Abstract
Purpose
To determine the incidence, ophthalmic manifestations, and survival among children with neuroblastoma in a defined population.
Design
Population-based retrospective cohort.
Methods
The medical records of all pediatric (< 19 years) residents of Olmsted County, Minnesota diagnosed with neuroblastoma from January 1, 1969, through December 31, 2008, were retrospectively reviewed.
Results
Fourteen children were diagnosed with neuroblastoma as residents of Olmstead County, Minnesota, during the 40-year period, yielding an age- and gender-adjusted incidence of 11.8 (95% confidence interval [CI]: 5.6 -18.0) per million patients < 15 years of age. The calculated incidence for patients presenting before the age of 5 in this cohort was 1 in 5970 children (95% CI: 3920 – 12580 children). The mean age at diagnosis for the 14 study patients was 22.5 months (range, .4 - 42.6 months). Six (43%) of the 14 (95% CI: 18% -71%) had ocular manifestations, including orbital metastasis in 6 (100%), proptosis and ecchymosis in 4 (67%), ptosis in 2 (33%), and strabismus in 1 (17%). The Kaplan-Meier rate of survival for all 14 children was 57% at 1 year (95% CI: 36% – 90%) and 50% at 5 years (95% CI: 30% - 84%), while the 6 with eye findings had a survival rate of 17% at 9 months (95% CI: 3-100%).
Conclusions
The incidence of neuroblastoma in this population was 11.8 per million patients < 15 years, with ophthalmic involvement observed in 6 (43%) of the 14 study patients. Orbital metastasis in the 6 children in this cohort was associated with poor prognosis.
Neuroblastoma is the most common extracranial solid tumor among children under the age of 5 years, with a published incidence for that age of approximately 1 in 7000 children.1-4 Neuroblastic tumors are derived from primordial neural crest cells and ultimately populate the sympathetic ganglia, adrenal medulla, and other sites.4 Recent advancements in the understanding of tumor biology have aided in the diagnosis and medical management of this disease. However, cases of widespread metastasis, at times signaled by proptosis, ecchymosis, and other signs of orbital involvement, continue to have a poor prognosis.5 Ophthalmic manifestations are well documented and include proptosis,1,6,7 periorbital ecchymosis,1,6,7 Horner syndrome,8-11 opsoclonus/myoclonus,12 ocular motility defects,1,13 ptosis,13 and blindness.6,14
Retrospective reviews conducted at large referral centers have found orbital metastasis in 10-20% of cases with neuroblastoma;1,6,7 however, there have been no population-based studies with which to compare these findings. The purposes of this study are to report the incidence and ophthalmic manifestations of neuroblastoma among a cohort of patients < 19 years diagnosed as residents of Olmsted County, Minnesota, over a defined 40-year period, and to calculate survival rates based on ophthalmic involvement.
Subjects and Methods
The medical records of all pediatric (< 19 years) patients residing in Olmsted County when diagnosed with neuroblastoma from January 1, 1969, through December 31, 2008, were retrospectively reviewed. Potential cases of neuroblastoma were identified using the resources of the Rochester Epidemiology Project, a medical record linkage system designed to capture data on any patient-physician encounter in Olmsted County, Minnesota.15 The racial distribution of Olmsted County residents in 1990 was 95.7% Caucasian, 3.0% Asian-American, 0.7% African-American, 0.3% each Native American and other. The population of this county (106,470 in 1990) is relatively isolated from other urban areas, and essentially all medical care is provided to residents by the Mayo Clinic or the Olmsted Medical Group and their affiliated hospitals.
All diagnosis were entered into the Rochester Epidemiology Project database and residency status was verified by specially-trained personnel. Children not living in Olmsted County at the time of their diagnosis were excluded. Neuroblastoma was defined in this study by clinical diagnosis, based on tumor biopsy results, catecholamine levels, and other systemic findings. Data collected included age at diagnosis, presenting symptoms, tumor staging, treatment received, ophthalmic involvement, and final outcome.
The cumulative probability of death was estimated using the Kaplan-Meier method.16 Continuous data were presented as a mean with the range. Categorical data were presented as counts and percentage. Annual age- and gender-specific incidences rates were constructed using the age- and gender-specific population figures for the county from the US Census. Estimates from the State of Minnesota were used to aid with linear interpolation of the 1970, 1980, 1990, and 2000 census years. The 95% confidence intervals were calculated with assumptions based on the Poisson distribution.
Results
A total of 14 patients < 19 years of age were diagnosed with neuroblastoma as residents of Olmsted County, Minnesota, during the 40-year study period, yielding an annual age- and gender-adjusted incidence of 11.8 cases per million children < 15 years of age. All 14 cases presented before the age of 5, leading to an age-adjusted incidence of 33.5 cases per million, or 1 per 5970 children < 5 years.
Clinical information, including age at diagnosis, presenting symptoms, ophthalmic site, tumor stage, and final outcome where recorded for all 14 patients (Table). The mean age at diagnosis for all 14 was 22.5 months (range, 0.4 – 42.6 months) and 8 (57%) were female. The adrenal gland was the primary site in 10 (72%) of the 14, 3 (21%) were in the abdomen, and 1 (7%) was located in both the mediastinum and thoracic spine. Eight (57%) of the 14 were diagnosed as stage IV, with 2 (14%) each of stage II, III, and IVS.
Table 1. Historical and clinical characteristics of 14 patients < 19 years diagnosed with Neuroblastoma in Olmsted County, MN, 1969-2008.
| Case No. (year at diagnosis) | Age at diag. (mon) | Gender | Presenting symptoms | Primary site | Ophthalmic site/manifestations | Ophthalmic complications | Treatment | F/U duration (months) | Final outcome | Stage |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (1969) | 42.6 | F | Bilateral periorbital ecchymosis | R adrenal | Bilateral orbits/painful proptosis | Scleral hemorrhage | Chemotherapy | 5.2 | Pt deceased | IV |
| 2 (1970) | 21.5 | M | Decreased appetite, listless, afebrile, hematuria | L adrenal | Right orbit/Ptosis, right subconjunctival hemorrhage secondary to right temporal mass | Sluggish pupillary response | Radiation and chemotherapy | 3.1 | Pt Deceased | IV |
| 3 (1971) | 16.5 | F | Fiver, rhinorrhea, cough, swollen L inguinal nodes | L adrenal | None | Resection, L adrenal and L inguinal nodes | 309.6 | Remission | IV | |
| 4 (1971) | 4.6 | F | Fever, vomiting, palpated RUQ mass | R adrenal | None | Resection, R adrenal mass and L supraclavicular mass | 454.6 | Remission | IVS | |
| 5 (1976) | 29.7 | F | Listless, purpura, thrombocytopenia, anemia | L adrenal | Bilateral orbits/Right proptosis, with ecchymosis and ptosis. Bilateral temporal tumor nodules | Difficulty depressing and abducting the right eye | Resection of L adrenal, L metastatic lymph nodes, L nephrectomy. Radiation and chemotherapy | 3.9 | Pt deceased | IV |
| 6 (1977) | 34.8 | F | Irritability, dehydration, fever, lethargy | L adrenal | Bilateral orbits/Left proptosis with inward and medial displacement of the left globe. Bilateral metastasis | Left esotropia with III, IV, and VI nerve palsies | Resection of L adrenal, L kidney, and L peri-aortic lymph nodes. Radiation and chemotherapy | 8.7 | Pt deceased | IV |
| 7 (1981) | 24.5 | M | Fatigue, fever, poor appetite, swollen L testicle | L adrenal | Left orbit/Left proptosis and ecchymosis | None | Radiation and chemotherapy | 3.5 | Pt deceased | IV |
| 8 (1987) | 29.5 | M | Bilateral periorbital ecchymosis, tender abdomen, ataxic gait | R abdomen | Bilateral orbits/Bilateral periorbital ecchymosis. Left eye swelling with hemorrhage. | None | Resection of R abdominal mass and peri-aortic lymph nodes | 9.1 | Pt deceased | IV |
| 9 (1988) | 37.9 | M | Hydronephrosis, noted abdominal mass | R abdomen | None | Resection of abdominal mass. chemotherapy | 245.4 | Remission | III | |
| 10 (1990) | 36.2 | F | Decreased appetite, irritability, palpatable LUQ mass. | Abdomen | None | Chemotherapy | 17.5 | Pt deceased | IV | |
| 11 (1993) | 31.3 | F | Ataxia, irritability, fever, urinary incontinence | R adrenal | None | Resection of R adrenal tumor. Chemotherapy | 142.1 | Remission | III | |
| 12 (1997) | 4.9 | M | 6 wk history of cough, nasal congestion. Chest x-ray showed mass | Mediastinum, thoracic spine | None | Resection of epidural tumor and mediastinal mass. | 144.0 | Remission | II | |
| 13 (1997) | .6 | M | Irritability, vomiting, diarrhea | L adrenal | None | Resection of L adrenal tumor | 142.9 | Remission | II | |
| 14 (2004) | .4 | F | Pre-natal ultrasound | R adrenal | None | Resection of R adrenal tumor. Chemotherapy | 60.6 | Remission | IVS |
Ophthalmic involvement was noted in 6 (43%) (95% CI: 18% - 71%) of the 14 cases and was the presenting symptom in 2 (14%). Metastasis to the orbit was seen in all 6 cases with ocular involvement. There were 4 bilateral presentations and 1 each affecting the left and right orbit respectively. Proptosis and ecchymosis occurred in 4 (67%) of the 6 cases, ptosis was observed in 2 (33%), and 1 (17%) patient had strabismus. The 9-month survival in patients with orbital metastasis was 17% (95% CI: 3-100%) with an average age at diagnosis of 30.3 months (range, 21.5 – 42.6 months).
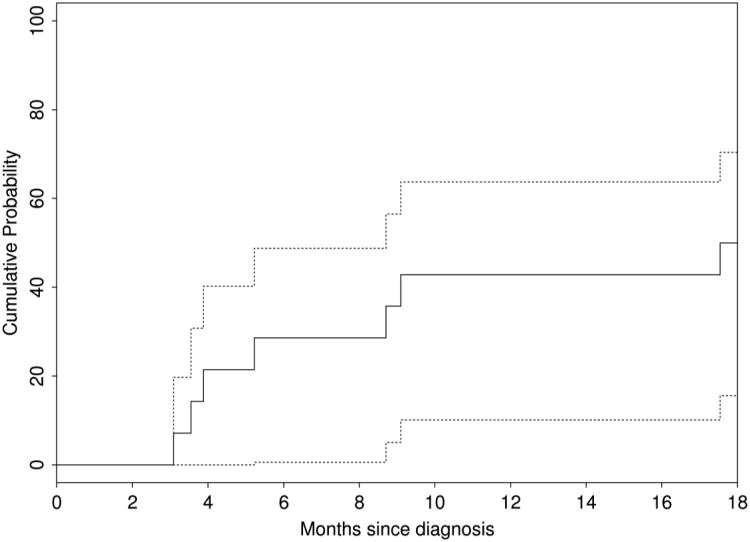
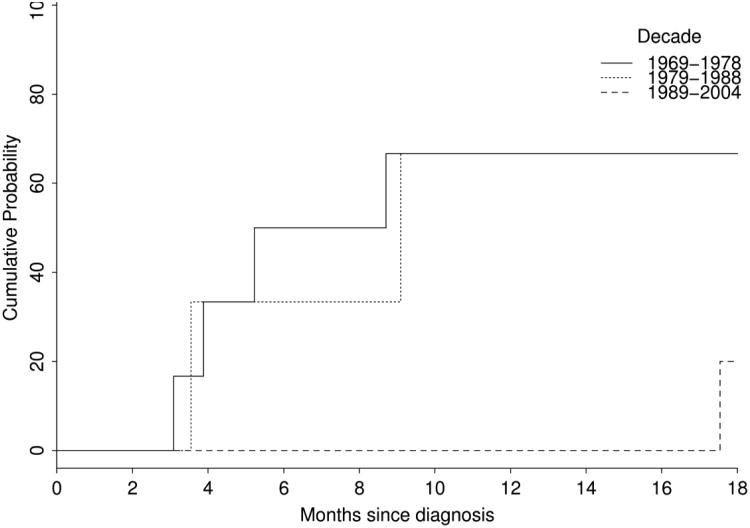
Seven of the 14 cases achieved clinical remission yielding a survival rate of 50% (95% CI: 30-84%) at 18 months (Figure 1). Survival was heavily age- and stage-dependent, and overall survival varied with the decade of the study, with markedly improved survival noted in cases presenting after 1980 (Figure 2). The 4 cases that presented before 1 year of age had a 100% 5-year survival, while the 10 cases presenting after their first birthday had a 5-year survival of only 30%. The 5-year survival for all 8 stage IV presentations was 13%. The mean survival for all 14 cases was 110.7 months (range, 3.1 – 454.6 months), while average survival for those with ocular involvement was only 5.6 months (range, 3.1 – 9.1 months).
Figure 1.
Kaplan-Meier cumulative probability of survival in pediatric patients diagnosed with Neuroblastoma in Olmsted County, MN, 1969-2008.
Figure 2.
Kaplan-Meier cumulative probability of survival by decade of diagnosis in pediatric patients diagnosed with Neuroblastoma in Olmsted County, MN, 1969-2008.
Discussion
Neuroblastoma was diagnosed in 14 children or 11.8 per million patients less than 15 years of age as resident of Olmsted County, Minnesota, during the 40-year study period. Ocular involvement occurred in 6 (43%) with orbital metastasis observed in all 6 patients. The Kaplan-Meier rate of survival for all 14 children was 50% at 5-years and 0% at 1 year for the 6 children with orbital metastasis. None of the children had Horner syndrome or opsoclonus.
Neuroblastoma accounts for 8% to 10% of all childhood cancers, with a gender-and race-adjusted published incidence of 9.8 annual cases per children < 15 years.17 Prior studies of incidence are lower than that reported in this study; however, our findings are within the upper range reported by the Surveillance, Epidemiology and End Results Program (SEER) of the United States National Cancer Institute, which recorded incidence rates ranging from 7/106 to 12.6/106 in developed countries.3,18 In addition, our results are similar to the 11.1/106 obtained in a comprehensive review of all cases of neuroblastoma in Denmark from 1943-1980.19 Most cases of neuroblastoma present by the age of 5 and it is rare to see the disease present after the age of 10.4 Current reports have estimated that 1 in 7000 children < 5 years will be diagnosed with neuroblastoma.1-4 The calculated incidence for patients presenting before the age of 5 in this cohort was 1 in 5970 children (95% CI: 3,920 – 12,580 children).
Nearly half (43%) of the patients in this series manifested orbital metastasis, which is significantly higher than the 10-20% orbital metastasis currently reported in literature.1,6,7 Five (83%) of the 6 patients were diagnosed in the first 12 years of the study and none presented during the last 20 years. These 5 cases all presented with advanced stage disease, which may explain the increased occurrence of ocular metastasis. However, Musarella et al7 reviewed 405 cases from 1919 through approximately 1980 and observed ocular involvement in only 80 (19.8%). More recently, Belgaumi et al6 reviewed cases from 1971-1994 and observed ocular involvement in 47 (10.4%) of 450 cases; however, their study only included cases that presented with ophthalmic involvement. Orbital involvement was the presenting sign in only 2 (14%) of the 14 cases during this study. It is likely that their overall percentage of ocular involvement would have been higher had they recorded ophthalmic involvement observed after diagnosis. Survival in this group was 0% at 12 months, owing largely to the advanced stage of disease that defines orbital metastasis.20
Orbital metastatic neuroblastoma, presenting with proptosis and periorbital ecchymosis, is considered one of the classic signs of neuroblastoma in children.4,6 In this cohort, proptosis and ecchymosis were the two most frequent ocular manifestations of neuroblastoma, each observed in 4 (67%) of the 6 cases. This result is slightly higher than the published range of 38% to 60% for this finding in cases of ophthalmic involvement 6,7,13 and may be due in part to the number of cases (5) that presented in advanced stage during the first decade of the study.
Horner syndrome and opsoclonus are infrequent manifestations of neuroblastic tumor mass effect on the sympathetic innervation of the eye.9-11,21 Horner syndrome is associated with localized neuroblastoma and consequently demonstrates markedly better survival rates than those seen in cases of orbital metastasis.7 The absence of Horner syndrome in this cohort is consistent with the findings of Weinstein et al21 and Wilhelm et al,22 who found no underlying mass lesion among their patients with pediatric Horner syndrome, and Jeffrey et al11 who found only 3 (4.1%) of 73 cases due to a neuroblastoma. Despite the frequent association of Horner syndrome and neuroblastoma in the literature,8-10,23 Musarella et al7 and Jaffe et al24 have concluded that as few as 3.5-13% of children with neuroblastoma have an associated Horner syndrome, while only 2.2% present with Horner syndrome as the initial symptom. Although rare, the gravity of pediatric Horner syndrome due to neuroblastoma understates the importance of a careful work-up to preclude an underlying mass lesion. Presentations of idiopathic pediatric Horner syndrome warrant a thorough physical exam, including palpation of the neck, abdomen, and axilla, and spot urine testing of homovanillic acid (HVA) and vanillylmandelic acid (VMA).10 Further investigation, including imaging, should be based on physical findings (including acquired or increasing iris heterochromia), and the relative incidence of neuroblastoma by age.11
Opsoclonus-myoclonus, a well-documented but rare sign of neuroblastoma, was also not observed in this cohort.7,12,25 Musarella et al7 observed opsoclonus in only 9 (2.2%) of 405 cases. Belgaumi et al,6 describing a cohort of 450 patients, and others,13,14,26 did not observe this paraneoplastic symptom. A population-based study of opsoclonus-myoclonus in the United Kingdom over a two-year period found an incidence of 0.18 cases per million total population per year.25 Only 4 of the 15 cases of opsoclonus-myoclonus were observed to have an underlying neuroblastoma. Given the small sample size of this study, it is not surprising that opsoclonus-myoclonus was not observed in this cohort.
The two most important clinical factors for predicting outcomes in neuroblastoma are the stage of the disease and age at diagnosis.2-4,7,27,28 According to the International Neuroblastoma Staging System, orbital involvement is a sign of stage IV disease.20 In this study, 8 (57%) of 14 cases presented as stage IV, and their 5-year survival was 13%. Published reports of 3- or 5- year survival rates in patients with stage IV disease show significant improvement in outcomes in recent years, reflecting improved efficacy of treatment regiments.29 Survival rates for patients diagnosed with stage IV disease after 1 year of age range from 2.5% mid-century30 to 16% in the 1980s3 and 38% in the 1990s.31 In this cohort, only 2 patients were diagnosed with stage IV disease after 1985, explaining in part the lower than expected survival in these patients.
The 4 cases in this study that presented before their first birthday had a 5-year survival of 100%, while the 10 diagnosed after 1 year had a 5-year survival of 30%. Comparative data from the SEER program reports a 5-year survival of 90.2% for those diagnosed at less than 1 year, while only 66.1% of those diagnosed between the ages of 1 through 4 years achieved a 5-year survival.17 It is likely that a larger sample size would have yielded results closer to those in other published reports.
There are several limitations to the findings in this study. First, the small sample size makes it difficult to calculate accurate incidence rates with which to compare to other studies. However, the extended duration of the study, the accuracy of residency status analysis made possible by the Rochester Epidemiology Project, and the relatively infrequent occurrence of Olmsted County residents seeking medical care in other urban centers allows for an accurate calculation from this population. The small sample size also decreases the likelihood of identifying relatively infrequent ophthalmic manifestations of neuroblastoma such as Horner syndrome and opsoclonus-myoclonus. However, theses signs are infrequently noted in patients with neuroblastoma.6,13,21,22,25,26 Finally, the largely Caucasion patient population of Olmsted County (95.7%) best translates to semiurban, white populations in the United States and thereby may limit the generalizability of calculated incidence and survival rates to more diverse populations. Statistics from the Pediatric Oncology Group (POG) indicate that neuroblastoma is 9.5% more common among African-Americans patients, which suggest that a more diverse patient population may have had a higher incidence of neuroblastoma.4
Neuroblastoma was diagnosed in 11.8 per million children < 15 years in this population, with a mean 5-year survival of 50.0%. Ocular involvement, consisting solely of orbital metastasis, was observed in 6 (43%) of the 14 cases, with a mean survival of 4.4 months (range, 3.1 – 9.1). Proptosis and ecchymosis, seen in 4 (67%) of the 6 cases, were the most common signs of orbital involvement, and were the presenting sign of neuroblastoma in 2 (14%) of the 14 cases. The Kaplan-Meier survival rate for the entire cohort was 50% at 5 years (95% CI: 30-84%) while none of the 6 patients with ocular involvement survived.
Acknowledgments
This project was supported in part by an unrestricted grant from Research to Prevent Blindness, Inc., New York, NY and made possible by the Rochester Epidemiology Project (Grant #R01-AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases).
This study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Group.
Biographies
Brian G. Mohney, MD, is Professor of Ophthalmology and Program Director for Pediatric Ophthalmology and Adult Strabismus Fellowship at Mayo Clinic.

Stephen J. Smith graduated from Grove City College in May 2006 with a BS in molecular biology. He is currently a second year medical student at Mayo Medical School, planning to graduate in 2012.

Footnotes
Disclosure Contributions of the authors: Design of the study: (SS, BM); Conducting the study: (SS, BM); Data analysis: (SS, ND, BS, BM); Preparation of the manuscript: (SS, ND, BS, BM); Review of the manuscript: (SS, BS, BM); Approval of the manuscript: (SS, ND, BS, BM).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahmed S, Goel S, Khandwala M, Agrawal A, Chang B, Simmons IG. Neuroblastoma with orbital metastasis Ophthalmic presentation and role of ophthalmologists. Eye. 2006;20:466–470. doi: 10.1038/sj.eye.6701912. [DOI] [PubMed] [Google Scholar]
- 2.Woods WG, Gao RN, Shuster JJ, et al. Screening of Infants and Mortality Due to Neuroblastoma. N Engl J Med. 2002;346:1041–1046. doi: 10.1056/NEJMoa012387. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein ML, Leclerc JM, Bunin G, et al. A population-based study of neuroblastoma incidence, survival and mortality in North America. J Clin Oncol. 1992;10:323–329. doi: 10.1200/JCO.1992.10.2.323. [DOI] [PubMed] [Google Scholar]
- 4.Castleberry RP. Biology and Treatment of Neuroblastoma. Pediatr Clin North Am. 1997;44:919–937. doi: 10.1016/s0031-3955(05)70537-x. [DOI] [PubMed] [Google Scholar]
- 5.Shields JA, Shields CL, Brotman HK, et al. Cancer Metastatic to the Orbit, The 2000 Robert M. Curts Lecture. Ophthalmic Plastic and Reconstructive Surgery. 2001;17(5):346–354. doi: 10.1097/00002341-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Belgaumi AF, Kauffman WM, Jenkins JJ, et al. Blindness in Children with Neuroblastoma. Cancer. 1997;80(10):1997–2004. [PubMed] [Google Scholar]
- 7.Musarell MA, Chan HS, DeBoer G, Gallie BL. Ocular Involvement in Neuroblastoma: Prognostic Implications. Ophthalmology. 1984;91:936–940. doi: 10.1016/s0161-6420(84)34211-7. [DOI] [PubMed] [Google Scholar]
- 8.Zafeiriou DI, Economou M, Koliouskas D, et al. Congenital Horner's Syndrome associated with cervical neuroblastoma. Eur J of Paediatric Neurology. 2006;10:90–92. doi: 10.1016/j.ejpn.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Saur C, Levinsohn MW. Horner's Syndrome in Childhood. Neurology. 1976;26:216–220. doi: 10.1212/wnl.26.3.216. [DOI] [PubMed] [Google Scholar]
- 10.Mahoney NR, Liu GT, Menacker SJ, et al. Pediatric Horner Syndrome: Etiologies and Roles of Imaging and Urine Studies to Detect Neuroblastoma and Other Responsible Mass Lesions. Am J Ophthalmol. 2006;142:651–659. doi: 10.1016/j.ajo.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 11.Jeffery AR, Ellis FJ, Repka MX, Buncic JR. Pediatric Horner Syndrome. J AAPOS. 1998;2:159–167. doi: 10.1016/s1091-8531(98)90008-8. [DOI] [PubMed] [Google Scholar]
- 12.Farrelly C, Daneman A, Chan H, Martin D. Occult Neuroblastoma Presenting with Opsomyoclonus: Utility of Computed Comography. AJR. 1984;142:807–810. doi: 10.2214/ajr.142.4.807. [DOI] [PubMed] [Google Scholar]
- 13.Alfano JE. Ophthalmological aspects of neuroblastomatosis: a study of 53 verified cases. Trans Am Acad Ophthalmol Otalaryngol. 1968;72:830–848. [PubMed] [Google Scholar]
- 14.Shubert EE, Oliver GL, Jaco NT. Metastatic neuroblastoma causing bilateral blindness. Canad J Ophthal. 1969;4:100–103. [PubMed] [Google Scholar]
- 15.Kurland L, Molgaard CA. The patient record in epidemiology. Sci Am. 1981;245(4):54–63. doi: 10.1038/scientificamerican1081-54. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 17.Horner MJ, Ries LAG, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2006. National Cancer Institute; Bethesda, MD: 2009. http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site. [Google Scholar]
- 18.Davis S, Rogers MAM, Pendergrass TW. The incidence and epidemiologic characteristics of neuroblastoma in the United States. Am J Epidemiol. 1987;126:1063–1074. doi: 10.1093/oxfordjournals.aje.a114745. [DOI] [PubMed] [Google Scholar]
- 19.Carlsen NLT. Epidemiological investigations of neuroblastomas in Denmark 1943-1980. Br J Cancer. 1986;54:977–988. doi: 10.1038/bjc.1986.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brodeur GM, Seeger RC, Barrett A, et al. International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J Clin Oncol. 1988;6:1874–1881. doi: 10.1200/JCO.1988.6.12.1874. [DOI] [PubMed] [Google Scholar]
- 21.Weinstein JM, Zweifel TJ, Thompson HS. Congenital Horner syndrome. Arch Ophthalmol. 1980;98:1074–1078. doi: 10.1001/archopht.1980.01020031064011. [DOI] [PubMed] [Google Scholar]
- 22.Wilhelm H, Ochsner H, Kopycziok E, et al. Horner's syndrome: a retrospective analysis of 90 cases and recommendations for clinical handling. German J Ophthalmol. 1992;1:96–102. [PubMed] [Google Scholar]
- 23.Woodruff G, Buncic JR, Morin JD. Horner's Syndrome in Children. J Pediatr Ophthalmol Strab. 1988;25:40–44. doi: 10.3928/0191-3913-19880101-11. [DOI] [PubMed] [Google Scholar]
- 24.Jaffe N, Cassady JR, Filler RM, et al. Heterochromasia and Horner's syndrome associated with cervical and mediastinal neuroblastoma. J Pediatr. 1975;87:75–77. doi: 10.1016/s0022-3476(75)80072-2. [DOI] [PubMed] [Google Scholar]
- 25.Pang KK, Sousa CD, Lang B, Pike MG. A prospective study of the presentation and management of dancing eye syndrome/opsoclonus-myoclonus syndrome in the United Kingdom. Eur J of Paediatric Neurology. 2009 doi: 10.1016/j.ejpn.2009.03.002. forthcoming. [DOI] [PubMed] [Google Scholar]
- 26.Blake J, Fitzpatrick C. Eye signs in neuroblastoma. Trans Ophthalmol Soc U K. 1972;92:825–833. [PubMed] [Google Scholar]
- 27.Carlsen NLT, Christensen IJ, Schroeder H, et al. Prognostic factors in neuroblastomas treated in Denmark from 1943 to 1980. A statistical estimate of prognosis based on 253 cases. Cancer. 1986;58:2726. doi: 10.1002/1097-0142(19861215)58:12<2726::aid-cncr2820581229>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 28.Coldman AJ, Fryer CJH, Elwood JM, Sonley MJ. Neuroblastoma: Influence of age at diagnosis, stage, tumor site, and sex on prognosis. Cancer. 1980;46:1896–1901. doi: 10.1002/1097-0142(19801015)46:8<1896::aid-cncr2820460833>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 29.Gutierrez JC, Fischer AC, Sola JE, Perez EA, Koniaris LG. Markedly improving survival of neuroblastom: a 30-year analysis of 1,646 patients. Pediatr Surg Int. 2007;23(7):636–646. doi: 10.1007/s00383-007-1933-7. [DOI] [PubMed] [Google Scholar]
- 30.Breslwo N, McCann B. Statistical estimation of prognosis for children with neuroblastoma. Cancer Res. 1971;31:2098–2103. [PubMed] [Google Scholar]
- 31.Kaneko M, Tsuchida Y, Mugishima H, et al. Intensified chemotherapy increases the survival rates in patients with stage 4 neuroblastoma with MYCN amplification. J Pediatr Hematol Oncol. 2002;24(8):613–621. doi: 10.1097/00043426-200211000-00004. [DOI] [PubMed] [Google Scholar]