038/sj.onc.1204948. [DOI] [PubMed] [Google Scholar]
- Hadchity E, Aloy MT, Paulin C, Armandy E, Watkin E, Rousson R, Gleave M, Chapet O, Rodriguez-Lafrasse C. Heat shock protein 27 as a new therapeutic target for radiation sensitization of head and neck squamous cell carcinoma. Mol Ther. 2009;17:1387–1394. doi: 10.1038/mt.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JS, Neef DW, Thiele DJ. A stress regulatory network for co-ordinated activation of proteasome expression mediated by yeast heat shock transcription factor. Mol Microbiol. 2006;60:240–251. doi: 10.1111/j.1365-2958.2006.05097.x. [DOI] [PubMed] [Google Scholar]
- Hamelin C, Cornut E, Poirier F, Pons S, Beaulieu C, Charrier JP, Haïdous H, Cotte E, Lambert C, Piard F, Ataman-Önal Y, Choquet-Kastylevsky G. Identification and verification of heat shock protein 60 as a potential serum marker for colorectal cancer. FEBS J. 2011;278:4845–4859. doi: 10.1111/j.1742-4658.2011.08385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Havasi A, Li Z, Wang Z, et al. Hsp27 inhibits Bax activation and apoptosis via a phosphatidylinositol 3-kinase-dependent mechanism. J Biol Chem. 2008;283(18):12305–12313. doi: 10.1074/jbc.M801291200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi N, Peacock JW, Beraldi E, Zoubeidi A, Gleave ME, Ong CJ. Hsp27 silencing coordinately inhibits proliferation and promotes Fas-induced apoptosis by regulating the PEA-15 molecular switch. Cell Death Differ. 2012;19(6):990–1002. doi: 10.1038/cdd.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich JC, Tuukkanen A, Schroeder M, Fahrig T, Fahrig R. RP101 (brivudine) binds to heat shock protein HSP27 (HSPB1) and enhances survival in animals and pancreatic cancer patients. J Cancer Res Clin Oncol. 2011;137:1349–1361. doi: 10.1007/s00432-011-1005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez MP, Sullivan WP, Toft DO. The assembly and intermolecular properties of the hsp70-Hop-hsp90 molecular chaperone complex. J Biol Chem. 2002;277(41):38294–38304. doi: 10.1074/jbc.M206566200. [DOI] [PubMed] [Google Scholar]
- Hessling M, Richter K, Buchner J. Dissection of the ATP-induced conformational cycle of the molecular chaperone Hsp90. Nat Struct Mol Biol. 2009;16(3):287–293. doi: 10.1038/nsmb.1565. [DOI] [PubMed] [Google Scholar]
- Hietakangas V, et al. Phosphorylation of serine 303 is a prerequisite for the stress-inducible SUMO modification of heat shock factor 1. Mol Cell Biol. 2003;23:2953–2968. doi: 10.1128/MCB.23.8.2953-2968.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino M, Kurogi K, Okubo MA, Murata-Hori M, Hosoya H. Small heat shock protein 27 (HSP27) associates with tubulin/microtubules in HeLa cells. Biochem Biophys Res Commun. 2000;271(1):164–169. doi: 10.1006/bbrc.2000.2553. [DOI] [PubMed] [Google Scholar]
- Horwitz J, Huang Q, Ding L. The native oligomeric organization of alpha-crystallin, is it necessary for its chaperone function? Exp Eye Res. 2004;79(6):817–21. doi: 10.1016/j.exer.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Horwitz J, Huang Q-L, Ding L-L. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HS, Lin JH, Huang WC, Hsu TW, Su K, Chiou SH, Tsai YT, Hung SC. Chemoresistance of lung cancer stemlike cells depends on activation of Hsp27. Cancer. 2011;117:1516–1528. doi: 10.1002/cncr.25599. [DOI] [PubMed] [Google Scholar]
- Hunt CR, Dix DJ, Sharma GG, Pandita RK, Gupta A, Funk M, Pandita TK. Genomic instability and enhanced radiosensitivity in Hsp70.1- and Hsp70.3-deficient mice. Mol Cell Biol. 2004;24:899–911. doi: 10.1128/MCB.24.2.899-911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz MD, Kaur P, Nagaraja GM, Bausero MA, Manola J, Asea A. Radiation therapy induces circulating serum Hsp72 in patients with prostate cancer. Radiother Oncol. 2010;95:350–358. doi: 10.1016/j.radonc.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang YJ, Lee SP, Kim SY, et al. Expression of heat shock protein 60 kDa is upregulated in cervical cancer. Yonsei Med J. 2009;50(3):399–406. doi: 10.3349/ymj.2009.50.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia TD, Craig EA. Four small heat shock proteins are related to each other and to mammalian α-crystallin. Proc Natl Acad Sci USA. 1982;79:2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Kobayashi M, Yoda M, et al. Hsc70/Hsp90 chaperone machinery mediates ATP-dependent RISC loading of small RNA duplexes. Mol Cell. 2010;39(2):292–299. doi: 10.1016/j.molcel.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Jäättelä M. Over-expression of Hsp70 confers tumorigenicity to mouse fibrosarcoma cells. Int J Cancer. 1995;60:689–693. doi: 10.1002/ijc.2910600520. [DOI] [PubMed] [Google Scholar]
- Jaattela M, Wissing D, Bauer PA, Li GC. Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. The EMBO Journal. 1992;11(10):3507–3512. doi: 10.1002/j.1460-2075.1992.tb05433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaattela M, Wissing D, Kokholm K, Kallunki T, Egeblad M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. Embo J. 1998;17(21):6124–34. doi: 10.1093/emboj/17.21.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob U, Gaestel M, Engels K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- Jin X, et al. Heat shock transcription factor 1 is a key determinant of HCC development by regulating hepatic steatosis and metabolic syndrome. Cell Metab. 2011;14:91–103. doi: 10.1016/j.cmet.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomary C, Cullen J, Jones SE. Inactivation of the Akt survival pathway during photoreceptor apoptosis in the retinal degeneration mouse. Invest Ophthalmol Vis Sci. 2006;47(4):1620–9. doi: 10.1167/iovs.05-1176. [DOI] [PubMed] [Google Scholar]
- Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- Kamada M, So A, Muramaki M, Rocchi P, Beraldi E, Gleave M. Hsp27 knockdown using nucleotide-based therapies inhibit tumor growth and enhance chemotherapy in human bladder cancer cells. Mol Cancer Ther. 2007;6(1):299–308. doi: 10.1158/1535-7163.MCT-06-0417. [DOI] [PubMed] [Google Scholar]
- Kamal A, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, et al. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamradt MC, Chen F, Cryns VL. The small heat shock protein alpha B-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J Biol Chem. 2001;276(19):16059–16063. doi: 10.1074/jbc.C100107200. [DOI] [PubMed] [Google Scholar]
- Kamradt MC, Chen F, Sam S, Cryns VL. The small heat shock protein alpha B-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. J Biol Chem. 2002;277(41):38731–38736. doi: 10.1074/jbc.M201770200. [DOI] [PubMed] [Google Scholar]
- Kamradt MC, Lu M, Werner ME, et al. The small heat shock protein alpha B-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J Biol Chem. 2005;280(12):11059–11066. doi: 10.1074/jbc.M413382200. [DOI] [PubMed] [Google Scholar]
- Kanagasabai R, Krishnamurthy K, Druhan LJ, Ilangovan G. Forced expression of heat shock protein 27 (Hsp27) reverses P-glycoprotein (ABCB1)-mediated drug efflux and MDR1 gene expression in Adriamycin-resistant human breast cancer cells. J Biol Chem. 2011;286:33289–33300. doi: 10.1074/jbc.M111.249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa Y, Isomoto H, Oka M, et al. Expression of heat shock protein (Hsp) 70 and Hsp 40 in colorectal cancer. Med Oncol. 2003;20(2):157–164. doi: 10.1385/MO:20:2:157. [DOI] [PubMed] [Google Scholar]
- Kang SH, Kang KW, Kim K-H, Kwon B, Kim S-K, Lee H-Y, Kong S-Y, Lee ES, Jang S-G, Yoo BC. Upregulated HSP27 in human breast cancer cells reduces Herceptin susceptibility by increasing Her2 protein stability. BMC Cancer. 2008;8:286. doi: 10.1186/1471-2407-8-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar RK, Cheung CH, Chang JY, Kanwar JR. Recent advances in anti-survivin treatments for cancer. Curr Med Chem. 2010;17(15):1509–1515. doi: 10.2174/092986710790979935. [DOI] [PubMed] [Google Scholar]
- Kase S, Parikh JG, Rao NA. Expression of heat shock protein 27 and alpha-crystallins in human retinoblastoma after chemoreduction. Br J Ophthalmol. 2009;93(4):541–544. doi: 10.1136/bjo.2008.145508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M, Koninkx J, Schumacher U. Heat shock protein expression in human tumours grown in severe combined immunodeficient mice. Cancer Lett. 2000;161(1):113–120. doi: 10.1016/s0304-3835(00)00601-7. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH, Karp JE, Litzow MR, Mesa RA, Hogan W, Steensma DP, Flatten KS, Loegering DA, Schneider PA, Peterson KL, Maurer MJ, Smith BD, Greer J, Chen Y, Reid JM, Ivy SP, Ames MM, Adjei AA, Erlichman C, Karnitz LM. Phase I and pharmacological study of cytarabine and tanespimycin in relapsed and refractory acute leukemia. Haematologica. 2011;96:1619–1626. doi: 10.3324/haematol.2011.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Nagaraja GM, Asea A. Combined lentiviral and RNAi technologies for the delivery and permanent silencing of the hsp25 gene. Methods Mol Biol. 2011;787:121–136. doi: 10.1007/978-1-61779-295-3_10. [DOI] [PubMed] [Google Scholar]
- Khaleque MA, et al. Heat shock factor 1 represses estrogen-dependent transcription through association with MTA1. Oncogene. 2008;27:1886–1893. doi: 10.1038/sj.onc.1210834. [DOI] [PubMed] [Google Scholar]
- Khaleque MA, et al. Induction of heat shock proteins by heregulin beta1 leads to protection from apoptosis and anchorage-independent growth. Oncogene. 2005;24:6564–6573. doi: 10.1038/sj.onc.1208798. [DOI] [PubMed] [Google Scholar]
- Khalil AA, Kabapy NF, Deraz SF, Smith C. Heat shock proteins in oncology: Diagnostic biomarkers or therapeutic targets? Biochim Biophys Acta. 2011;1816(2):89–104. doi: 10.1016/j.bbcan.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Kim EH, Lee HJ, Lee DH, et al. Inhibition of heat shock protein 27-mediated resistance to DNA damaging agents by a novel PKC delta-V5 heptapeptide. Cancer Res. 2007;67(13):6333–6341. doi: 10.1158/0008-5472.CAN-06-4344. [DOI] [PubMed] [Google Scholar]
- Kim JY, Son YO, Park SW, Bae JH, Chung JS, Kim HH, Chung BS, Kim SH, Kang CD. Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Exp Mol Med. 2006;38:474–484. doi: 10.1038/emm.2006.56. [DOI] [PubMed] [Google Scholar]
- Kim LS, Kim JH. Heat shock protein as molecular targets for breast cancer therapeutics. J Breast Cancer. 2011;14:167–174. doi: 10.4048/jbc.2011.14.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Jeong SJ, Kim B, Yun SM, Choi do Y, Kim SH. Melatonin synergistically enhances cisplatin-induced apoptosis via the dephosphorylation of ERK/p90 ribosomal S6 kinase/heat shock 27 protein in SK-OV-3 cells. J Pineal Res. 2012;52:244–252. doi: 10.1111/j.1600-079X.2011.00935.x. [DOI] [PubMed] [Google Scholar]
- Kirkegaard T, Roth AG, Petersen NH, et al. Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature. 2010;463(7280):549–553. doi: 10.1038/nature08710. [DOI] [PubMed] [Google Scholar]
- Knapinska AM, Gratacos FM, Krause CD, et al. Chaperone Hsp27 modulates AUF1 proteolysis and AU-rich element-mediated mRNA degradation. Mol Cell Biol. 2011;31(7):1419–1431. doi: 10.1128/MCB.00907-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis J, Madaras B, Toth EK, Fust G, Prohaszka Z. Serum level of soluble 70-kD heat shock protein is associated with high mortality in patients with colorectal cancer without distant metastasis. Cell Stress Chaperones. 2010;15(2):143–151. doi: 10.1007/s12192-009-0128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren J, 3rd, Jinwal UK, Jin Y, et al. Facilitating Akt clearance via manipulation of Hsp70 activity and levels. J Biol Chem. 2010;285(4):2498–505. doi: 10.1074/jbc.M109.057208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koteiche HA, McHaourab HS. Mechanism of chaperone function in small heat-shock proteins. Phosphorylation-induced activation of two-mode binding in alphaB-crystallin. J Biol Chem. 2003;278(12):10361–10367. doi: 10.1074/jbc.M211851200. [DOI] [PubMed] [Google Scholar]
- Kwon SM, Kim SA, Yoon JH, Ahn SG. Transforming growth factor beta1-induced heat shock protein 27 activation promotes migration of mouse dental papilla-derived MDPC-23 cells. J Endod. 2010;36:1332–1335. doi: 10.1016/j.joen.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Lamb J, Crawford ED, Peck D, et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313(5795):1929–1935. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- Lamoureux F, Thomas C, Yin MJ, Kuruma H, Beraldi E, Fazli L, Zoubeidi A, Gleave ME. Clusterin inhibition using OGX-011 synergistically enhances Hsp90 inhibitor activity by suppressing the heat shock response in castrate-resistant prostate cancer. Cancer Res. 2011;71:5838–5849. doi: 10.1158/0008-5472.CAN-11-0994. [DOI] [PubMed] [Google Scholar]
- Lancet JE, Gojo I, Burton M, Quinn M, Tighe SM, Kersey K, Zhong Z, Albitar MX, Bhalla K, Hannah AL, Baer MR. Phase I study of the heat shock protein 90 inhibitor alvespimycin (KOS-1022, 17-DMAG) administered intravenously twice weekly to patients with acute myeloid leukemia. Leukemia. 2010;24:699–705. doi: 10.1038/leu.2009.292. [DOI] [PubMed] [Google Scholar]
- Lander AD, Kimble J, Clevers H, Fuchs E, Montarras D, Buckingham M, Calof AL, Trumpp A, Oskarsson T. What does the concept of the stem cell niche really mean today? BMC Biology. 2012;10:19–34. doi: 10.1186/1741-7007-10-19. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry J, Chretien P, Lambert H, Hickey E, Weber LA. Heat shock resistance confered by expression of the human HSP 27 gene in rodent cells. J Cell Biol. 1989;109:7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer R, Ott K, Specht K, Becker K, Lordick F, Burian M, Herrmann K, Schrattenholz A, Cahill MA, Schwaiger M, Hofler H, Wester HJ. Protein expression profiling in esophageal adenocarcinoma patients indicates association of heat-shock protein 27 expression and chemotherapy response. Clin Cancer Res. 2008;14:8279–8287. doi: 10.1158/1078-0432.CCR-08-0679. [DOI] [PubMed] [Google Scholar]
- Laszlo A, Fleischer I. Heat-induced perturbations of DNA damage signaling pathways are modulated by molecular chaperones. Cancer Res. 2009;69:2042–2049. doi: 10.1158/0008-5472.CAN-08-1639. [DOI] [PubMed] [Google Scholar]
- Launay N, Tarze A, Vicart P, Lilienbaum A. Serine 59 phosphorylation of {alpha}B-crystallin down-regulates its anti-apoptotic function by binding and sequestering Bcl-2 in breast cancer cells. J Biol Chem. 2010;285(48):37324–32. doi: 10.1074/jbc.M110.124388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Lin TW, Ko TP, Wang AH. The hexameric structures of human heat shock protein 90. PLoS One. 2011;6(5):e19961. doi: 10.1371/journal.pone.0019961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Lee YS. Repeated-dose toxicity of HSP27-binding heptapeptide in mice. Drug Chem Toxicol. 2010;33(3):284–290. doi: 10.3109/01480540903483425. [DOI] [PubMed] [Google Scholar]
- Lee J, Lim KT. Inhibitory effect of SJSZ glycoprotein (38 kDa) on expression of heat shock protein 27 and 70 in chromium (VI)-treated hepatocytes. Mol Cell Biochem. 2012;359:45–57. doi: 10.1007/s11010-011-0998-8. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim EH, Seo WD, Choi TH, Cheon GJ, Lee YJ, Lee YS. Heat shock protein 27-targeted heptapeptide of the PKCΔ catalytic V5 region sensitizes tumors with radio- and chemoresistance. Int J Radiat Oncol Biol Phys. 2011;80:221–230. doi: 10.1016/j.ijrobp.2010.11.069. [DOI] [PubMed] [Google Scholar]
- Lemieux P, Oesterreich S, Lawrence JA, et al. The small heat shock protein hsp27 increases invasiveness but decreases motility of breast cancer cells. Invasion Metastasis. 1997;17(3):113–123. [PubMed] [Google Scholar]
- Leu JI, Pimkina J, Pandey P, Murphy ME, George DL. HSP70 inhibition by the small-molecule 2-phenylethynesulfonamide impairs protein clearance pathways in tumor cells. Mol Cancer Res. 2011;9(7):936–947. doi: 10.1158/1541-7786.MCR-11-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DW, Liu JP, Mao YW, et al. Calcium-activated RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and is abrogated by alpha B-crystallin through inhibition of RAS activation. Mol Biol Cell. 2005;16(9):4437–4453. doi: 10.1091/mbc.E05-01-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JJ, Akhtar NJ. Human lens high-molecular-weight alpha-crystallin aggregates. Biochem Biophys Res Commun. 2000;275(2):354–359. doi: 10.1006/bbrc.2000.3306. [DOI] [PubMed] [Google Scholar]
- Lin TY, Chang JT, Wang HM, Chan SH, Chiu CC, Lin CY, Fan KH, Liao CT, Chen IH, Liu TZ, Li HF, Cheng AJ. Proteomics of the radioresistant phenotype in head-and-neck cancer: Gp96 as a novel prediction marker and sensitizing target for radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:246–256. doi: 10.1016/j.ijrobp.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat shock proteins. Ann. Rev. Genet. 1988;22:631–637. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lo WY, Lai CC, Hua CH, Tsai MH, Huang SY, Tsai CH, Tsai FJ. S100A8 is identified as a biomarker of HPV18-infected oral squamous cell carcinomas by suppression subtraction hybridization, clinical proteomics analysis, and immunohistochemistry staining. J Proteome Res. 2007;6:2143–2151. doi: 10.1021/pr060551+. [DOI] [PubMed] [Google Scholar]
- Liu JP, Schlosser R, Ma WY, et al. Human alphaA- and alphaB-crystallins prevent UVA-induced apoptosis through regulation of PKCalpha, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res. 2004;79(6):393–403. [PubMed] [Google Scholar]
- Mambula SS, Calderwood SK. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol. 2006;177:7849–7857. doi: 10.4049/jimmunol.177.11.7849. [DOI] [PubMed] [Google Scholar]
- Mantovani A, et al. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Mao YW, Liu JP, Xiang H, Li DW. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11(5):512–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- Margel D, Pevsner-Fisher M, Baniel J, Yossepowitch O, Cohen IR. Stress proteins and cytokines are urinary biomarkers for diagnosis and staging of bladder cancer. Eur Urol. 2011;59:113–119. doi: 10.1016/j.eururo.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Marigo I, et al. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- Markossian KA, Yudin IK, Kurganov BI. Mechanism of Suppression of Protein Aggregation by alpha-Crystallin. Int J Mol Sci. 2009;10(3):1314–13145. doi: 10.3390/ijms10031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey AJ, Williamson DS, Browne H, et al. A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in HCT116 colon carcinoma cells. Cancer Chemother Pharmacol. 2010;66(3):535–45. doi: 10.1007/s00280-009-1194-3. [DOI] [PubMed] [Google Scholar]
- Matassa DS, Amoroso MR, Maddalena F, Landriscina M, Esposito F. New insights into TRAP1 pathway. Am J Cancer Res. 2012;2(2):235–248. [PMC free article] [PubMed] [Google Scholar]
- Mazumdar A, et al. Transcriptional repression of oestrogen receptor by metastasis-associated protein 1 corepressor. Nat Cell Biol. 2001;3:30–37. doi: 10.1038/35050532. [DOI] [PubMed] [Google Scholar]
- McClellan AJ, Xia Y, Deutschbauer AM, Davis RW, Gerstein M, Frydman J. Diverse cellular functions of the Hsp90 molecular chaperone uncovered using systems approaches. Cell. 2007;131(1):121–135. doi: 10.1016/j.cell.2007.07.036. [DOI] [PubMed] [Google Scholar]
- McClung HM, Golembieski WA, Schultz CR, Jankowski M, Schultz LR, Rempel SA. Deletion of the SPARC acidic domain or EGF-like module reduces SPARC-induced migration and signaling through p38 MAPK/HSP27 in glioma. Carcinogenesis. 2012;33:275–284. doi: 10.1093/carcin/bgr276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Préville X, Chareyron P, Briolay J, Klemenz R, Arrigo A-P. Constitutive expression of human hsp27, Drosophila hsp27, or human alpha B-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol. 1995;154(1):363–374. [PubMed] [Google Scholar]
- Mehlen P, Préville X, Kretz-Remy C, Arrigo A-P. Human hsp27, Drosophila hsp27 and human αB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these protein against TNFα-induced cell death. EMBO J. 1996a;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Schulze-Osthoff K, Arrigo AP. Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. The Journal of Biological Chemistry. 1996b;271(28):16510–16514. doi: 10.1074/jbc.271.28.16510. [DOI] [PubMed] [Google Scholar]
- Merendino AM, Bucchieri F, Campanella C, et al. Hsp60 is actively secreted by human tumor cells. PLoS One. 2010;5(2):e9247. doi: 10.1371/journal.pone.0009247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels AA, Kanon B, Konings AW, Ohtsuka K, Bensaude O, Kampinga HH. Hsp70 and Hsp40 chaperone activities in the cytoplasm and the nucleus of mammalian cells. J Biol Chem. 1997;272(52):33283–33289. doi: 10.1074/jbc.272.52.33283. [DOI] [PubMed] [Google Scholar]
- Michiel M, Skouri-Panet F, Duprat E, et al. Abnormal assemblies and subunit exchange of alphaB-crystallin R120 mutants could be associated with destabilization of the dimeric substructure. Biochemistry. 2009;48(2):442–453. doi: 10.1021/bi8014967. [DOI] [PubMed] [Google Scholar]
- Mickler M, Hessling M, Ratzke C, Buchner J, Hugel T. The large conformational changes of Hsp90 are only weakly coupled to ATP hydrolysis. Nat Struct Mol Biol. 2009;16(3):281–286. doi: 10.1038/nsmb.1557. [DOI] [PubMed] [Google Scholar]
- Mimnaugh EG, Xu W, Vos M, et al. Simultaneous inhibition of hsp 90 and the proteasome promotes protein ubiquitination, causes endoplasmic reticulum-derived cytosolic vacuolization, and enhances antitumor activity. Mol Cancer Ther. 2004;3(5):551–566. [PubMed] [Google Scholar]
- Mitra A, Shevde LA, Samant RS. Multi-faceted role of HSP40 in cancer. Clin Exp Metastasis. 2009;26(6):559–567. doi: 10.1007/s10585-009-9255-x. [DOI] [PubMed] [Google Scholar]
- Moon A, Bacchini P, Bertoni F, Olvi LG, Santini-Araujo E, Kim YW, Park YK. Expression of heat shock proteins in osteosarcomas. Pathology. 2010;42:421–42. doi: 10.3109/00313025.2010.493866. [DOI] [PubMed] [Google Scholar]
- Mori-Iwamoto S, Kuramitsu Y, Ryozawa S, Mikuria K, Fujimoto M, Maehara S, Maehara Y, Okita K, Nakamura K, Sakaida I. Proteomics finding heat shock protein 27 as a biomarker for resistance of pancreatic cancer cells to gemcitabine. Int J Oncol. 2007;31:1345–1350. [PubMed] [Google Scholar]
- Moulick K, Ahn JH, Zong H, et al. Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat Chem Biol. 2011;7(11):818–826. doi: 10.1038/nchembio.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier N, Arrigo AP. Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones. 2002;7(2):167–176. doi: 10.1379/1466-1268(2002)007<0167:acashs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyano JV, Evans JR, Chen F, et al. AlphaB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J Clin Invest. 2006;116(1):261–270. doi: 10.1172/JCI25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchemwa FC, Nakatsura T, Fukushima S, Nishimura Y, Kageshita T, Ihn H. Differential expression of heat shock protein 105 in melanoma and melanocytic naevi. Melanoma Res. 2008;18:166–171. doi: 10.1097/CMR.0b013e3282fe9a16. [DOI] [PubMed] [Google Scholar]
- Multhoff G. Heat shock protein 70 (Hsp70): membrane location, export and immunological relevance. Methods. 2007;43(3):229–237. doi: 10.1016/j.ymeth.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Murshid A, et al. Protein kinase A binds and activates heat shock factor 1. PLoS One. 2010;5:e13830. doi: 10.1371/journal.pone.0013830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshid A, et al. Heat shock proteins and cancer vaccines: developments in the past decade and chaperoning in the decade to come. Expert Rev Vaccines. 2011;10:1553–1568. doi: 10.1586/erv.11.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadin SB, Vargas-Roig LM, Drago G, Ibarra J, Ciocca DR. Hsp27, Hsp70 and mismatch repair proteins hMLH1 and hMSH2 expression in peripheral blood lymphocytes from healthy subjects and cancer patients. Cancer Lett. 2007;252:131–146. doi: 10.1016/j.canlet.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Nadin SA, Ciocca DR. Participation of heat shock proteins in DNA repair mechanisms in cancer. In: Thomas Allison E., editor. DNA Repair: Damage, Repair Mechanisms and Aging. Nova Science Publisher, Inc; New York: 2010. pp. 165–186. Chapter 7. Hardcover ISBN 978-1-61668-914-8. eBook ISBN 978-1-61728-055-9. [Google Scholar]
- Nagaraja GM, Kaur P, Neumann W, Asea EE, Bausero MA, Multhoff G, Asea A. Silencing Hsp25/Hsp27 gene expression augments proteasome activity and increases CD8+ T-cell-mediated tumor killing and memory responses. Cancer Prev Res (Phila) 2012;5:122–137. doi: 10.1158/1940-6207.CAPR-11-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Kato H, Miyazaki T, Fukuchi M, Masuda N, Fukai Y, Sohda M, Ahmad F, Kuwano H. Tumor immune systems in esophageal cancer with special reference to heat-shock protein 70 and humoral immunity. Anticancer Res. 2009;29:1595–1606. [PubMed] [Google Scholar]
- Nakajima M, Kato H, Miyazaki T, Fukuchi M, Masuda N, Fukai Y, Sohda M, Inose T, Sakai M, Sano A, Tanaka N, Ahmad F, Kuwano H. Prognostic significance of heat shock protein 110 expression and T lymphocyte infiltration in esophageal cancer. Hepatogastroenterology. 2011;58:1555–1560. doi: 10.5754/hge09758. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Adachi S, Yasuda I, Yamauchi T, Kawaguchi J, Itani M, Yoshioka T, Matsushima-Nishiwaki R, Hirose Y, Kozawa O, Moriwaki H. Phosphorylation status of heat shock protein 27 plays a key role in gemcitabine-induced apoptosis of pancreatic cancer cells. Cancer Lett. 2011;313:218–225. doi: 10.1016/j.canlet.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Neckers L, Mimnaugh E, Schulte TW. Hsp90 as an anti-cancer target. Drug Resist Updat. 1999;2(3):165–172. doi: 10.1054/drup.1999.0082. [DOI] [PubMed] [Google Scholar]
- Neckers L, Ivy SP. Heat shock protein 90. Curr Opin Oncol. 2003;15:419–424. doi: 10.1097/00001622-200311000-00003. [DOI] [PubMed] [Google Scholar]
- O’callaghan-Sunol C, et al. Hsp27 modulates p53 signaling and suppresses cellular senescence. Cancer Res. 2007;67:11779–11788. doi: 10.1158/0008-5472.CAN-07-2441. [DOI] [PubMed] [Google Scholar]
- Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med. 2002;8(4 Suppl):S55–61. doi: 10.1016/s1471-4914(02)02316-x. [DOI] [PubMed] [Google Scholar]
- Norton JA, Weinberger PM, Waller JL, Merkley MA, Jackson LL, Dynan WS. Significance of HSPB1 expression in head and neck squamous cell carcinoma: a meta-analysis of published literatures. Laryngoscope. 2010;120(Suppl 4):S172. doi: 10.1002/lary.21636. [DOI] [PubMed] [Google Scholar]
- Oba M, Yano S, Shuto T, Suico MA, Eguma A, Kai H. IFN-gamma down-regulates Hsp27 and enhances hyperthermia-induced tumor cell death in vitro and tumor suppression in vivo. Int J Oncol. 2008;32:1317–1324. doi: 10.3892/ijo_32_6_1317. [DOI] [PubMed] [Google Scholar]
- O’Callaghan-Sunol C, Gabai VL, Sherman MY. Hsp27 modulates p53 signaling and suppresses cellular senescence. Cancer Res. 2007;67(24):11779–11788. doi: 10.1158/0008-5472.CAN-07-2441. [DOI] [PubMed] [Google Scholar]
- Oh HJ, Easton D, Murawski M, Kaneko Y, Subjeck JR. The chaperoning activity of hsp110. Identification of functional domains by use of targeted deletions. J Biol Chem. 1999;274:15712–15718. doi: 10.1074/jbc.274.22.15712. [DOI] [PubMed] [Google Scholar]
- Oka M, Sato S, Soda H, et al. Autoantibody to heat shock protein Hsp40 in sera of lung cancer patients. Jpn J Cancer Res. 2001;92(3):316–320. doi: 10.1111/j.1349-7006.2001.tb01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono A, Kumai T, Koizumi H, Nishikawa H, Kobayashi S, Tadokoro M. Overexpression of heat shock protein 27 in squamous cell carcinoma of the uterine cervix: a proteomic analysis using archival formalin-fixed, paraffin-embedded tissues. Hum Pathol. 2009;40:41–49. doi: 10.1016/j.humpath.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Padival AK, Crabb JW, Nagaraj RH. Methylglyoxal modifies heat shock protein 27 in glomerular mesangial cells. FEBS Lett. 2003;551(1-3):113–118. doi: 10.1016/s0014-5793(03)00874-3. [DOI] [PubMed] [Google Scholar]
- Pandey P, Farber R, Nakazawa A, et al. Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene. 2000;19(16):1975–1981. doi: 10.1038/sj.onc.1203531. [DOI] [PubMed] [Google Scholar]
- Pandey P, Saleh A, Nakazawa A, et al. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. Embo J. 2000;19(16):4310–4322. doi: 10.1093/emboj/19.16.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraiso KH, Haarberg HE, Wood E, Rebecca VW, Chen YA, Xiang Y, Ribas A, Lo RS, Weber JS, Sondak VK, John JK, Sarnaik AA, Koomen JM, Smalley KS. The HSP90 inhibitor XL888 overcomes BRAF inhibitor resistance mediated through diverse mechanisms. Clin Cancer Res. 2012 Apr 23; doi: 10.1158/1078-0432.CCR-11-2612. 2012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcellier A, Brunet M, Schmitt E, et al. HSP27 favors ubiquitination and proteasomal degradation of p27Kip1 and helps S-phase re-entry in stressed cells. Faseb J. 2006;20(8):1179–1181. doi: 10.1096/fj.05-4184fje. [DOI] [PubMed] [Google Scholar]
- Parcellier A, Schmitt E, Gurbuxani S, et al. HSP27 is a ubiquitin-binding protein involved in I-kappaBalpha proteasomal degradation. Mol Cell Biol. 2003;23(16):5790–5802. doi: 10.1128/MCB.23.16.5790-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Cho SG, Kim CK, et al. Heat shock protein hsp72 is a negative regulator of apoptosis signal-regulating kinase 1. Mol Cell Biol. 2002;22(22):7721–7730. doi: 10.1128/MCB.22.22.7721-7730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Park CH, Choi BR, Lim MS, Heo SH, Kim CH, Kang SG, Whang KU, Cho MK. Expression of heat shock protein 105 and 70 in malignant melanoma and benign melanocytic nevi. J Cutan Pathol. 2009;36:511–516. doi: 10.1111/j.1600-0560.2008.01085.x. [DOI] [PubMed] [Google Scholar]
- Pasta SY, Raman B, Ramakrishna T, Rao Ch M. The IXI/V motif in the C-terminal extension of alpha-crystallins: alternative interactions and oligomeric assemblies. Mol Vis. 2004;10:655–62. [PubMed] [Google Scholar]
- Patil SB, Pawar MD, Bitar KN. Direct association and translocation of PKC-alpha with calponin. Am J Physiol Gastrointest Liver Physiol. 2004;286(6):G954–63. doi: 10.1152/ajpgi.00477.2003. [DOI] [PubMed] [Google Scholar]
- Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP. Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol. 2002;22(3):816–834. doi: 10.1128/MCB.22.3.816-834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C, Simon S, Gibert B, Virot S, Manero F, Arrigo AP. Dynamic processes that reflect anti-apoptotic strategies set up by HspB1 (Hsp27) Exp Cell Res. 2010;316(9):1535–1552. doi: 10.1016/j.yexcr.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Pei H, Huang L, Liu L, Zhu H, Zeng L, Xiao Z. Experimental study of HSP27 differential expression in left sided colon cancer and right sided colon cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36:277–285. doi: 10.3969/j.issn.1672-7347.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Pekarek LA, et al. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181:435–440. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng MD, Cairns L, van den IP, Prescott A, Hutcheson AM, Quinlan RA. Intermediate filament interactions can be altered by HSP27 and alphaB-crystallin. J Cell Sci. 1999;112(Pt 13):2099–2112. doi: 10.1242/jcs.112.13.2099. [DOI] [PubMed] [Google Scholar]
- Petersen NH, Kirkegaard T, Olsen OD, Jaattela M. Connecting Hsp70, sphingolipid metabolism and lysosomal stability. Cell Cycle. 2010;9(12):2305–2309. doi: 10.4161/cc.9.12.12052. [DOI] [PubMed] [Google Scholar]
- Pfosser A, et al. Liposomal Hsp90 cDNA induces neovascularization via nitric oxide in chronic ischemia. Cardiovasc Res. 2005;65:728–736. doi: 10.1016/j.cardiores.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Plescia J, Salz W, Xia F, et al. Rational design of shepherdin, a novel anticancer agent. Cancer Cell. 2005;7(5):457–468. doi: 10.1016/j.ccr.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Georgiades A, Thulin T, de Faire U, Frostegard J. Serum heat shock protein 70 levels predict the development of atherosclerosis in subjects with established hypertension. Hypertension. 2003;42(3):235–238. doi: 10.1161/01.HYP.0000086522.13672.23. [DOI] [PubMed] [Google Scholar]
- Powers MV, et al. Dual targeting of HSC70 and HSP72 inhibits HSP90 function and induces tumor-specific apoptosis. Cancer Cell. 2008;14:250–262. doi: 10.1016/j.ccr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Powers MV, et al. Targeting HSP70: the second potentially druggable heat shock protein and molecular chaperone? Cell Cycle. 2011;9:1542–1550. doi: 10.4161/cc.9.8.11204. [DOI] [PubMed] [Google Scholar]
- Preville X, Salvemini F, Giraud S, et al. Mammalian small stress proteins protect against oxidative stress through their ability to increase glucose-6-phosphate dehydrogenase activity and by maintaining optimal cellular detoxifying machinery. Exp Cell Res. 1999;247(1):61–78. doi: 10.1006/excr.1998.4347. [DOI] [PubMed] [Google Scholar]
- Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phenotypic variation. Nature. 2002;417(6889):618–624. doi: 10.1038/nature749. [DOI] [PubMed] [Google Scholar]
- Ran R, Lu A, Zhang L, et al. Hsp70 promotes TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa B survival signaling. Genes Dev. 2004;18(12):1466–1481. doi: 10.1101/gad.1188204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane MJ, Coxon PY, Powell DW, et al. p38 Kinase-dependent MAPKAPK-2 activation functions as 3-phosphoinositide-dependent kinase-2 for Akt in human neutrophils. J Biol Chem. 2001;276(5):3517–3523. doi: 10.1074/jbc.M005953200. [DOI] [PubMed] [Google Scholar]
- Rane MJ, Pan Y, Singh S, et al. Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem. 2003;278(30):27828–27835. doi: 10.1074/jbc.M303417200. [DOI] [PubMed] [Google Scholar]
- Rajan A, Kelly RJ, Trepel JB, Kim YS, Alarcon SV, Kummar S, Gutierrez M, Crandon S, Zein WM, Jain L, Mannargudi B, Figg WD, Houk BE, Shnaidman M, Brega N, Giaccone G. A phase I study of PF-04929113 (SNX-5422), an orally bioavailable heat shock protein 90 inhibitor, in patients with refractory solid tumor malignancies and lymphomas. Clin Cancer Res. 2011;17:6831–6839. doi: 10.1158/1078-0432.CCR-11-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravagnan L, Gurbuxani S, Susin SA, et al. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3(9):839–843. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Levin ER. Heat shock protein 27 is required for sex steroid receptor trafficking to and functioning at the plasma membrane. Mol Cell Bio. 2010;30:3249–3261. doi: 10.1128/MCB.01354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerole AL, Gobbo J, De Thonel A, et al. Peptides and aptamers targeting HSP70: a novel approach for anticancer chemotherapy. Cancer Res. 2011;71(2):484–495. doi: 10.1158/0008-5472.CAN-10-1443. [DOI] [PubMed] [Google Scholar]
- Richards EH, Hickey E, Weber LA, Master JR. Effect of overexpression of the small heat shock protein HSP27 on the heat and drug sensitivities of human testis tumor cells. Cancer Res. 1996;56:2446–2451. [PubMed] [Google Scholar]
- Richardson PG, Badros AZ, Jagannath S, et al. Tanespimycin with bortezomib: activity in relapsed/refractory patients with multiple myeloma. Br J Haematol. 2010;150(4):428–437. doi: 10.1111/j.1365-2141.2010.08264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritossa F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia. 1962;18:571–573. [Google Scholar]
- Ritossa FM. Experimental Activation of Specific Loci in Polytene Chromosomes of Drosophila. Exp Cell Res. 1964;35:601–607. doi: 10.1016/0014-4827(64)90147-8. [DOI] [PubMed] [Google Scholar]
- Rocchi P, Beraldi E, Ettinger S, et al. Increased Hsp27 after androgen ablation facilitates androgen-independent progression in prostate cancer via signal transducers and activators of transcription 3-mediated suppression of apoptosis. Cancer Res. 2005;65(23):11083–11093. doi: 10.1158/0008-5472.CAN-05-1840. [DOI] [PubMed] [Google Scholar]
- Rocchi P, Jugpal P, So A, et al. Small interference RNA targeting heat-shock protein 27 inhibits the growth of prostatic cell lines and induces apoptosis via caspase-3 activation in vitro. BJU Int. 2006;28:28. doi: 10.1111/j.1464-410X.2006.06425.x. [DOI] [PubMed] [Google Scholar]
- Rogalla T, Ehrnsperger M, Preville X, et al. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem. 1999;274(27):18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- Rohde M, Daugaard M, Jensen MH, Helin K, Nylandsted J, Jaattela M. Members of the heat-shock protein 70 family promote cancer cell growth by distinct mechanisms. Genes Dev. 2005;19(5):570–582. doi: 10.1101/gad.305405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum EE, Brehm KS, Vasiljevic E, Liu CH, Hardie RC, Colley NJ. XPORT-Dependent Transport of TRP and Rhodopsin. Neuron. 2011;72(4):602–615. doi: 10.1016/j.neuron.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396(6709):336–342. doi: 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- Saha S, Das KP. Relationship between chaperone activity and oligomeric size of recombinant human alphaA- and alphaB-crystallin: a tryptic digestion study. Proteins. 2004;57(3):610–617. doi: 10.1002/prot.20230. [DOI] [PubMed] [Google Scholar]
- Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the apaf-1 apoptosome by hsp70. Nat Cell Biol. 2000;2(8):476–483. doi: 10.1038/35019510. [DOI] [PubMed] [Google Scholar]
- Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146(3):353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S. Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of Jurkat cells. Embo J. 1999;18(8):2040–2048. doi: 10.1093/emboj/18.8.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster TA, Lindquist S, Queitsch C. Under cover: causes, effects and implications of Hsp90-mediated genetic capacitance. Bioessays. 2004;26(4):348–362. doi: 10.1002/bies.20020. [DOI] [PubMed] [Google Scholar]
- Santagata S, et al. High levels of nuclear heat-shock factor 1 (HSF1) are associated with poor prognosis in breast cancer. Proc Natl Acad Sci U S A. 2011;108:18378–18383. doi: 10.1073/pnas.1115031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarge KD, et al. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol. Cell. Biol. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Harada K, Sasaki M, Yasaka T, Nakanuma Y. Heat shock proteins 27 and 70 are potential biliary markers for the detection of cholangiocarcinoma. Am J Pathol. 2012;180:123–130. doi: 10.1016/j.ajpath.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Schäfer C, Seeliger H, Bader DC, Assmann G, Buchner D, Guo Y, Ziesch A, Palagyi A, Ochs S, Laubender RP, Jung A, De Toni EN, Kirchner T, Göke B, Bruns C, Gallmeier E. Heat shock protein 27 as a prognostic and predictive biomarker in pancreatic ductal adenocarcinoma. J Cell Mol Med. 2011 Oct 18; doi: 10.1111/j.1582-4934.2011.01473.x. 2011. doi: 10.1111/j.1582-4934.2011.01473.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling D, Gehrmann M, Steinem C, et al. Binding of heat shock protein 70 to extracellular phosphatidylserine promotes killing of normoxic and hypoxic tumor cells. Faseb J. 2009;23(8):2467–2477. doi: 10.1096/fj.08-125229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecht R, Erbse AH, Bukau B, Mayer MP. Mechanics of Hsp70 chaperones enables differential interaction with client proteins. Nat Struct Mol Biol. 2011;18(3):345–351. doi: 10.1038/nsmb.2006. [DOI] [PubMed] [Google Scholar]
- Schultz CR, Golembieski WA, King DA, Brown SL, Brodie C, Rempel SA. Inhibition of HSP27 alone or in combination with pAKT inhibition as therapeutic approaches to target SPARC-induced glioma cell survival. Mol Cancer. 2012 Apr 5;11(1):20. doi: 10.1186/1476-4598-11-20. 2012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlackova L, Spacek M, Holler E, Imryskova Z, Hromadnikova I. Heat-shock protein expression in leukemia. Tumour Biol. 2011;32:33–44. doi: 10.1007/s13277-010-0088-7. [DOI] [PubMed] [Google Scholar]
- Shan ZX, et al. miR-1/miR-206 regulate Hsp60 expression contributing to glucose-mediated apoptosis in cardiomyocytes. FEBS Lett. 2010;584:3592–3600. doi: 10.1016/j.febslet.2010.07.027. [DOI] [PubMed] [Google Scholar]
- Sharma A, Upadhyay AK, Bhat MK. Inhibition of Hsp27 and Hsp40 potentiates 5-fluorouracil and carboplatin mediated cell killing in hepatoma cells. Cancer Biol Ther. 2009;8(22):2106–2113. doi: 10.4161/cbt.8.22.9687. [DOI] [PubMed] [Google Scholar]
- Sherman MY, et al. Oncogenes induce senescence with incomplete growth arrest and suppress the DNA damage response in immortalized cells. Aging Cell. 2010;10:949–961. doi: 10.1111/j.1474-9726.2011.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YY, Lee H, Nakagawara A, Juan HF, Jeng YM, Tsay YG, Lin DT, Hsieh FJ, Pan CY, Hsu WM, Liao YF. Nuclear GRP75 binds retinoic acid receptors to promote neuronal differentiation of neuroblastoma. PLoS One. 2012;6(10):e26236. doi: 10.1371/journal.pone.0026236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S, Fontaine JM, Martin JL, et al. Myopathy-associated alpha B-crystallin mutants: Abnormal phosphorylation, intracellular location, and interactions with other small heat shock proteins. J Biol Chem. 2007;82:34276–34287. doi: 10.1074/jbc.M703267200. [DOI] [PubMed] [Google Scholar]
- Singh U, et al. A DNA sequence directed mutual transcription regulation of HSF1 and NFIX involves novel heat sensitive protein interactions. PLoS One. 2009;4:e5050. doi: 10.1371/journal.pone.0005050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalnikova H, Martinkova J, Hrabakova R, Halada P, Dziechciarkova M, Hajduch M, Gadher SJ, Hammar A, Enetoft D, Ekefjard A, Forsstrom-Olsson O, Kovarova H. Cancer drug-resistance and a look at specific proteins: Rho GDP-dissociation inhibitor 2, Y-box binding protein 1, and HSP70/90 organizing protein in proteomics clinical application. J Proteome Res. 2011;10:404–415. doi: 10.1021/pr100468w. [DOI] [PubMed] [Google Scholar]
- Slaby O, Sobkova K, Svoboda M, Garajova I, Fabian P, Hrstka R, Nenutil R, Sachlova M, Kocakova I, Michalek J, Smerdova T, Knoflickova D, Vyzula R. Significant overexpression of Hsp110 gene during colorectal cancer progression. Oncol Rep. 2009;21:1235–1241. doi: 10.3892/or_00000346. [DOI] [PubMed] [Google Scholar]
- Sloan EK, Ciocca DR, Pouliot N, Natoli A, Restall C, Henderson MA, Fanelli MA, Cuello-Carrión FD, Gago FE, Anderson RL. Stromal cell expression of caveolin-1 predicts outcome in breast cancer. Am J Pathol. 2009;174:2035–2043. doi: 10.2353/ajpath.2009.080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger PK, Pelham HRB. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988;54:855–864. doi: 10.1016/s0092-8674(88)91219-6. [DOI] [PubMed] [Google Scholar]
- Spizzo R, et al. SnapShot: MicroRNAs in Cancer. Cell. 2009;137:586–586 e581. doi: 10.1016/j.cell.2009.04.040. [DOI] [PubMed] [Google Scholar]
- Srinivasan A, Nagineni C, Bhat S. alpha A-crystallin is expressed in non-ocular tissues. J Biol Chem. 1992;267:23337–23341. [PubMed] [Google Scholar]
- Stangl S, Gehrmann M, Riegger J, et al. Targeting membrane heat-shock protein 70 (Hsp70) on tumors by cmHsp70.1 antibody. Proc Natl Acad Sci U S A. 2011;108(2):733–738. doi: 10.1073/pnas.1016065108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel F, Baldwin AJ, Painter AJ, et al. Quaternary dynamics and plasticity underlie small heat shock protein chaperone function. Proc Natl Acad Sci U S A. 2010;107(5):2007–2012. doi: 10.1073/pnas.0910126107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straume O, Shimamura T, Lampa MJ, et al. Suppression of heat shock protein 27 induces long-term dormancy in human breast cancer. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1017909109. 10.1073/pnas.1017909109 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stope MB, Schubert T, Staar D, Rönnau C, Streitbörger A, Kroeger N, Kubisch C, Zimmermann U, Walther R, Burchardt M. Effect of the heat shock protein HSP27 on androgen receptor expression and function in prostate cancer cells. World J Urol. 2012 Feb 24; doi: 10.1007/s00345-012-0843-z. 2012. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Sun TX, Liang JJ. Intermolecular exchange and stabilization of recombinant human alphaA- and alphaB-crystallin. J Biol Chem. 1998;273(1):286–290. doi: 10.1074/jbc.273.1.286. [DOI] [PubMed] [Google Scholar]
- Sun J, Liao JK. Induction of angiogenesis by heat shock protein 90 mediated by protein kinase Akt and endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2004;24:2238–2244. doi: 10.1161/01.ATV.0000147894.22300.4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Welsh MJ, Benndorf R. Conformational changes resulting from pseudophosphorylation of mammalian small heat shock proteins--a two-hybrid study. Cell Stress Chaperones. 2006;11(1):61–70. doi: 10.1379/CSC-149R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhou M, Fu D, et al. Ubiquitination of heat shock protein 27 is mediated by its interaction with Smad ubiquitination regulatory factor 2 in A549 cells. Exp Lung Res. 2011;37:568–573. doi: 10.3109/01902148.2011.619627. [DOI] [PubMed] [Google Scholar]
- Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11(7):515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- Takara K, Yamamoto K, Matsubara M, Minegaki T, Takahashi M, Yokoyama T, Okumura K. Effects of α-adrenoceptor antagonists on ABCG2/BCRP-mediated resistance and transport. PLoS One. 2012;7:e30697. doi: 10.1371/journal.pone.0030697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto L, Emmons T, Horwitz J. The C-terminal region of a-crystallin: involvement in protection against heat-induced denaturation. Biochem J. 1993;294:435–438. doi: 10.1042/bj2940435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, et al. Expression of heat shock proteins and heat shock protein messenger ribonucleic acid in human prostate carcinoma in vitro and in tumors in vivo. Cell Stress Chaperones. 2005;10:46–58. doi: 10.1379/CSC-44R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YJ, Zheng W. Chaperones and the maturation of steroid hormone receptor complexes. Oncotarget. 2011;2:104–106. doi: 10.18632/oncotarget.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchénio T, Havard M, Martinez LA, Dautry F. Heat shock-independent induction of multidrug resistance by heat shock factor 1. Mol Cell Biol. 2006;26:580–591. doi: 10.1128/MCB.26.2.580-591.2006. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teimourian S, Jalal R, Sohrabpour M, Goliaei B. Down-regulation of Hsp27 radiosensitizes human prostate cancer cells. Int J Urol. 2006;13:1221–1225. doi: 10.1111/j.1442-2042.2006.01483.x. [DOI] [PubMed] [Google Scholar]
- Theriault JR, Lambert H, Chavez-Zobel AT, Charest G, Lavigne P, Landry J. Essential role of the NH2-terminal WD/EPF motif in the phosphorylation-activated protective function of mammalian Hsp27. J Biol Chem. 2004;279(22):23463–23471. doi: 10.1074/jbc.M402325200. [DOI] [PubMed] [Google Scholar]
- Tozawa-Ono A, Yoshida A, Yokomachi N, Handa R, Koizumi H, Kiguchi K, Ishizuka B, Suzuki N. Heat shock protein 27 and p16 immunohistochemistry in cervical intraepithelial neoplasia and squamous cell carcinoma. Hum Cell. 2012 Feb 3; doi: 10.1007/s13577-011-0040-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10(8):537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YP, Teng SC, Wu KJ. Direct regulation of HSP60 expression by c-MYC induces transformation. FEBS Lett. 2008;582(29):4083–4088. doi: 10.1016/j.febslet.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Tutar Y. Hsp70 in oncology. Recent Pat DNA Gene Seq. 2011;5:214–218. doi: 10.2174/187221511797636293. [DOI] [PubMed] [Google Scholar]
- Vaishampayan UN, Burger AM, Sausville EA, Heilbrun LK, Li J, Horiba MN, Egorin MJ, Ivy P, Pacey S, Lorusso PM. Safety, efficacy, pharmacokinetics, and pharmacodynamics of the combination of sorafenib and tanespimycin. Clin Cancer Res. 2010;16:3795–3804. doi: 10.1158/1078-0432.CCR-10-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedle EM, Khattak I, Ang CW, Nedjadi T, Jenkins R, Park BK, Kalirai H, Dodson A, Azadeh B, Terlizzo M, Grabsch H, Mueller W, Myint S, Clark P, Wong H, Greenhalf W, Neoptolemos JP, Rooney PS, Costello E. Low molecular weight heat shock protein HSP27 is a prognostic indicator in rectal cancer but not colon cancer. Gut. 2010;59:1501–1510. doi: 10.1136/gut.2009.196626. [DOI] [PubMed] [Google Scholar]
- Urushibara M, Kageyama Y, Akashi T, Otsuka Y, Takizawa T, Koike M, Kihara K. HSP60 may predict good pathological response to neoadjuvant chemoradiotherapy in bladder cancer. Jpn J Clin Oncol. 2007;37:56–61. doi: 10.1093/jjco/hyl121. [DOI] [PubMed] [Google Scholar]
- Viaud S, Ullrich E, Zitvogel L, Chaput N. Exosomes for the treatment of human malignancies. Horm Metab Res. 2008;40(2):82–88. doi: 10.1055/s-2007-1022548. [DOI] [PubMed] [Google Scholar]
- Visone R, Croce CM. MiRNAs and cancer. Am J Pathol. 2009;174:1131–1138. doi: 10.2353/ajpath.2009.080794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh N, Larkin A, Swan N, et al. RNAi knockdown of Hop (Hsp70/Hsp90 organising protein) decreases invasion via MMP-2 down regulation. Cancer Lett. 2011;306(2):180–189. doi: 10.1016/j.canlet.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Wang MH, Grossmann ME, Young CY. Forced expression of heat-shock protein 70 increases the secretion of Hsp70 and provides protection against tumour growth. Br J Cancer. 2004;90(4):926–931. doi: 10.1038/sj.bjc.6601583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhang P, Shi C, Yang Y, Qin H. Immunohistochemical detection of HSP27 and hnRNP K as prognostic and predictive biomarkers for colorectal cancer. Med Oncol. 2011 Aug 23; doi: 10.1007/s12032-011-0037-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Wang W, Xu X, Wang W, Shao W, Li L, Yin W, Xiu L, Mo M, Zhao J, He Q, He J. The expression and clinical significance of CLIC1 and HSP27 in lung adenocarcinoma. Tumour Biol. 2011b;32:1199–1208. doi: 10.1007/s13277-011-0223-0. [DOI] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Wei L, Liu TT, Wang HH, Hong HM, Yu AL, Feng HP, Chang WW. Hsp27 participates in the maintenance of breast cancer stem cells through regulation of epithelial-mesenchymal transition and nuclear factor-κB. Breast Cancer Res. 2011 Oct 24;13(5):R101. doi: 10.1186/bcr3042. 2011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerheide SD, et al. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323:1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood T, Wu C. Activation of drosophila heat shock factor: conformational changes associated with monomer-to-trimer transition. Mol. Cell. Biol. 1993;13:3481–3486. doi: 10.1128/mcb.13.6.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell L, Lindquist S. Inhibiting the transcription factor HSF1 as an anticancer strategy. Expert Opin Ther Targets. 2009;13:469–478. doi: 10.1517/14728220902832697. [DOI] [PubMed] [Google Scholar]
- Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5(10):761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A. 1994;91(18):8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmus MM, Boelens WC, Otte-Holler I, et al. Small heat shock protein HspB8: its distribution in Alzheimer’s disease brains and its inhibition of amyloid-beta protein aggregation and cerebrovascular amyloid-beta toxicity. Acta Neuropathol. 2006;111(2):139–149. doi: 10.1007/s00401-005-0030-z. [DOI] [PubMed] [Google Scholar]
- Workman P, et al. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann N Y Acad Sci. 2007;1113:202–216. doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- Wu C. Heat shock transcription factors: structure and regulation. Ann Rev Cell Dev Biol. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- Wu R, Kausar H, Johnson P, Montoya-Durango DE, Merchant M, Rane MJ. Hsp27 regulates Akt activation and polymorphonuclear leukocyte apoptosis by scaffolding MK2 to Akt signal complex. J Biol Chem. 2007;282(30):21598–21608. doi: 10.1074/jbc.M611316200. [DOI] [PubMed] [Google Scholar]
- Wu Y, Liu J, Zhang Z, et al. HSP27 regulates IL-1 stimulated IKK activation through interacting with TRAF6 and affecting its ubiquitination. Cell Signal. 2009;21(1):143–150. doi: 10.1016/j.cellsig.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Wu P, Zhang H, Qi L, Tang Q, Tang Y, Xie Z, Lv Y, Zhao S, Jiang W. Identification of ERp29 as a biomarker for predicting nasopharyngeal carcinoma response to radiotherapy. Oncol Rep. 27:987–994. doi: 10.3892/or.2011.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Liu Y, Rocchi P, Wang M, Fan Y, Qu F, Iovanna JL, Peng L. Targeting heat shock factor 1 with a triazole nucleoside analog to elicit potent anticancer activity on drug-resistant pancreatic cancer. Cancer Lett. 2012 May 28;318(2):145–153. doi: 10.1016/j.canlet.2011.09.043. 2012. [DOI] [PubMed] [Google Scholar]
- Xu L, Chen S, Bergan RC. MAPKAPK2 and HSP27 are downstream effectors of p38 MAP kinase-mediated matrix metalloproteinase type 2 activation and cell invasion in human prostate cancer. Oncogene. 2006;25:2987–2998. doi: 10.1038/sj.onc.1209337. [DOI] [PubMed] [Google Scholar]
- Xu X, Wang W, Shao W, Yin W, Chen H, Qiu Y, Mo M, Zhao J, Deng Q, He J. Heat shock protein-60 expression was significantly correlated with the prognosis of lung adenocarcinoma. J Surg Oncol. 2011;104:598–603. doi: 10.1002/jso.21992. [DOI] [PubMed] [Google Scholar]
- Yan LJ, Christians ES, Liu L, Xiao X, Sohal RS, Benjamin IJ. Mouse heat shock transcription factor 1 deficiency alters cardiac redox homeostasis and increases mitochondrial oxidative damage. Embo J. 2002;21(19):5164–72. doi: 10.1093/emboj/cdf528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JC, Agashe VR, Siegers K, Hartl FU. Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol. 2004;5(10):781–791. doi: 10.1038/nrm1492. [DOI] [PubMed] [Google Scholar]
- Yang F, Yin Y, Wang F, Wang Y, Zhang L, Tang Y, Sun S. miR-17-5p Promotes migration of human hepatocellular carcinoma cells through the p38 mitogen-activated protein kinase-heat shock protein 27 pathway. Hepatology. 2010;51:1614–1623. doi: 10.1002/hep.23566. [DOI] [PubMed] [Google Scholar]
- Yang X, Wang J, Zhou Y, Wang Y, Wang S, Zhang W. Hsp70 promotes chemoresistance by blocking Bax mitochondrial translocation in ovarian cancer cells. Cancer Lett. 2012 doi: 10.1016/j.canlet.2012.01.030. 10.1016/j.canlet.2012.01.030 [doi] [DOI] [PubMed] [Google Scholar]
- Yeh CH, Tseng R, Hannah A, et al. Clinical correlation of circulating heat shock protein 70 in acute leukemia. Leuk Res. 2010;34(5):605–609. doi: 10.1016/j.leukres.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CH, Tseng R, Zhang Z, et al. Circulating heat shock protein 70 and progression in patients with chronic myeloid leukemia. Leuk Res. 2009;33(2):212–217. doi: 10.1016/j.leukres.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, et al. A novel role of microRNA in late preconditioning: upregulation of endothelial nitric oxide synthase and heat shock protein 70. Circ Res. 2009;104:572–575. doi: 10.1161/CIRCRESAHA.108.193250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon YJ, Kim JA, Shin KD, Shin DS, Han YM, Lee YJ, Lee JS, Kwon BM, Han DC. KRIBB11 inhibits HSP70 synthesis through inhibition of heat shock factor 1 function by impairing the recruitment of positive transcription elongation factor b to the hsp70 promoter. J Biol Chem. 2011;286:1737–1747. doi: 10.1074/jbc.M110.179440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Nagahara M, Kuo C, Turner RR, Soon-Shiong P, Hoon DS. Lymphovascular invasion of colorectal cancer is correlated to SPARC expression in the tumor stromal microenvironment. Epigenetics. 2011;6:1001–1011. doi: 10.4161/epi.6.8.16063. [DOI] [PubMed] [Google Scholar]
- Yu Z, Zhi J, Peng X, Zhong X, Xu A. Clinical significance of HSP27 expression in colorectal cancer. Mol Med Report. 2010;3:953–958. doi: 10.3892/mmr.2010.372. [DOI] [PubMed] [Google Scholar]
- Zanini C, Pulerà F, Carta F, Giribaldi G, Mandili G, Maule MM, Forni M, Turrini F. Proteomic identification of heat shock protein 27 as a differentiation and prognostic marker in neuroblastoma but not in Ewing’s sarcoma. Virchows Arch. 2008;452:157–167. doi: 10.1007/s00428-007-0549-6. [DOI] [PubMed] [Google Scholar]
- Zantema A, Vries MV-D, Maasdam D, Bol S, Eb Avd. Heat shock protein 27 and αB-cristallin can form a complex, which dissociates by heat shock. J Biol Chem. 1992;267(18):12936–12941. [PubMed] [Google Scholar]
- Zhang Y, et al. Protein kinase A regulates molecular chaperone transcription and protein aggregation. Plos One Epub. 2011 doi: 10.1371/journal.pone.0028950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Luo D, Miao R, et al. Hsp90-Akt phosphorylates ASK1 and inhibits ASK1-mediated apoptosis. Oncogene. 2005;24(24):3954–3963. doi: 10.1038/sj.onc.1208548. [DOI] [PubMed] [Google Scholar]
- Zhang L, Pang E, Loo RR, Rao J, Go VL, Loo JA, Lu QY. Concomitant inhibition of HSP90, its mitochondrial localized homologue TRAP1 and HSP27 by green tea in pancreatic cancer HPAF-II cells. Proteomics. 2011a;11:4638–4647. doi: 10.1002/pmic.201100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Wong LL, Koay ES. Phosphorylation of Ser78 of Hsp27 correlated with HER-2/neu status and lymph node positivity in breast cancer. Mol Cancer. 2007;6:52–57. doi: 10.1186/1476-4598-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YH, et al. Upregulation of lactate dehydrogenase A by ErbB2 through heat shock factor 1 promotes breast cancer cell glycolysis and growth. Oncogene. 2009;28:3689–3701. doi: 10.1038/onc.2009.229. [DOI] [PubMed] [Google Scholar]
- Zhuang H, Jiang W, Cheng W, Qian K, Dong W, Cao L, Huang Q, Li S, Dou F, Chiu JF, Fang XX, Lu M, Hua ZC. Down-regulation of HSP27 sensitizes TRAIL-resistant tumor cell to TRAIL-induced apoptosis. Lung Cancer. 2010;68:27–38. doi: 10.1016/j.lungcan.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Tassi L, Lane W, Mendelsohn ME. Specific binding of the transglutaminase, platelet factor XIII, to HSP27. J Biol Chem. 1994;269(35):22379–22384. [PubMed] [Google Scholar]
- Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94(4):471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- Zimmermann M, Nickl S, Lambers C, Hacker S, Mitterbauer A, Hoetzenecker K, Rozsas A, Ostoros G, Laszlo V, Hofbauer H, Renyi-Vamos F, Klepetko W, Dome B, Ankersmit HJ. Discrimination of clinical stages in non-small cell lung cancer patients by serum HSP27 and HSP70: A multi-institutional case-control study. Clin Chim Acta. 2012 Mar 23; doi: 10.1016/j.cca.2012.03.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8(1):59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- Zou J, et al. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- Zoubeidi A, Zardan A, Beraldi E, et al. Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res. 2007;67(21):10455–65. doi: 10.1158/0008-5472.CAN-07-2057. [DOI] [PubMed] [Google Scholar]
- Zoubeidi A, Zardan A, Wiedmann RM, Locke J, Beraldi E, Fazli L, Gleave ME. Hsp27 promotes insulin-like growth factor-I survival signaling in prostate cancer via p90Rsk-dependent phosphorylation and inactivation of BAD. Cancer Res. 2010;70:2307–2317. doi: 10.1158/0008-5472.CAN-09-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]