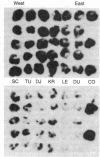
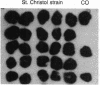
Abstract
Genetic and molecular investigations were carried out with Eurasian Drosophila melanogaster populations on the P-M system of hybrid dysgenesis. In 27 strains sampled from France to Middle Asia, a clear gradient exists between Western Europe, in which most modern strains are of the Q type, and eastern areas, in which M-cytotype strains predominate. Molecular analysis on individual flies was performed with two complementary probes of the cloned 2.9-kilobase P element. The results provide evidence for a gradually decreasing frequency of P elements from west to east, but the presence of P-homologous sequences has been ascertained in all of the wild M-cytotype populations analyzed. Moreover, some active P elements with GD sterility potential were revealed in the majority of M-cytotype populations when tested with a highly sensitive reference line. The gradual change in distribution of the polymorphic P family in Eurasia is discussed in relation to the structure of the elements together with the theories of P-M evolution and is interpreted as the present invasion of Eurasian populations by these elements.
Keywords: transposable elements, hybrid dysgenesis, DNA hybridization, geographical variation
Full text
PDF


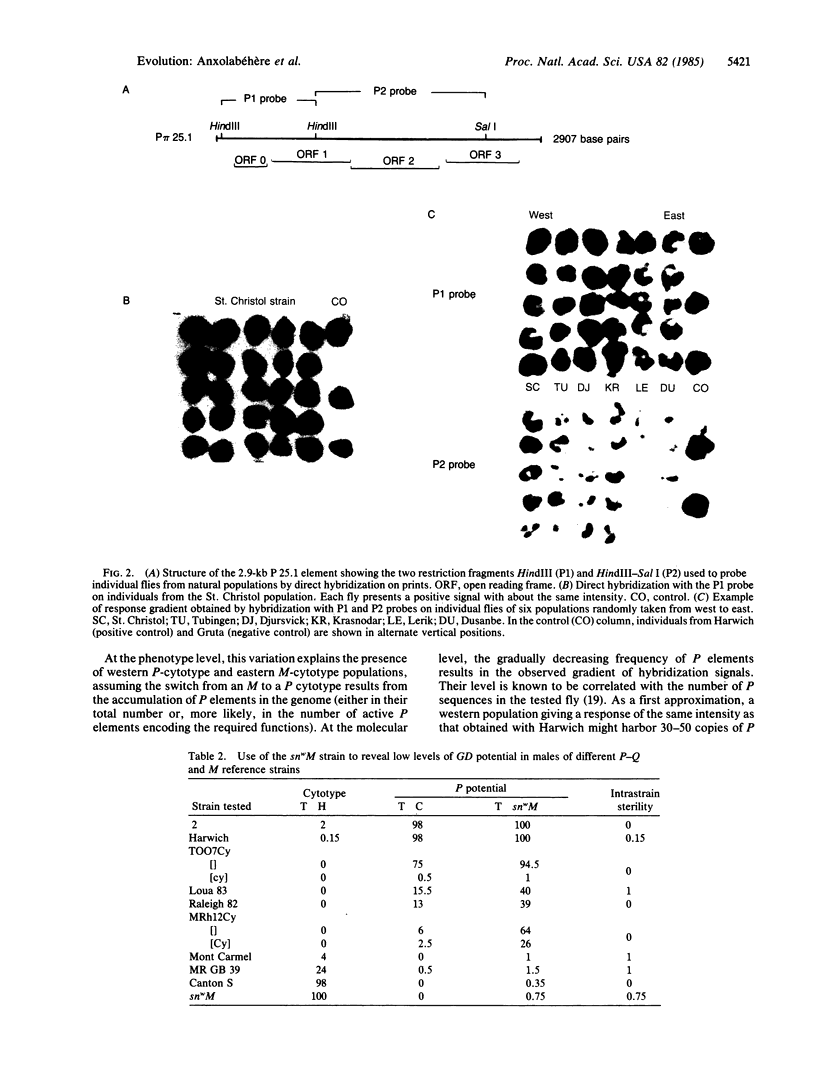

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anxolabéhère D., Nouaud D., Périquet G. Cytotype polymorphism of the P-M system in two wild populations of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7801–7803. doi: 10.1073/pnas.79.24.7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham P. M., Kidwell M. G., Rubin G. M. The molecular basis of P-M hybrid dysgenesis: the role of the P element, a P-strain-specific transposon family. Cell. 1982 Jul;29(3):995–1004. doi: 10.1016/0092-8674(82)90463-9. [DOI] [PubMed] [Google Scholar]
- Brookfield J. F., Montgomery E., Langley C. H. Apparent absence of transposable elements related to the P elements of D. melanogaster in other species of Drosophila. 1984 Jul 26-Aug 1Nature. 310(5975):330–332. doi: 10.1038/310330a0. [DOI] [PubMed] [Google Scholar]
- Bucheton A., Paro R., Sang H. M., Pelisson A., Finnegan D. J. The molecular basis of I-R hybrid dysgenesis in Drosophila melanogaster: identification, cloning, and properties of the I factor. Cell. 1984 Aug;38(1):153–163. doi: 10.1016/0092-8674(84)90536-1. [DOI] [PubMed] [Google Scholar]
- Daniels S. B., Strausbaugh L. D., Ehrman L., Armstrong R. Sequences homologous to P elements occur in Drosophila paulistorum. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6794–6797. doi: 10.1073/pnas.81.21.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels W. R. A trans-acting product needed for P factor transposition in Drosophila. Science. 1984 Dec 7;226(4679):1194–1196. doi: 10.1126/science.6095450. [DOI] [PubMed] [Google Scholar]
- Engels W. R. Germline hypermutability in Drosophila and its relation to hybrid dysgenesis and cytotype. Genetics. 1981 Jul;98(3):565–587. doi: 10.1093/genetics/98.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels W. R. The P family of transposable elements in Drosophila. Annu Rev Genet. 1983;17:315–344. doi: 10.1146/annurev.ge.17.120183.001531. [DOI] [PubMed] [Google Scholar]
- Kidwell M. G. Evolution of hybrid dysgenesis determinants in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1655–1659. doi: 10.1073/pnas.80.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell M. G., Kidwell J. F., Sved J. A. Hybrid Dysgenesis in DROSOPHILA MELANOGASTER: A Syndrome of Aberrant Traits Including Mutation, Sterility and Male Recombination. Genetics. 1977 Aug;86(4):813–833. doi: 10.1093/genetics/86.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare K., Rubin G. M. Structures of P transposable elements and their sites of insertion and excision in the Drosophila melanogaster genome. Cell. 1983 Aug;34(1):25–35. doi: 10.1016/0092-8674(83)90133-2. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Kidwell M. G., Bingham P. M. The molecular basis of P-M hybrid dysgenesis: the nature of induced mutations. Cell. 1982 Jul;29(3):987–994. doi: 10.1016/0092-8674(82)90462-7. [DOI] [PubMed] [Google Scholar]
- Schaefer R. E., Kidwell M. G., Fausto-Sterling A. Hybrid Dysgenesis in DROSOPHILA MELANOGASTER: Morphological and Cytological Studies of Ovarian Dysgenesis. Genetics. 1979 Aug;92(4):1141–1152. doi: 10.1093/genetics/92.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons M. J., Raymond J. D., Culbert T. P., Laverty T. R. Analysis of dysgenesis-induced lethal mutations on the X chromosome of a Q strain of Drosophila melanogaster. Genetics. 1984 May;107(1):49–63. doi: 10.1093/genetics/107.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchen P., Fuchs R. P., Sage E., Leng M. Chemically modified nucleic acids as immunodetectable probes in hybridization experiments. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3466–3470. doi: 10.1073/pnas.81.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]