Abstract
Background
Multiple studies demonstrate poor adherence to medications prescribed for chronic illnesses, including osteoporosis, but few interventions have been proven to enhance adherence. We examined the effectiveness of a telephone-based counseling program rooted in motivational interviewing to improve medication adherence for osteoporosis.
Methods
We conducted a one year randomized controlled clinical trial. Participants were recruited from a large pharmacy benefits program for Medicare beneficiaries. All potentially eligible individuals had been newly prescribed a medication for osteoporosis. Consenting persons were randomized to either a program of telephone-based counseling (n = 1,046) using a motivational interviewing framework or a control group (n = 1,041) that received mailed educational materials. Medication adherence was the primary outcome compared across treatment arms and was measured as the median (interquartile range, IQR) medication possession ratio (MPR), calculated as the ratio of days with filled prescriptions to total days of follow-up.
Results
The groups were balanced at baseline, with a mean age of 78 years; 94% were female. In an intention-to-treat analysis, median adherence was 49% (IQR 7, 88) in the intervention arm and 41% (1.5, 86.0) in the control arm (P = 0.074 by Kruskal-Wallis test). There were no differences in self-reported fractures.
Conclusions
In this randomized controlled trial, we did not find a statistically significant improvement in osteoporosis medication adherence using a telephonic motivational interviewing intervention.
INTRODUCTION
Adherence with many chronic medications is poor and appears worse for conditions that do not produce daily symptoms.1 A meta-analysis involving over 50,000 patients found that people with osteoporosis adhere to 48% of days with a prescribed treatment during the first year of therapy.2 More than 2 million fractures associated with osteoporosis or osteopenia occur annually in the US at an estimated medical cost of $19 billion.3 Medications have been shown to reduce fracture risk in many populations;4 thus, improving adherence to osteoporosis regimens is a public health priority.5
Non-adherence is a complex behavior with many potential causes,6 including concerns about medication safety, lack of confidence in a medication’s benefits or in the patient’s ability to adhere, forgetfulness, complexity of treatment regimen and drug affordability.1, 7–9 The multitude of reasons underpinning medication non-adherence, and the failure of many unidimensional programs suggest that a successful intervention needs to be multi-faceted and tailored for a given individual.10 These features are characteristic of one-on-one counseling interventions. Several medication adherence interventions using counseling based on motivational interviewing have been successful in other clinical areas.11, 12
Motivational interviewing is a client-centered counseling method based on the stages of change model of health behavior.13, 14 The counselor interacts with the patient to identify the reasons for problematic health behaviors, and then shapes the counseling to address the issues most likely to help that particular person. We developed a motivational interviewing program that could be delivered by health educators via the telephone. Medicare beneficiaries starting prescription osteoporosis medications were recruited and randomized to receive either motivational interviewing counseling sessions or mailed educational materials.
METHODS
Study Design and Oversight
The OPTIMA (Osteoporosis Telephonic Intervention to Improve Medication Adherence) Trial was a large pragmatic randomized effectiveness trial, which has been described in detail previously.15 The trial was conducted in accordance with the trial protocol, which was reviewed by the Partners Healthcare Institutional Review Board. The study was deemed exempt from Institutional Review Board oversight. However, a safety officer appointed by the National Institutes of Health (the funding agency) reviewed safety data over the course of the trial. The study was designed by the authors; data were collected in a blinded fashion and analyzed by a statistical programmer blinded to treatment assignment. The Principal Investigator (DHS) vouches for the accuracy and completeness of the data and analysis.
Study Participants
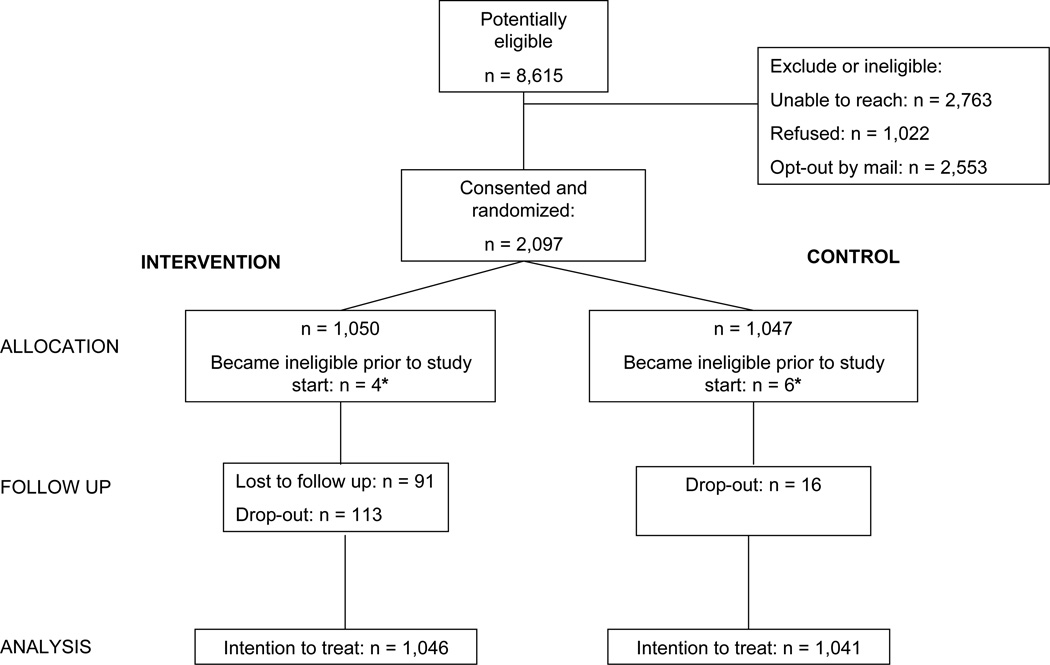
We collaborated with a state-run pharmacy benefits program for low income older adults residing in the state of Pennsylvania.16 Over 200,000 state residents are enrolled annually and receive medications for a nominal co-payment. Monthly administrative claims files identifying beneficiaries starting a prescription medication for osteoporosis (e.g., estrogen replacement therapy, a bisphosphonate, teriparatide, calcitonin, or raloxifene) were transferred securely to the study team. Subjects received a letter inviting them to participate in the program and providing them the opportunity to opt out of further contact. Potentially eligible subjects who did not opt out were contacted and recruitment was attempted if they met additional study eligibility criteria, including living in the community, and being able to understand spoken English. Subjects gave verbal consent to be part of a program for osteoporosis and were then randomized to the intervention or control arms (see Figure 1). Since all subjects in both arms received enhanced care, subjects were not aware of their treatment arm allocation.
Figure 1.
The CONSORT diagram illustrates the assembly of the study cohort and its follow-up through the trial procedures. Ten subjects (4 in the intervention arm and 6 in the control arm) died between recruitment and the start of follow-up 30 days later and were excluded from analyses.
Intervention
Approximately 30 days after randomization, all subjects in both arms began to receive mailings regarding osteoporosis. Over the course of the study, all subjects received seven informational mailings covering topics such as exercise, fall prevention, and recommended calcium intake (see Supplemental File for mailings). The subjects in the intervention arm also received motivational interviewing counseling sessions via telephone with one of seven health educators. We aimed for ten counseling sessions per subject over the course of the study. Each session had a specific educational topic (e.g., discussing medications with your doctor, calcium and vitamin D supplementation, fall prevention, managing medication side effects) and included a series of open ended questions to elicit subjects’ attitudes toward medication adherence and to determine barriers to long-term osteoporosis medication use The health educators received training in motivational interviewing through a half-day training program at study initiation, including role-playing, lecture, and discussion. The motivational interviewing counseling was reinforced through telephone conferences 1–2 times per month with a behavioral scientist and clinical expert. In addition, three times over the course of the 2.5 years of the study period, health educators recorded client telephone calls (with subject’s consent) which were then reviewed and graded by a motivational interviewing trainer. The trainer gave structured feedback to each health educator using an assessment tool.17
Outcomes
The primary outcome of the study was adherence to prescription osteoporosis medications, as measured by the medication possession ratio (MPR) during the 12 months of follow-up.18 The MPR can be measured using different methods; we calculated it as:19
We used pharmacy claims data from the collaborating state-run pharmacy benefits program to calculate the MPR. Paid medication claims based on filled prescriptions form the basis for these data. The MPR is widely recognized as a valid measure of adherence.18
Secondary outcomes included persistence with prescription osteoporosis medication, defined as days from initial prescription until the first period in which the subject experienced an interruption in prescription filling lasting longer than 60 days.18 We also assessed self-reported fractures, falls, and depression, and satisfaction with the program using an exit survey.
Statistical analyses
Primary analysis
The analysis was carried out according to a pre-determined statistical analysis plan. We compared the baseline characteristics of the two treatment arms using the Student t-test for continuous variables and a Kruskal-Wallis test for categorical items. The primary analyses of outcomes used an intention to treat approach that evaluated all subjects in the assigned treatment arms until the completion of the 12 month follow-up period without regard to drop-out (unless subjects died or lost eligibility for the state-run pharmacy benefits program, in which case they were censored at the time of these events). The distribution of MPRs was not normal and thus we compared the median (interquartile range; IQR) MPR across the two treatment arms. For each treatment arm, we also compared the distribution of MPRs, calculated as the percentage of patients in each decile of MPR.
The sample size was chosen to ensure 90% power to detect an absolute difference of 10% in adherence (measured by MPR) between the two arms.
Secondary analyses
We examined the secular trends of MPR by treatment arm across the study follow-up period, assessing whether the effect of the intervention varied over the course of the 12 month follow-up period. Follow-up time was partitioned into 60-day increments and an interaction term between follow-up time and treatment arm was tested in a linear regression model, with MPR as the dependent variable.
We also compared the intervention’s effects in subgroups, such as age 65–74 versus 75 and over, those with a history of a prior fracture versus none, white race versus other, and married versus not.
During the study period, Medicare Part D was introduced. Some pharmacy claims were unavailable from Medicare Prescription Drug Plans who did not share complete data with the state-run pharmacy benefits program. We conducted sensitivity analyses to determine the potential effects of incomplete data. Subjects were censored when the state-run pharmacy program noted that a beneficiary entered a “non-participating” Medicare Prescription Drug Plan.
Persistence with prescription osteoporosis treatments was illustrated using Kaplan-Meier survival curves and compared across study arms using the log-rank test. The proportions of subjects in each treatment arm self-reporting fractures, falls, and poor or fair health were compared using the Kruskal-Wallis test.
The statistical analysis was performed using SAS software, version 9.1.
RESULTS
Patients
We recruited and enrolled 2,097 subjects into the trial (Figure 1). Ten subjects died between enrollment and the start of follow-up and were excluded, leaving 2,087 for analysis. The recruited subjects closely resembled the total eligible pool (see Supplemental Table). As noted in Table 1, the baseline characteristics of subjects in the two arms were similar. Across both study arms, the mean age was 78 years and 94% were female. Most subjects were single or widowed. The mean number of comorbid conditions was 5.2 and the mean number of different medications used in the year prior to the trial was 10.4. Prior fractures, falls, activity limitations, and poor eyesight were common and similar across treatment arms. The most common prescription osteoporosis treatment in both arms was weekly bisphosphonates. The only statistically significant difference across the arms was in the distribution of races, with slightly more Whites in the intervention arm. Thirty-six subjects in the intervention and 39 in the control arm died during follow-up.
Table 1.
Baseline characteristics of study population
| Intervention | Control | |
|---|---|---|
| N (%) or mean (± standard deviation) | ||
| N | 1046 | 1041 |
| Female gender | 986 (94%) | 971 (93%) |
| Age, years* | 77.8 (± 6.4) | 77.7 (± 6.6) |
| Race | ||
| White | 942 (90%) | 909 (87%) |
| Non-white | 104 (10%) | 132 (13%) |
| Marital status | ||
| Married | 228 (22%) | 231 (22%) |
| Divorced or separated | 103 (10%) | 124 (12%) |
| Widowed or single | 715 (68%) | 686 (66%) |
| Number of medications | 10.2 (± 6) | 10.6 (± 6) |
| Comorbidities | 5.2 (± 3.4) | 5.3 (± 3.3) |
| Prior fracture | 303 (30%) | 285 (29%) |
| Poor eyesight | 210 (20%) | 208 (21%) |
| Activity limitation | 730 (71%) | 709 (71%) |
| Prior falls, at least one | 412 (40%) | 378 (38%) |
| Osteoporosis medication | ||
| Bisphosphonate, IV | 2 (0.2%) | 2 (0.2%) |
| Bisphosphonate, oral, daily | 7 (0.7%) | 4 (0.4%) |
| Bisphosphonate, oral, weekly | 664 (64%) | 666 (64%) |
| Other, oral or intranasal | 355 (34%) | 358 (34%) |
| Other, injectable | 18 (2%) | 11 (1%) |
Other osteoporosis medications included raloxifene, calcitonin, teriparatide, and estrogen replacement therapy.
In the intervention arm, the median number of completed calls was 8 (interquartile range 6 to 9), and the median duration of a counseling session was 14 minutes (interquartile range 10 to 19).
Primary End Point
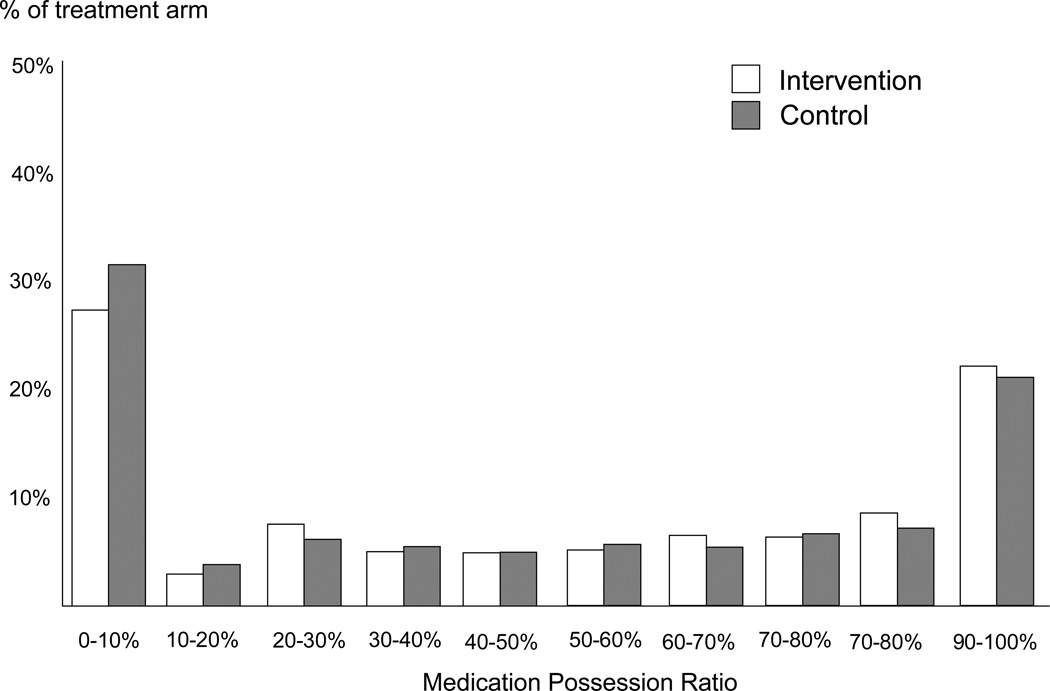
Over the 12 months of follow-up, the median MPR was 49% (IQR 7–88%) in the intervention arm and 41% (IQR 1–86%) in the control arm (P from Kruskal-Wallis test = 0.074) (see Table 2). There was a suggestion of difference in MPR between intervention and control group at the extremes of the distribution: 34% of the intervention groups versus 39% in the control group had MPR < 20% while 33% in the intervention group versus 30% in the control group had MPR >80%, but this difference was small and did not reach statistical significance (P from chi-square trend test across all deciles = 0.078) (see Figure 2). The trend in the median medication possession ratios for six 60-day intervals during the follow-up year is shown in Figure 3.
Table 2.
Median medication possession ratio in entire cohort and in select, prespecified strata
| Intervention | Control | ||||
|---|---|---|---|---|---|
| N | MPR- Median (IQR) |
N | MPR- Median (IQR) |
P value┼ | |
| Intention to treat | 1046 | 49 (7, 88) | 1041 | 41 (2, 86) | 0.074 |
| Subgroup analyses | P value (interaction) | ||||
| Age, years | 0.045 | ||||
| 65–74 | 346 | 48 (7, 87) | 362 | 31 (0, 80) | |
| 75 and over | 700 | 49 (6, 88) | 679 | 46 (2, 90) | |
| Gender | 0.27 | ||||
| Female | 986 | 50 (7, 88) | 971 | 41 (2, 86) | |
| Male | 60 | 20 (0, 81) | 70 | 38 (0, 83) | |
| Prior fracture | 0.058 | ||||
| Yes | 303 | 46 (7, 87) | 285 | 51 (12, 85) | |
| No | 723 | 50 (7, 88) | 708 | 35 (0, 86) | |
| Marital status | 0.17 | ||||
| Married | 228 | 52 (7, 90) | 231 | 32 (0, 83) | |
| Other** | 818 | 48 (7, 87) | 810 | 44 (3, 87) | |
| Race | 0.065 | ||||
| White | 942 | 50 (7, 90) | 909 | 40 (0, 87) | |
| Non-white | 104 | 35 (4, 76) | 132 | 43 (11, 79) | |
MPR, medication possession ratio; IQR, interquartile range
P-values derived from Wilcoxon Rank Sum test.
Other refers to widowed, divorced, or single.
Figure 2.
This histogram displays the distribution of adherence for intervention (white) and control arm (grey), by decile of medication possession ratio.
Figure 3.
This histogram displays the median medication possession ratios for six 60-day intervals, by treatment assignment, with intervention in purple and control in red. The interaction effect between treatment arm and sequential 60-day periods during follow-up was not statistically significant (P for interaction = 0.60).
The sensitivity analyses performed to test the effect of data drop-out through enrollment in non-participating Medicare Part D Plans showed higher median MPRs for both groups than in the primary analysis (intervention = 61% and control = 54%).
Secondary End Points
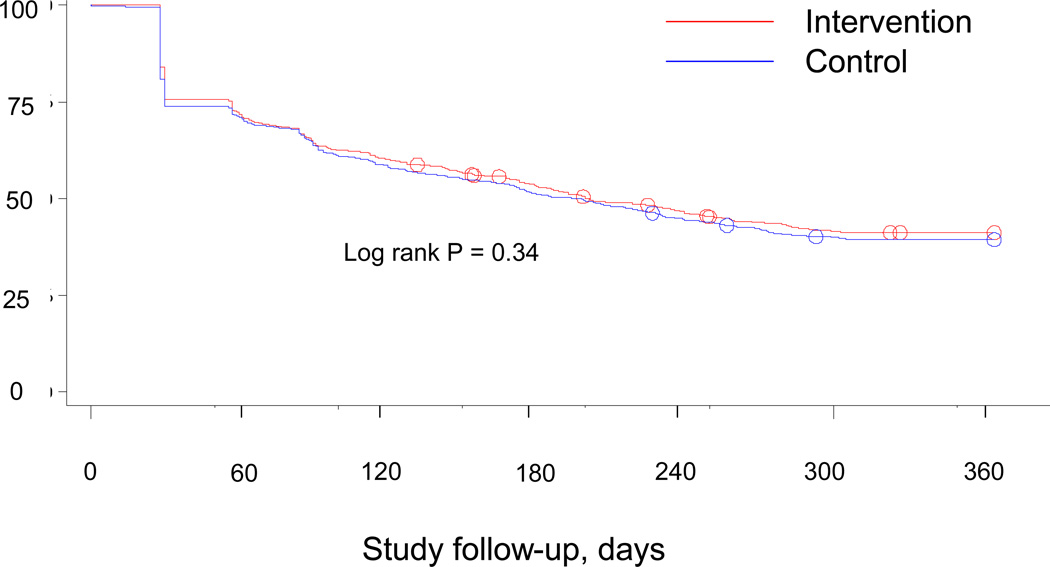
There were no statistically significant differences noted in secondary outcomes, including self-reported fractures (intervention 11% and control 11%), self-reported falls (intervention 38% and control 36%) or poor or fair general health (intervention 40% and control 41%). In addition, persistence with osteoporosis medications appeared similar across the two groups (see Figure 4, log rank p-value 0.34).
Figure 4.
This figure illustrates the persistence over time in use of medications for osteoporosis. The intervention arm is show in red and the control arm in blue.
Subgroup Analyses
The effectiveness of the intervention appeared to differ modestly across several of the subgroups (Table 2). The intervention was associated with improvement in MPR for subjects age 65–74 (median MPR intervention = 48%, compared to MPR control = 31%) compared to little improvement in those 75 and over (median MPR intervention = 49%, MPR control = 46%, P for interaction = 0.045). Among those without a prior fracture, the intervention appeared more effective (median MPR intervention = 50%, MPR control = 35%) compared with those with a prior fracture median (median MPR intervention 46%, MPR control = 51%, P for interaction = 0.058). As well, the intervention produced somewhat larger effects among those of White race (median MPR intervention = 50%, MPR control = 40%) compared with non-Whites (median MPR intervention = 35%, MPR control = 43%, P for interaction = 0.061). We note that in the latter two subgroup analyses (by prior fracture history and by race) the interactions terms did not reach statistical significance.
The per patient intervention costs were $280.94, including training of the health educators, recruitment of subjects, telephone calls, mailings, and data storage.
DISCUSSION
Medication non-adherence results in sub-optimal clinical outcomes and excess health care costs in many chronic conditions, including osteoporosis.20, 21,22, 23 We attempted to improve prescription medication adherence for osteoporosis through a pragmatic randomized controlled trial in collaboration with a public prescribing benefits program. The intervention used principles of motivational interviewing and was delivered by telephone. Subjects in the intervention arm did not experience a statistically significant increase in median medication possession ratio, a well accepted measure of adherence,, as compared with controls.
We used motivational interviewing as the behavioral framework for the medication adherence counseling. It was carried out by trained health educators who underwent extensive training, using regular feedback and structured assessments.17 Motivational interviewing has been widely used for addiction counseling and more recently, has been adopted for other health care settings.14 It formed the basis for a successful intervention that improved adherence with anti-hypertensive therapy, improving adherence by 14% and producing reductions in blood pressure.12 Adherence with anti-retroviral therapy improved by 4.5% using a motivational interviewing based counseling program.11 However, not all similar programs have been successful.24 Our intervention was the first telephone-based motivational interviewing program targeting medication adherence that we are aware of. Even though our intervention did not achieve a statistically significant improvement, these prior studies and the effects seen in select prespecified subgroups in our trial suggest that motivational interviewing shows promise as a counseling model for medication adherence and should be investigator further
We had estimated the sample size of this trial based on a 10% increase in medication adherence, which was deemed to be clinically important. We observed an increase in adherence of 8%. Thus the trial did not document a statistically significant difference between randomization groups and did not achieve the clinically important increase in adherence in adherence we sought to identify. Our follow-up may have been inadequate to detect a change in fracture rate attributable to a modest change in medication use. We note that the a priori power calculation for the trial was based on the adherence endpoint and not the fracture endpoint., as detecting a difference in fractures was unlikely based on our hypothesized 10% improvement in adherence.23, 25, 26 These findings have several important implications. First, while our results were not statistically significant, we demonstrated that a relatively simple intervention has the potential to achieve modest improvements in medication adherence, particularly in select prespecified subgroups.
Second, the intervention’s structure has relevance for other programs that aim to improve adherence with recommended regimens. We used health care and pharmacy insurance claims data to identify people starting a medication for osteoporosis, segment them for analyses, and collect outcomes data. Since the expansion of Medicare to include drug benefits, the Center for Medicare and Medicaid Services has the most extensive database of longitudinal health care utilization on a large portion of the US population. These data should facilitate case finding and follow-up in care improvement programs as demonstrated in our intervention trial. Similar capabilities are in place in most large managed care programs and for most health insurers that cover prescription benefits. This trial also demonstrates the power of health care and pharmacy insurance claims data to conduct a large pragmatic trial. We recruited over 2,000 subjects, followed them for a year, and conducted extensive analyses on a budget of less than one million US dollars (direct costs over five years). This economy of scale could only be achieved through relying on administrative data collected as part of routine health care delivery.
The use of routinely collected data presents important methodologic challenges. We relied on pharmacy claims data to identify recent initiators of an osteoporosis medication. These data need to be processed and then we allowed potential subjects to opt-out of recruitment. This meant that by the time subjects were recruited and received their first intervention call, a median of 113 days had passed since they filled their first prescription. While data shown in Figure 3 suggest that the intervention’s effect increased over the study period, the lag period may have limited the benefit of our intervention, since many subjects had already discontinued use of their osteoporosis medication at the time of their first call (see Figure 4). If a health care delivery system were to deliver the intervention itself, data would be more immediately available and lag times would be reduced. In addition, by relying on pharmacy claims data, we do not know that patients actually took the medicine, only that they filled prescriptions. Finally, we were only able to enroll a fraction of those who were potentially eligible. The groups were similar with respect to age, gender, race, and number of prescription medications (see Supplemental Table).
In conclusion, we conducted a randomized controlled trial of over 2,000 patients that did not demonstrate a statistically significant improvement in medication adherence associated with a telephone-based motivational interviewing counseling intervention. Subgroup analyses suggest that the intervention may be more effective in specific populations including patients > 75 years old as compared with those 65–74. Further research is necessary to determine how to best target this intervention. The study also demonstrated the potential utility of routinely collected prescription data to identify new users of a particular medication class and follow them for outcomes. Given the widespread nature of medication non-adherence, this work may provide useful information for further studies to improve the appropriateness of prescription drug use.
Supplementary Material
Acknowledgments
Dr. Solomon had access to all of the data and takes full responsibility for its accuracy. In the last three years, Dr. Solomon has received research grants regarding rheumatoid arthritis from Amgen, Abbott, and Lilly. He has received an educational grant for a clinical research course from Bristol-Myers Squibb. He serves as an unpaid member of a Data Safety Monitoring Board for a Pfizer-sponsored trial on analgesics. As well, he serves as an unpaid member of an Executive Committee for a Pfizer-sponsored trial on non-steroidal anti-inflammatory drugs. Dr. Shrank receives research support from CVS Caremark. Dr. Brookhart has received research support from Amgen and Rockwell Medical and has been an unpaid member of advisory boards for Pfizer and Amgen. Dr. Brookhart has received consulting fees from World Health Information Consultants, McKesson Health Solutions, Kaiser Permanente, and Crimson Health. We appreciate the input of Douglas C. Bauer, MD, MPH (Professor of Medicine and Epidemiology & Biostatistics, University of California – San Francisco) who served as the NIH appointed Safety Officer for this study and received payment from the NIH for these services.
Support: National Institutes of Health--NIAMS (P60 AR047782). Other salary support from the National Institutes of Health includes K24 AR055989 (to DHS), K23 HL090505 (to WS), K24 AR057827 (to EL), K24 AR02123 (to JNK), and K25 AG027400 (to MAB).
REFERENCES
- 1.Osterberg L, Blaschke T. Adherence to medication. New England Journal of Medicine. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [see comment]. [DOI] [PubMed] [Google Scholar]
- 2.Kothawala P, Badamgarav E, Ryu S, Miller RM, Halbert RJ. Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc. 2007 Dec;82(12):1493–1501. doi: 10.1016/S0025-6196(11)61093-8. [DOI] [PubMed] [Google Scholar]
- 3.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007 Mar;22(3):465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 4.Favus MJ. Bisphosphonates for osteoporosis. N Engl J Med. Nov 18;363(21):2027–2035. doi: 10.1056/NEJMct1004903. [DOI] [PubMed] [Google Scholar]
- 5.Wilkes MM, Navickis RJ, Chan WW, Lewiecki EM. Bisphosphonates and osteoporotic fractures: a cross-design synthesis of results among compliant/persistent postmenopausal women in clinical practice versus randomized controlled trials. Osteoporos Int. Apr;21(4):679–688. doi: 10.1007/s00198-009-0991-1. [DOI] [PubMed] [Google Scholar]
- 6.Shrank WH, Hoang T, Ettner SL, et al. The implications of choice: prescribing generic or preferred pharmaceuticals improves medication adherence for chronic conditions. Arch Intern Med. 2006 Feb 13;166(3):332–337. doi: 10.1001/archinte.166.3.332. [DOI] [PubMed] [Google Scholar]
- 7.Madden JM, Graves AJ, Ross-Degnan D, Briesacher BA, Soumerai SB. Cost-related medication nonadherence after implementation of Medicare Part D, 2006–2007. Jama. 2009 Oct 28;302(16):1755–1756. doi: 10.1001/jama.2009.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shrank WH, Cadarette SM, Cox E, et al. Is there a relationship between patient beliefs or communication about generic drugs and medication utilization? Med Care. 2009 Mar;47(3):319–325. doi: 10.1097/MLR.0b013e31818af850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choudhry NK, Fischer MA, Avorn J, et al. The Implications of Therapeutic Complexity on Adherence to Cardiovascular Medications. Arch Intern Med. Jan 10; doi: 10.1001/archinternmed.2010.495. [DOI] [PubMed] [Google Scholar]
- 10.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2) doi: 10.1002/14651858.CD000011.pub3. CD000011. [DOI] [PubMed] [Google Scholar]
- 11.Golin CE, Earp J, Tien HC, Stewart P, Porter C, Howie L. A 2-arm, randomized, controlled trial of a motivational interviewing-based intervention to improve adherence to antiretroviral therapy (ART) among patients failing or initiating ART. J Acquir Immune Defic Syndr. 2006 May;42(1):42–51. doi: 10.1097/01.qai.0000219771.97303.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogedegbe G, Chaplin W, Schoenthaler A, et al. A practice-based trial of motivational interviewing and adherence in hypertensive African Americans. Am J Hypertens. 2008 Oct;21(10):1137–1143. doi: 10.1038/ajh.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller WR. Motivational interviewing: research, practice, and puzzles. Addict Behav. 1996 Nov-Dec;21(6):835–842. doi: 10.1016/0306-4603(96)00044-5. [DOI] [PubMed] [Google Scholar]
- 14.Rollnick S, Butler CC, Stott N. Helping smokers make decisions: the enhancement of brief intervention for general medical practice. Patient Educ Couns. 1997 Jul;31(3):191–203. doi: 10.1016/s0738-3991(97)01004-5. [DOI] [PubMed] [Google Scholar]
- 15.Solomon DH, Gleeson T, Iversen M, et al. A blinded randomized controlled trial of motivational interviewing to improve adherence with osteoporosis medications: design of the OPTIMA trial. Osteoporos Int. Jan;21(1):137–144. doi: 10.1007/s00198-009-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aging PDo. Prescription Assistance. Web page describing PACE. [Accessed February 1, 2011]; Available at: http://www.aging.state.pa.us/portal/server.pt/community/prescription_assistance/17942. [Google Scholar]
- 17.Moyers TB, Martin T, Manuel JK, Hendrickson SM, Miller WR. Assessing competence in the use of motivational interviewing. J Subst Abuse Treat. 2005 Jan;28(1):19–26. doi: 10.1016/j.jsat.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007 Jan-Feb;10(1):3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 19.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006 Aug;15(8):565–574. doi: 10.1002/pds.1230. discussion 575–567. [DOI] [PubMed] [Google Scholar]
- 20.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006 Sep 25;166(17):1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 21.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005 Jun;43(6):521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 22.Siris ES, Harris ST, Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006 Aug;81(8):1013–1022. doi: 10.4065/81.8.1013. [DOI] [PubMed] [Google Scholar]
- 23.Siris ES, Selby PL, Saag KG, Borgstrom F, Herings RM, Silverman SL. Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med. 2009 Feb;122(2 Suppl):S3–S13. doi: 10.1016/j.amjmed.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Samet JH, Horton NJ, Meli S, et al. A randomized controlled trial to enhance antiretroviral therapy adherence in patients with a history of alcohol problems. Antivir Ther. 2005;10(1):83–93. doi: 10.1177/135965350501000106. [DOI] [PubMed] [Google Scholar]
- 25.Patrick AR, Brookhart MA, Losina E, et al. The complex relation between bisphosphonate adherence and fracture reduction. J Clin Endocrinol Metab. Jul;95(7):3251–3259. doi: 10.1210/jc.2009-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curtis JR, Westfall AO, Cheng H, Lyles K, Saag KG, Delzell E. Benefit of adherence with bisphosphonates depends on age and fracture type: results from an analysis of 101,038 new bisphosphonate users. J Bone Miner Res. 2008 Sep;23(9):1435–1441. doi: 10.1359/JBMR.080418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.