Abstract
Patient: Male, 36
Final Diagnosis: Levamisole-induced vasculopathy
Symptoms: Purpuric skin lesions
Medication: Levamisole
Clinical Procedure: —
Specialty: Internal Medicine
Objective:
Unusual clinical course
Background:
Levamisole has been detected in seized cocaine samples and a levamisole-induced vasculopathy (LIV) has been described, mainly focused on skin.
Case Report:
A 36-year-old Caucasian man with history of antibodies to hepatitis C infection (negative hepatitis C virus RNA and negative HIV serology), smoking, and intravenous use of cocaine and brown heroin, presented to the hospital with purpuric skin lesions on extremities and earlobes. One month before the current presentation, a skin punch biopsy of one of these lesions was performed, showing histopathologic findings suggestive of mixed cryoglobulinemia. Laboratory testing revealed leukopenia, renal failure, and nephrotic syndrome. Antimyeloperoxidase antineutrophil cytoplasmic antibodies (MPO-ANCA) were positive. The previous skin punch biopsy was revised and demonstrated pathologic findings consistent with leukocytoclastic vasculitis. An analysis of a cocaine sample for personal use, provided by the patient, was performed using mass spectrometry-gas chromatography and levamisole was detected. Three boluses of intravenous methylprednisolone were administered, followed by oral prednisone 1 mg/Kg per day. Skin lesions and renal function improved.
Conclusions:
To our knowledge, this is the first report of nephrotic syndrome induced by levamisole-adulterated cocaine, proven by cocaine sample toxicology. Lack of renal biopsy is a limitation of this report.
Keywords: nephrotic syndrome, vasculitis, cocaine, levamisole
Background
Levamisole is a veterinary antihelminthic agent, previously used to treat nephrotic syndrome, various autoimmune disorders, and colon and breast cancers in humans. Because of its adverse effects profile, levamisole was withdrawn for use in humans in United States in 1999 but is still available for veterinary use. The U.S. Drug Enforcement Agency first detected levamisole in cocaine in April 2005 [1] and first report of cocaine/levamisole-induced vasculopathy (LIV) was in June 2010 [2].
According to 2009 estimates, approximately two-thirds of the cocaine entering the U.S. was contaminated with levamisole [3]. In Europe, levamisole has been detected in seized cocaine samples in the U. K., Italy, and Spain. During the second half of 2009, surveillance program results suggest widespread cocaine consumption in Spain, with levamisole adulteration ranging from 3% to 20% [4].
Levamisole may have an inhibitory action on monoamine oxidase and catechol-O-methyltransferase, the enzymes that metabolize catecholamine neurotransmitters. It is theoretically possible that cocaine and levamisole may have a synergistic action on nicotinic acetylcholine receptors, resulting in increased nicotinic and dopaminergic effects. Recent reports have suggested that, due to its ability to act as a hapten, levamisole may cause increased formation of antibodies to various antigens and therefore lead to an immune response [5].
Cocaine itself has been reported to be associated with an antineutrophil cytoplasmic antibodies (ANCA)-positive pseudo-vasculitis [3]. LIV is a diagnosis of exclusion, but this entity should be strongly considered in patients with a history of cocaine abuse who present with a tetrad of: cutaneous manifestations consisting of palpable retiform purpura (lesions tend to be stellate with a bright erythematous border and necrotic appearing center [6]) or bullae, with ear involvement (the most pathognomonic site), arthralgias, leukopenia, and positive ANCA in high titers (although not specific for the condition), when other infectious or idiopathic vasculitides have been excluded [7]. Biopsy findings range from leukocytoclastic and thrombotic vasculitis to vascular occlusion without true vasculitis. Although neutropenia is an expected and well-recognized association with LIV, it is not necessary to make the diagnosis, nor is it an inevitable consequence of levamisole exposure [2].
The time from last cocaine use to onset of the condition may be relatively rapid, but many of these affected individuals are chronic, habitual cocaine users, suggesting a large cumulative exposure to cocaine and, by association, levamisole, possibly over an extended period of time [8].
Case Report
A 36-year-old Caucasian man with history of antibodies to hepatitis C infection (negative hepatitis C virus RNA and negative HIV serology), smoking, and intravenous use of cocaine and brown heroin, on treatment with methadone, presented to the hospital with purpuric skin lesions on extremities and earlobes. The patient had been admitted to the hospital 4 months before due to intravenous drug-induced cellulitis and abscess on his forearms and legs. He had received treatment with amoxicillinclavulanic acid, and skin lesions had improved. At that time, the patient reported anorexia and weight loss and blood tests had revealed leukopenia and iron deficiency anemia ( attributable to inadequate diet and malnutrition). A transthoracic echocardiogram had ruled out infectious endocarditis. One month before the current presentation, purpuric lesions on extremities and earlobes had appeared and a skin punch biopsy had been performed. The histopathologic findings were suggestive of mixed cryoglobulinemia. Blood tests showed polyclonal hypergammaglobulinemia and deterioration of renal function (serum creatinine 1.6 mg/dl, normal range of 0.9–1.3 mg/dl). Previous laboratory tests performed 6 years before revealed creatinine 1.1 mg/dl and inactive urinary sediment.
The patient reported last cocaine use 15 days before the current admission. His complaints were: anorexia, weakness and loss of functional independence with confinement to bed, sore throat, dysphagia, arthralgias, myalgias and impairment of skin lesions, evolving to expand and become ulcers, leading to wound infection.
On physical examination, the patient was afebrile and skin revealed a purpuric and violaceous, non-blanching rash in a retiform pattern with areas of necrosis and infected ulcers, located on helix and earlobes and upper and lower extremities (Figures 1 and 2). There were no mucosal lesions or peripheral lymphadenopathy and no heart murmur or rub. A hepatosplenomegaly was revealed.
Figure 1.
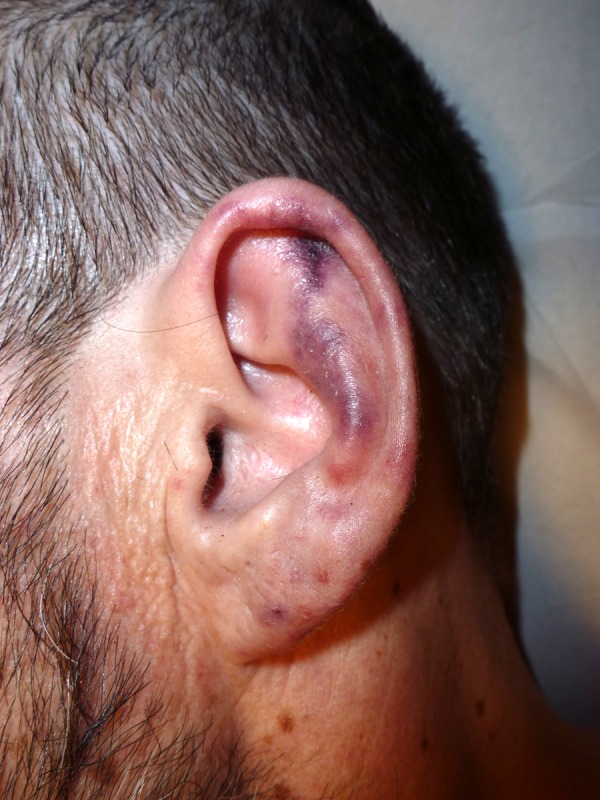
Lesion on helix and earlobe.
Figure 2.
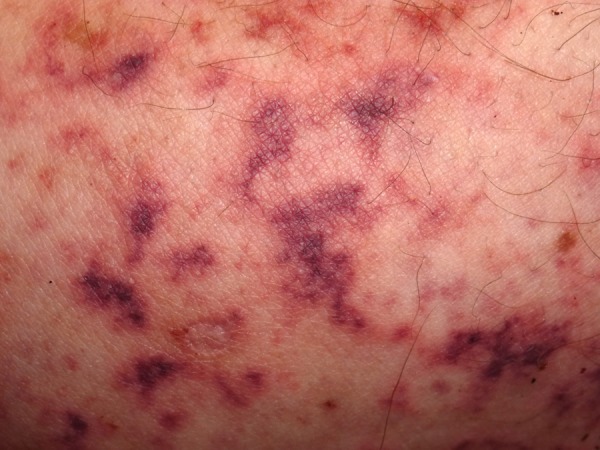
Purpuric and retiform rash on lower extremity.
Investigations
Laboratory testing showed a white blood cell (WBC) count of 2720 per mm3 (normal range of 4300–11000 per mm3), hemoglobin 5.6 g/dl (normal range of 12.5–17.5 g/dl); hepatic function was normal; renal function revealed serum creatinine 2.71 mg/dl, glomerular filtration rate (GFR) 28ml/min per 1.73 m2; urinary sediment showed hematuria and 4750 mg of protein excretion in 24-hour urine collection. Erythrocyte sedimentation rate was 121 mm per hour and C-reactive protein was 200 mg/dl.
Test result for cryoglobulin was negative; anti-nuclear antibody (ANA) positive at 1:80 dilution with a homogeneous pattern; complement C3 89mg/dl and C4 14 mg/dl (normal range of 19–152 mg/dl and 16–43 mg/dl, respectively); enzyme-linked immunosorbent assay (ELISA) for antiproteinase 3 antineutrophil cytoplasmic antibodies (PR3-ANCA) was negative at 3 AU/ml (normal 0–5 AU/ml) and for antimyeloperoxidase (MPO-ANCA) was positive at 145 AU/ml (normal 0–5 AU/ml). Anti-glomerular basement membrane antibodies were negative. Anticardiolipin antibodies (ACA) were normal (IgG 2 GPL/ml and IgM 2 MPL/ml, normal range of 0–10 and 0–7 MPL/ml, respectively). In our patient population, testing for anti-human neutrophil elastase (HNE) antibodies was not available.
The skin punch biopsy performed 1 month before the current admission (suggestive of mixed cryoglobulinemia) was revised and demonstrated pathologic findings consistent with leukocytoclastic vasculitis. Serum cryoglobulins and hepatitis C virus RNA were negative. Syphilis and hepatitis B serology were negative and tuberculin skin test was 0 mm.
An ultrasound scan showed slightly enlarged kidneys and no other remarkable findings were present.
Although a renal biopsy was not performed, the renal damage was suggestive of nephrotic syndrome induced by probable glomerulonephritis.
The patient provided a cocaine sample for personal use and an analysis was performed using mass spectrometry – gas chromatography. Levamisole was detected in the cocaine sample.
Treatment
The patient received a 3-week course of amoxicillin-clavulanic acid for infected skin ulcers. In addition, 6 packed red cell transfusions were required due to severe anemia.
Considering signs of inflammation and end-organ damage (nephrotic syndrome induced by probable glomerulonephritis) in a toxic context, 3 boluses of intravenous methylprednisolone were administered, followed by oral prednisone 1 mg/Kg per day. Sulfamethoxazole-trimethoprim was started for Nocardia and Pneumocystis jiroveci prophylaxis. An angiotensin-converting enzyme (ACE) inhibitor was added to decrease protein loss in the urine.
The patient was discharged with tapering down of prednisone.
Outcome and follow-up
Three months later, the patient denied cocaine use since last admission. On physical examination, he had gained weight and was fully active, skin lesions had disappeared, and he reported only migrating joint pains. WBC count had normalized. Renal function had improved, reaching serum creatinine of 1.83 mg/dl (GFR 45 ml/min per 1.73 m2) and 1915 mg of protein excretion in 24-hour urine collection. Complement C4 was normal and MPO-ANCA decreased to 83 AU/ml.
Tapering off of prednisone is ongoing. Patient counseling has been provided to avoid cocaine use and therefore progression of renal damage can be reversed or at least, slowed or stopped.
Discussion
Apart from neutropenia, ANCA-positive vasculitis induced by levamisole-adulterated cocaine has been reported to be limited to the skin, without evidence of the involvement of other organ systems.[1] Moreover, Tran et al. suggested that the absence of end-organ involvement with isolated skin manifestations, along with a typical serologic profile, differentiates cocaine-levamisole cutaneous vasculopathy from idiopathic systemic vasculitis [9].
However, rare clinical findings in LIV include renal and pulmonary involvement [2]. McGrath et al. described 30 patients with ANCA-positivity associated with levamisole-contaminated cocaine use. Abnormal urinalysis (defined as dipstick proteinuria, hematuria, or the presence of cellular casts on microscopy) was present in 8 patients at diagnosis. Two developed severe acute kidney injury and 1 underwent renal biopsy, revealing pauci-immune focal necrotizing and crescentic glomerulonephritis; neither required renal replacement therapy. Despite improved renal function with immunosuppressive treatment, both were left with significant chronic kidney disease (estimated GFR <30 ml/min per 1.73 m2). There was little definitive evidence of combined pulmonary-renal disease in this cohort. All cases had positive anti-MPO antibodies. By immunofluorescence, all cases were perinuclear antineutrophil cytoplasmic antibodies (p-ANCA)-positive. The presence of hypocomplementemia with ANA and anti-ds DNA antibodies raised the possibility of drug-induced lupus in a subset of these patients. Lack of documentation of levamisole in patient samples is a limitation of the study [8].
Differential diagnosis includes infectious or primary vasculitis. In the study of Graf et al., 3 patients had antibodies to hepatitis C (none with detectable cryoglobulins), as did our case presented above. ELISA revealed multiple antibodies to neutrophil elastase, lactoferrin, cathepsin G, PR3, and MPO [1]. The finding of multiple ANCA positivity is a strong indication of a drug-induced vasculopathy as opposed to other primary vasculitides. Positivity for anti-HNE antibodies is an indication of the cocaine use associated with LIV [2].
In addition, cocaine and heroin themselves can induce renal damage. There is evidence from in vitro cellular and animal studies to support the existence of cocaine-induced renal changes through multifactorial pathophysiology: vasoconstrictive effects (inhibition of catecholamine reuptake at the presynaptic nerve terminal, increase of endothelins, activation of the renin-angiotensin-aldosterone system, increase of cellular oxidative stress and platelet aggregation), and nonspecific glomerular, interstitial, and tubular cell lesions. Case reports of renal infarction, anti-glomerular basement membrane antibody-mediated glomerulonephritis, and acute interstitial nephritis have been described in cocaine users. In addition, opioids can amplify cocaine-induced expression of tissue inhibitors of metalloproteinase-2, resulting in mesangial matrix accumulation. Focal segmental glomerulosclerosis and membranoproliferative glomerulonephritis have been found in renal biopsy of heroin users. Otherwise, chronic skin suppurative infections can lead to development of renal amyloidosis, in a similar manner to those who subcutaneously inject drugs (“skin popping”) [10]. However, in our patient, despite the absence of renal biopsy, the typical skin lesions and serologic spectrum, in addition to detection of levamisole in a cocaine sample, suggest cocaine-levamisole–induced vasculitis causing renal injury revealed as nephrotic syndrome.
Jenkins et al. reported the first case of vasculitis after levamisole snorting that was proven by urine toxicology [11]. The short half-life of levamisole (5.6 hours) and small amount of the parent drug (<5%) detected in urine, limits the utility of detection of this substance in determining the cause of this syndrome; thus, in a patient known to have used cocaine and with a high index of clinical suspicion, detection of levamisole should not be considered essential for diagnosis [2].
The natural course of LIV may be self-limited [3]. There is a lack of evidence that systemic corticosteroids modify the clinical course of LIV. Discontinuation of levamisole is a critical part of the treatment. Nonetheless, in individual patients with striking signs of inflammation, corticosteroids might be considered [2].
Conclusions
End-organ involvement is an uncommon presentation of ANCA-positive vasculitis induced by levamisole-adulterated cocaine.
To our knowledge, this is the first report of nephrotic syndrome induced by levamisole-adulterated cocaine, proven by cocaine sample toxicology. Lack of renal biopsy is a limitation of this report.
It is important to perform a differential diagnosis with other infectious or idiopathic vasculitis, considering that discontinuation of levamisole-adulterated cocaine can improve the clinical course of the condition without immunosuppressive therapy.
References:
- 1.Graf J, Lynch K, Yeh C, et al. Purpura, cutaneous necrosis and antineutrophil cytoplasmic antibodies associated with levamisole-adulterated cocaine. Arthritis Rheum. 2011;63(12):3998–4001. doi: 10.1002/art.30590. [DOI] [PubMed] [Google Scholar]
- 2.Pearson T, Bremmer M, Cohen J, et al. Vaculopathy related to cocaine adultered with levamisole: A review of the literature. Dermatol Online J. 2012;18(7):1. [PubMed] [Google Scholar]
- 3.Arora N. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345(1):45–51. doi: 10.1097/MAJ.0b013e31825b2b50. [DOI] [PubMed] [Google Scholar]
- 4.Ventura M, Caudevilla F, Vidal C. Grupo Investigadores SELECTO. Cocaína adulterada con levamisol: posibles implicaciones clínicas. [Levamisole-adulterated cocaine: potential clinical implications] Med Clin (Barc) 2011;136(8):365–68. doi: 10.1016/j.medcli.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 5.Arora N, Jain T, Bhanot R, et al. Levamisole-induced leukocytoclastic vasculitis and neutropenia in a patient with cocaine use: an extensive case with necrosis of skin, soft tissue, and cartilage. Addict Sci Clin Pract. 2012;7(1):19. doi: 10.1186/1940-0640-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geller L, Whang T, Mercer S, et al. Retiform purpura: a new stigmata of illicit drug use? Dermatol Oline J. 2011;17(2):7. [PubMed] [Google Scholar]
- 7.Poon SH, Baliog CR, Sams RN, et al. Syndrome of cocaine-levamisole-induced cutaneous vasculitis and immune-mediated leukopenia. Semin Arthritis Rheum. 2011;41(3):434–44. doi: 10.1016/j.semarthrit.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Mc Grath M, Isakova T, Rennke H, et al. Contamined cocaine and antineutrophil cytoplasmic antibody-associated disease. Clin J Am Soc Nephrol. 2011;6:2799–805. doi: 10.2215/CJN.03440411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran H, Tan D, Marnejon T. Cutaneous vasculopathy associated with levamisole-adulterated cocaine. Clin Med Res. 2013;11(1):26–30. doi: 10.3121/cmr.2012.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaffe J, Kimmel P. Chronic nephropathies of cocaine and heroin abuse: a critical review. Clin J Am Soc Nephrol. 2006;1:655–67. doi: 10.2215/CJN.00300106. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins J, Babu K, Hsu-Hung E, et al. ANCA-positive necrotizing vasculitis and thrombotic vasculopathy induced by levamisole-adultered cocaine: A distinctive clinicopathologic presentation. J Am Acad Dermatol. 2011:e14–16. doi: 10.1016/j.jaad.2010.09.778. [DOI] [PubMed] [Google Scholar]


