Summary
Objective
The objective was to characterize the contralateral non-surgical temporomandibular joint (TMJ) in mice that had an opposing osteoarthrosis(OA)-like joint induced by unilateral partial discectomy.
Methods
TMJs on one side in mice were subjected to partial discectomy. Both surgical and contralateral non-surgical TMJs were collected at 4, 8, 12 and 16 weeks post-surgery for histological examination. The morphology of the articular cartilage of the condyle was evaluated using a scoring system.
Results
A progression of articular cartilage degeneration was seen in the TMJs following unilateral partial discectomy, including increased proteoglycan staining in the extracellular matrix at 4 weeks, the appearance of chondrocyte clusters at 8 weeks, reduced proteoglycan staining and fibrillation at 12 weeks and the loss of articular cartilage at 16 weeks. In the contralateral non-surgical TMJs, increased proteoglycan staining occurred in the articular cartilage of the condyle at 8 weeks and continued to age.
Conclusion
The result indicated that OA-like changes in one TMJ by partial discectomy could initiate early onset articular cartilage degeneration in the contralateral non-surgical TMJ in mice.
Keywords: temporomandibular, articular cartilage, discectomy, contralateral, mouse
Introduction
The aim of this study was to characterize the contralateral non-surgical temporomandibular joint (TMJ) in mice that had an opposing osteoarthrosis (OA)-like joint that was induced by unilateral partial discectomy. In this study, we observed early onset degenerative joint changes in the contralateral non-surgical TMJ following surgically-induced OA of the opposing TMJ.
The TMJ is a pressure-bearing bilateral synovial joint. The fibrous disc in the joint acts as a cushion during joint movements and allows forces to be evenly distributed throughout the fossa of the temporal bone and the articular surfaces of the condyle. Derangement of the condyle-disc complex, erosion of the disc itself and/or disc displacement can initiate the articular cartilage degeneration of the condyle, eventually leading to the development of OA and temporomandibular disorder (TMD) (1-3). The results from a recent study with a mouse model support this observation (4). In that study, mice that had undergone partial discectomy developed early onset articular cartilage degeneration in the surgically-induced TMJ, revealing a typical OA-like joint at 16 weeks post-surgery. One plausible explanation is that the partial discectomy results in an uneven distribution of pressure as well as a decreased capacity for load absorption in the TMJ; thus resulting in an excessive amount of mechanical force on a small area of the articular surface. This, in turn, triggers a series of molecular events that lead to the development of OA in the TMJ.
Another unique aspect of the TMJ is that the left and right joints do not function independently as do other joints of the body, such as the knee, shoulder or hip. In fact, the two TMJs must work together to produce the movements necessary for speech, mastication, deglutition and facial expression (5). Consequently, we posed the question of whether OA-like changes in one TMJ lead to the pathological changes in the contralateral joint. We believe that mice that have undergone unilateral discectomy are excellent models with which to address this question. Mice grind or use their TMJs constantly, regardless of any pathologic condition in either TMJ. This allows mice to maintain a normal equilibrium of tooth wear and eruption. Since we had already developed a mouse model with unilateral surgically-induced OA of the TMJ, we had an excellent opportunity to characterize the contralateral non-surgical TMJ in this model. In this study, we examined the articular cartilage of the non-surgical and the surgical TMJs of mice that had undergone unilateral partial-discectomy and the TMJs of mice that had undergone sham surgery, for evidence of OA-like changes.
Methods
Partial discectomy of TMJs
The experimental procedure was performed following approval from the Institutional Animal Care and Use Committee. A detailed procedure of partial discectomy has been described in our previous publication (4). Briefly, C57BL male and female mice at the age of 8 weeks old were used for the surgery. The surgical procedures of the sham and discectomy were the same with the exception of no removal of any part of the disc in the sham surgery.
Histology
The condylar articular cartilages from the TMJs of sham surgery and discectomy mice were examined at 4, 8, 12 and 16-week intervals following surgery. Histologic examination of the contralateral non-surgical TMJ was also performed at each of the same timepoints.
The eight mice at each time point per experimental group were sacrificed, and their skulls were collected. The samples were fixed in 4% paraformaldehyde for 6 hours at room temperature and processed for paraffin embedding. Serial sectioning at a thickness of 6μm was performed. Approximately 100 sections were needed to represent the entire TMJ from anterior to posterior. The sections were stained with Safranin O/fast green.
Scoring system and statistical analysis
Every fifteenth section was collected and the pathologic condition of the joints was evaluated by a scoring system recommended by the OARSI histopathology initiative (6).
In this scoring system, normal mouse articular cartilage is scored as a 0. Loss of Safranin-O without structural changes is 0.5. Fibrillation without loss of cartilage is 1. Vertical clefts down to the layer immediately below the superficial layer and some loss of surface lamina is 2. Vertical clefts/erosion to the calcified cartilage extending to <25% of the articular surface is 3. Vertical clefts/erosion to the calcified cartilage extending to 25-50% of the articular surface is 4. Vertical clefts/erosion to the calcified cartilage extending to 50-75% of the articular surface is 5. Vertical clefts/erosion to the calcified cartilage extending >75% of the articular surface is 6. Increased proteoglycan staining was added to the study as an additional criterion, contributing a score of 0.5. There were 8 TMJs in each experimental group. Each joint was represented by 8 to 9 paraffin sections. Each section was scored, and an average score was calculated from the 8 or 9 individual scores. The 8 mean scores, representing each of the TMJs in each experimental group, were then averaged. Thus, one final mean score was calculated for each experimental group. A two-sample t test with a significance level of 0.05 was used to determine whether a significant difference between any two mean scores was present.
Results
A detailed description of the development of OA in the TMJ, induced by partial discectomy, has been described in our previous publication (4). In summary, increased proteoglycan staining was present throughout the articular cartilage and on the surface of the temporal fossa at 4 weeks following partial discectomy (see arrow, Figure 1). At 8 weeks following the discectomy, the articular cartilage displayed chondrocyte clustering and increased proteoglycan staining around chondrocytes (see arrow, Figure 1). At 12 weeks, the surgical joints exhibited diminished proteoglycan staining in addition to fibrillation of the articular cartilage in the superfacial cartilage layer (see arrow, Figure 1). At 16 weeks, the surgically-induced TMJs exhibited a dramatic reduction of proteoglycan staining and regions of missing articular cartilage were present in the condyle (see arrow, Figure1), which is typical of an OA-like joint.
Figure 1.
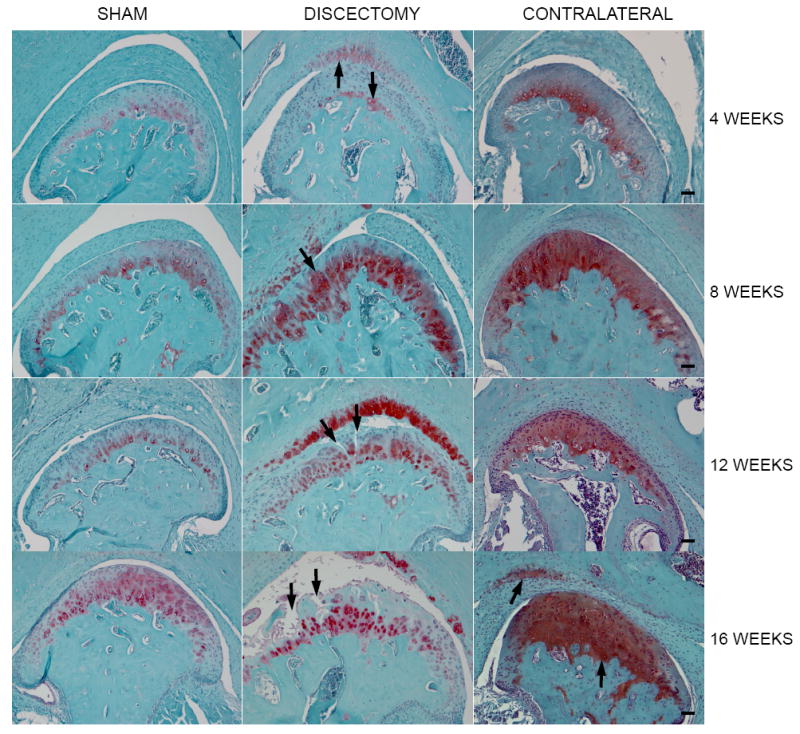
Histology of TMJs in mice. Each image shown in the figure is a representative section for each experimental group. Morphological changes of articular cartilage degeneration were seen in the discectomy mice, such as increased proteoglycan staining at early stage, chondrocyte clusters, reduced proteoglycan staining at late stage, fibrillation and the loss of articular cartilage. The morphological change was also seen in the contralateral non-surgical TMJs. However, only increased proteoglycan staining was present in the articular cartilage of the condyle at early stage and the increased staining was extended to the fossa of temporal bone at late stage. The morphological changes in each section were observed in all joints in each animal group. (Bar=50 μm) Table 1 Average scores for the sham, discectomy and contralateral non-surgical TMJs.
As the mice continued to age, the scores of the discectomy and contralateral non-surgical joints continued to increase, however, the rate of score increase in the non-surgical groups was significantly slower, compared to that in the discectomy groups
For contralateral non-surgical TMJs, no changes were visible at 4 weeks post-surgery. However, at 8 weeks post-surgery, increased proteoglycan staining was seen throughout the condylar articular cartilage of the contralateral non-surgical TMJs. At 12 weeks, proliferation of chondrocytes was observed in the contralateral non-surgical TMJs, which was similar in quality to that observed in the surgical TMJs at 8 weeks. At 16 weeks post-surgery, increased proteoglycan staining was visible throughout the articular cartilage of the condyle as well as on the articular surface of the temporal fossa in the contralateral non-surgical TMJs (see arrows, Figure 1).
No significant morphologic changes were observed in the subchondral plate and joint margins of any of the joints. There were no changes in the sham TMJ at any of the time points.
A statistical comparison between sham and contralateral non-surgical groups indicated that the scores were significantly increased in the non-surgical joints (Table 1), starting at 8 weeks following the surgery. However, the pattern of increase of the scores in the non-surgical joints exhibited a significantly lesser degree, compared to that in the discectomy joints (Table 1).
Table 1.
Histologic scores for TMJ in mice.
| weeks | Mean ± SD | p-value | |
|---|---|---|---|
| Sham | Contralateral | ||
| 4 | 0.00 ± 0.00 | 0.00 ± 0.00 | - |
| 8 | 0.00 ± 0.00 | 0.27 ± 0.11 | 0.0004 |
| 12 | 0.00 ± 0.00 | 0.34 ± 0.07 | < 0.0001 |
| 16 | 0.07 ± 0.06 | 1.01 ± 0.10 | < 0.0001 |
| weeks | Mean ± SD | p-value | |
| Contralateral | Discectomy | ||
| 4 | 0.00 ± 0.00 | 0.34 ± 0.14 | 0.0004 |
| 8 | 0.27 ± 0.11 | 0.47 ± 0.12 | 0.0092 |
| 12 | 0.34 ± 0.07 | 2.41 ± 0.63 | 0.0001 |
| 16 | 1.01 ± 0.10 | 3.80 ± 0.61 | < 0.0001 |
Discussion
The present study found that, in mice, articular cartilage degeneration of one TMJ causes morphological changes in the TMJ on the contralateral side. It has been reported that damage induced in one TMJ by condylectomy or discectomy, causes damage in the contralateral TMJ. For example, three months after condylectomy of the left TMJ in sheep, the right, non-surgical TMJ appears to have thicker epiphysial bone and irregularity of the condylar bone surface (7). In another study performed on rabbits, discectomy and condylectomy of left TMJs resulted in flattening of the condylar head of the right, non-surgical TMJs at 4 weeks following surgery. However, there were no obvious abnormalities in the non-surgical TMJs at 20 weeks following surgery (8). It seems that the damage to the non-surgical TMJs are not as severe and progressive in these experiments as our findings in this study. The main drawback of these studies is the small sample size and few time points. Our study evaluated a total of 64 mice--8 mice per experimental group at each of the four time points. The entire TMJ, for each of the mice, was examined by serial histology sectioning. We found that a typical OA-like TMJ developed at approximately16 weeks post-discectomy and no regeneration appeared in the disc following surgery. We also found that increased proteoglycan staining occurred in the contralateral non-surgical TMJ of the discectomy mice. The increased production of the proteoglycan is one of histopathological changes in early onset articular cartilage degeneration. However, the progressive rate of the articular cartilage degeneration on the non-surgical side was slower, compared to the discectomy side. This indicates that the intact disc can prevent the articular cartilage from being degraded in the contralateral joint.
OA is one of the common conditions that lead to TMD. Articular disc displacement is also the most frequent cause of articular cartilage degeneration, which eventually leads to OA (9-12). The clinical ramifications of our results in this study are two-fold: 1) Clinically, there is a great deal of concern that oral trauma may lead to the development or worsening of TMJ dysfunction (13). Thus, caution should be taken when treating oral trauma patients with underlying chronic orofacial pain symptoms. 2) Patients typically present with unilateral TMD symptoms often time. Data from our study has brought to light an issue in TMDs- -the unilaterally affected TMJ may likely influence the contralateral, “unaffected”, TMJ. Treatment protocols for patients with TMDs should address both the symptomatic TMJ as well as the “unaffected” TMJ. Specifically, bilateral MRI should be performed to evaluate both TMJs in patients presenting with unilateral TMJ dysfunction (14,15).
Acknowledgments
The funding source
This study was supported by a grant from the National Institutes of Health, NIAMS R01 AR051989 (to LX and LY).
Footnotes
Contributions
WC and LX participated in study conception and design, acquisition, analysis and interpretation of data, drafting and revision of the manuscript and final approval of the version to be submitted. JS, IP and YL performed experiments and participated in acquisition and analysis of data, drafting and final revision of the manuscript.
Competing interests
The authors have no conflicts of interest to declare.
Contributor Information
Wendy A. Cohen, Email: wendy_cohen@post.harvard.edu.
Jacqueline M. Servais, Email: jacqueline.servais@gmail.com.
Ilona Polur, Email: ilona.polur@gmail.com.
Yefu Li, Email: yefu_li@hms.harvard.edu.
Lin Xu, Email: lin_xu@hms.harvard.edu.
References
- 1.de Bont LG, Boering G, Liem RS, Eulderink F, Westesson PL. Osteoarthrosis and internal derangement of the temporomandibular joint. A light microscopic study. J Oral Maxillofac Surg. 1986;44:634–43. doi: 10.1016/s0278-2391(86)80075-1. [DOI] [PubMed] [Google Scholar]
- 2.Dimitroulis G. The prevalence of osteoarthrosis in cases of advanced internal derangement of the Temporomandibular Joint: a clinical, surgical and histological study. Int J OralMaxillofac Surg. 2005;34:345–9. doi: 10.1016/j.ijom.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka E, Detamore MS, Mercuri LG. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res. 2008;87:296–307. doi: 10.1177/154405910808700406. [DOI] [PubMed] [Google Scholar]
- 4.Xu L, Polur I, Lim C, et al. Early-onset osteoarthritis of mouse temporomandibular joint indiced by partial discectomy. Osteoarth Cartil. 2009;17:917–22. doi: 10.1016/j.joca.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okeson JP. Management of Temporomandibular Disorders and Occlusion. 7. St. Louis: CV Mosby Co; 2012. [Google Scholar]
- 6.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarth Cartil. 2010;(Suppl 3):S17–23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Miyamoto K, Vickers R, Ishimaru JI, Ogi N, Kurita K, Goss AN. Effect of unilateral condylectomy on the sheep temporomandibular joint. Br J Oral Maxillofac Surg. 1999;37:401–4. doi: 10.1054/bjom.1999.0193. [DOI] [PubMed] [Google Scholar]
- 8.Dimitroulis G, Slavin J. The effects of unilateral discectomy and condylectomy on the contralateral intact rabbit craniomandibular joint. J Oral Maxillofac Surg. 2006;64:1261–6. doi: 10.1016/j.joms.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Dimitroulis G. Temporomandibular disorders: a clinical update. Br Med J. 1998;317:190–4. doi: 10.1136/bmj.317.7152.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milam SB. Pathogenesis of degenerative temporomandibular joint arthritides. Odontology. 2005;93:7–15. doi: 10.1007/s10266-005-0056-7. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Gupta T, Barasz JA, et al. Analysis of microarchitectural changes in a mouse temporomandibular joint osteoarthritis model. Arch Oral Biol. 2009a;54:1091–8. doi: 10.1016/j.archoralbio.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagandora CK, Almarza AJ. TMJ disc removal: comparison between preclinical studies and clinical findings. J Dent Res. 2012;91:745–52. doi: 10.1177/0022034512453324. [DOI] [PubMed] [Google Scholar]
- 13.Israel HA, Ward JD, Horrell B, Scrivani SJ. Oral and maxillofacial surgery in patients with chronic orofacial pain. J Oral Maxillofac Surg. 2003;61:662–7. doi: 10.1053/joms.2003.50133. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Woodworth RE, Tallents RH, Katzberg RW, Guay JA. Bilateral internal derangements of temporomandibular joint: evaluation by magnetic resonance imaging. Oral Surg Oral Med Oral Pathol. 1988;65:281–5. doi: 10.1016/0030-4220(88)90109-0. [DOI] [PubMed] [Google Scholar]
- 15.Bertram S, Rudisch A, Innerhofer K, Pümpel E, Grubwieser G, Emshoff R. Diagnosing TMJ internal derangement and osteoarthritis with magnetic resonance imaging. J Am Dent Assoc. 2001;132(6):753–61. doi: 10.14219/jada.archive.2001.0272. [DOI] [PubMed] [Google Scholar]


