Abstract
Carol A Janney has received research support from Actigraph, Inc for this study. Andrea Fagiolini is/has been a consultant and/or speaker and/or has received grants from: Angelini, Astra Zeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Pfizer, Eli Lilly, Janssen, Lundbeck, Novartis, Otsuka, Roche and Sigma Tau. Holly A. Swartz has received honoraria from Servier, Astra Zeneca, SciMed, Bristol-Myers Squibb, Eli Lilly, and Sanofi. She has received royalties from UpToDate. John M. Jakicic has received an honorarium for scientific presentations from the Nestle Nutrition Institute and JennyCraig. He has served on the scientific advisory board for Alere WellBeing. He has also served as the Principal Investigator on a research grant awarded to the University of Pittsburgh from BodyMedia, Inc.. Robert G. Holleman and Caroline R. Richardson have no financial disclosures.
Keywords: accelerometry, actigraphy, bipolar disorder, adult, physical activity, sedentary behavior, NHANES 2003-2004, mental health services, outpatients
Background
The medical and economic burden of bipolar disorder (BP) is substantial. The World Health Organization ranks BP as “the sixth most disabling medical condition” (Murray and Lopez, 1997). In addition, BP is the most expensive behavioral health care diagnosis for patients and insurance plans (Peele BP et al., 2003) with mental as well as non-mental healthcare contributing to the 4-fold increase in total medical costs for patients with BP compared to patients without BP (Fagiolini AM et al., 2008, Bryant-Comstock et al., 2002). Hence public health efforts to reduce the tremendous health burden of BP on the individual and society are warranted.
BP is a chronic mood disorder characterized by episodes of depression, and hypomania or mania. Individuals with this disorder are symptomatic about half of their lives (Judd et al., 2003a, Judd et al., 2003b). Changes in mood are accompanied by extreme shifts in energy, activity, sleep, and behavior. Compared to the general population, adults with BP experience elevated rates of obesity, diabetes, cardiovascular disease, metabolic syndrome, and mortality (Kupfer DJ, 2005, Fagiolini et al., 2003, Kilbourne et al., 2007, Fagiolini et al., 2005). Lack of physical activity may be one modifiable factor associated with increased risk of these common medical comorbidities in adults with BP.
To date, physical activity studies of adults with BP have relied on self-reported rather than objective measures of physical activity (Cairney J et al., 2009, Strohle et al., 2007, Elmslie et al., 2001). Interestingly, differences between individuals with BP and individuals with and without other mental disorders were only noted if self-reported physical activity included occupational and leisure activities (Elmslie et al., 2001) but not leisure activities alone (Cairney J et al., 2009, Strohle et al., 2007). Unfortunately, self-reported measures of physical activity are problematic in general populations (Sallis and Saelens, 2000), but may even be less reliable and valid in individuals with BP due to high symptom burden and significant neurocognitive impairment associated with the disorder (Joffe et al., 2004, Sole et al., 2012). Hence, objectively measured physical activity studies are necessary to confirm and/or refute these self-reported findings in adults with BP (Cairney J et al., 2009).
Purpose
The objectives of this report are 1) to provide a profile of objectively measured physical activity and sedentary behavior among adult outpatients with BP, for the first time, and 2) to compare objective physical activity levels of adult outpatients with BP with a national sample of users and non-users of mental health services (MHS) matched on age, gender and BMI. It was hypothesized that adults with BP would have significantly lower physical activity levels than a national sample of MHS non-users and MHS users who represent a broader and less severe spectrum of mental health disorders.
Methods
Studies
This report is based on data from the Physical Activity and Function in Adults with Bipolar Disorder (PARC2) study and the National Health and Nutrition Examination Survey (NHANES) 2003-2004. All research procedures were approved by the Institutional Review Board at the University of Pittsburgh. PARC2 participants were recruited between January 2009 to November 2011 and signed informed consent documents prior to engaging in research procedures. Individuals were eligible for PARC2 if they were receiving treatment for BP at Western Psychiatric Institute and Clinic (WPIC) at the University of Pittsburgh, Pittsburgh, and were age ≥18 years. They were eligible for the study regardless of their clinical status (euthymic, depressed, hypomanic, or manic) or bipolar subtype (BP I, BP II, BP Not Otherwise Specified (NOS), BP NOS/Schizoaffective (SA) disorder). Participants were compensated $10 from January 2009 to July 2011 and $30 from August 2011 to November 2011. PARC2 data collection occurred between January 2009 and November 2011. Data was analyzed in 2012-2013.
NHANES 2003-2004 is a cross-sectional observational study using a stratified, multistage probability design to obtain a nationally representative sample of the civilian, non-institutionalized US population (Department of Health and Human Services Center for Disease Control and Prevention, 2005). NHANES data was collected in 2003-2004. The NHANES sample was restricted to those adults (≥18 years) with at least 3 days of valid actigraph data to match the eligibility criteria of the PARC2 study.
Assessments
PARC2 replicated the NHANES 2003-2004 physical activity monitoring protocol (Department of Health and Human Services Center for Disease Control and Prevention, 2006). Participants were instructed to wear the ActiGraph AM-7164 monitoring device (ActiGraph, Ft. Walton Beach, FL) on an elasticized belt over the non-dominant hip for seven consecutive days. ActiGraphs were set to measure the duration and intensity of uniaxial movement within one-minute epochs. If there were no activity counts for ≥ 60 minutes, the accelerometer was considered not worn for that interval of time. Each minute epoch was assigned an activity level based on the number of counts per minute; sedentary(<= 100 counts), light(101 - 1951 counts), or moderate/vigorous(≥ 1952 counts)(Hagstromer et al., 2007, Freedson et al., 1998, Matthews et al., 2008). Daily totals of sedentary behavior and activity levels (minutes/day) were averaged. Percentages of monitoring time were calculated by dividing the minutes engaged in each category by the total monitoring minutes for each participant. Valid and reliable data was defined as an accelerometer worn for 10 hours a day for 3 or more days (Trost et al., 2005, Matthews et al., 2012).
PARC2 participation involved 2 clinic visits scheduled one week apart. At study entry, participants received Actigraphs and completed self-assessments. At the second visit, participants returned the Actigraph and completed additional self-assessments. For descriptive purposes, the prior week’s mood symptoms were assessed by independent evaluators using the 17-item Hamilton Depression (HRSD17) scale (Hamilton, 1960) and an expanded 25-item Hamilton Depression (HRSD25) scale that includes reverse neurovegetative symptoms (Thase et al., 1991). The HRSD17 and HRSD25 yields scores that range from 0-52 and 0-72, respectively. Higher scores for HRSD17 and HRSD25 indicate a greater burden of depressive symptoms. HRSD17 symptoms were defined as ≤7 not depressed; 8-13 mild depressive symptoms; 14-19 moderate depressive symptoms; ≥20 severe depressive symptoms. Mania/hypomania was assessed with the Young Mania Rating Scale (YMRS)(Young et al., 1978) that yields scores from 0-60 with higher scores indicating higher levels of mania/hypomania. YMRS symptoms were defined as ≤6 not experiencing mania; 7-14 mild mania; 15-19 moderate mania; and ≥20 for severe mania. Psychiatric diagnoses were obtained from participants’ research records. All research diagnoses were made in accordance with DSM IV criteria using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (SCID)(n=55, 92%), Fourth edition(First MB et al., 1996) or Mini International Neuropsychiatric Interview (MINI) (n=5, 8%) (Sheehan et al., 1998).
Data Analysis
For descriptive purposes, BMI was categorized as healthy (18.5-24.9 kg/m2), overweight (25 to <30 kg/m2), obese (30 to <40 kg/m2), and extreme obesity (≥40 kg/m2). T-tests and Chi-Square Exact Tests were used to compare the continuous and categorical variables, respectively, by gender. PARC2 participants were 1:1 matched to users and non-users of MHS in NHANES 2003-2004 by gender, closest BMI, and age to account for the potential residual effects of these demographic variables on actigraphy comparisons. Conditional logistic regression models were used to determine if actigraphy measures and demographics differed between the PARC2 participants matched to 1) users of MHS, and 2) non-users of MHS. Descriptive summaries and statistical analyses were performed using Stata (release 9, StataCorp, College Station, TX) and SAS (version 9.2, SAS Institute, Triangle Park, NC).
Results
Sixty-nine of the 101 PARC2 participants were asked to participant in the actigraphy monitoring. Nine participants were excluded from the analysis due to either providing less than 3 days of valid actigraphy data (n=3), BP diagnosis relied on clinical diagnosis and not SCID or MINI (n=5), or both (n=1). Mean symptom scores for HRSD17, HRSD25, and YMRS did not clinically or statistically differ among those included and excluded from the PARC2 analytical sample (data not shown, p≥ 0.41).
The PARC2 analytical sample was comprised of 60 participants diagnosed with BP I (n=41), BP II (n=17), and BP NOS/SA (n=2). The majority of the participants were diagnosed as BPI (68%) and not experiencing any depressive (48%, n=29) or (hypo)manic (80%, n=48) symptoms or experiencing mild to moderate depressive symptoms (46%, n=28) or mild (hypo)manic (20%, n=12) symptoms (Table 1). Severe depressive symptoms with no (hypo)mania were experienced by 3% (n=2); and 2% (n= 1) had a mixed presentation characterized by severe depressive symptoms co-occurring with mild (hypo)manic symptoms. None of the participants were experiencing moderate to severe (hypo)manic symptoms. No association was observed between days of valid accelerometry data and moods [HRSD17 (rs = −0.10, p=0.46), HRSD25 (rs = −0.04, p=0.76), YMRS (rs = −0.002, p=0.99)], age (rs= − 0.04, p=0.73), BMI (rs= 0.06, p=0.65), or gender (p=0.07)].
Table 1.
Characteristics of PARC2 participants diagnosed with BP (n=60).
| Variable | Total Sample (n=60) |
Male (n=21) |
Female (n=39) |
p-value |
|---|---|---|---|---|
| Age (years) Mean ±STD Median 25th, 75th percentile Range |
45.3 ± 12.2 46.3 37.5, 54.9 18.7, 63.1 |
45.5 ± 13.0 48.2 40.6, 55.2 20.4, 62.8 |
45.2 ± 11.9 45.7 37.2, 54.6 18.7, 63.1 |
0.92a |
| Weight (lbs) Mean ±STD Median 25th, 75th percentile Range |
181.9 ± 46.4 171.1 148.1, 215.5 111.6, 309.8 |
194.8 ± 41.1 184.6 158.8, 216.4 144.6, 309.8 |
174.9 ± 48.0 161.0 138.2, 214.6 111.6, 297.0 |
0.11a |
| Body Mass Index (kg/m2) Mean ± STD Median 25th, 75th percentile Range |
28.9 ± 6.9 27.3 23.4, 33.7 18.9, 46.6 |
28.3 ±4.6 27.3 24.8, 29.9 22.1, 39.9 |
29.2 ± 8.0 27.4 22.0, 35.8 18.9, 46.6 |
0.61b |
| Body Mass Index [n(%)] Healthy (18.5-24.9 kg/m2) Overweight (25-29.9 kg/m2) Obese (30-39.9 kg/m2) Extreme Obesity (≥40 kg/m2) |
21 (35%) 21 (35%) 13 (23%) 4 (7%) |
6 (29%) 10 (48%) 5 (24%) 0 (0%) |
15 (38%) 11 (28%) 9 (23%) 4 (10%) |
0.32c |
| Diagnosis [n(%)] Bipolar I Bipolar II Bipolar NOS/SA |
41 (68%) 17 (28%) 2 (3%) |
14 (67%) 7 (33%) 0 (0%) |
27 (69%) 10 (26%) 2 (5%) |
0.69c |
| HRSD17 Mean ± STD Median 25th, 75th percentile Range |
7.9 ± 6.3 8.0 3.0, 10.0 0, 31.0 |
8.2 ± 7.1 7.0 3.0, 10.0 0, 27.0 |
7.7 ± 5.9 8.0 3.0, 10.0 0, 31.0 |
0.80a |
| HRSD25 Mean ± STD Median 25th, 75th percentile Range |
10.8 ± 8.4 10.0 4.5, 15.5 0, 42.0 |
11.2 ± 9.5 10.0 3.0, 15.0 0, 36.0 |
10.6 ± 7.8 10.0 6.0, 16.0 0, 42.0 |
0.78a |
| YMRS Mean ± STD Median 25th, 75th percentile Range |
3.2 ± 3.6 2.0 0, 5.5 0, 12.0 |
3.1 ± 3.3 2.0 0, 6.0 0, 10.0 |
3.3 ± 3.8 2.0 0, 5.0 0, 12.0 |
0.91a |
| Depression Symptoms Not experiencing (HRSD17≤7) Mild (8≤HRSD17≤13) Moderate (14≤HRSD17≤ 19) Severe (HRSD17≥20) |
29 (48%) 23 (38%) 5 (8%) 3 (5%) |
11 (52%) 7 (33%) 1 (5%) 2 (10%) |
18 (46%) 16 (41%) 4 (10%) 1 (3%) |
0.60c |
| Mania Symptoms Not experiencing (YRMS≤ 6) Mild (7≤YRMS≤14) Moderate (15≤YRMS≤19) Severe (YRMS≥20) |
48 (80%) 12 (20%) 0 (0%) 0 (0%) |
16 (76%) 5 (24%) 0 (0%) 0 (0%) |
32 (82%) 7 (18%) 0 (0%) 0 (0%) |
0.74c |
| Season [N(%)] Winter (Dec,Jan, February) Spring (March, April, May) Summer (June, July, August) Fall (Sept, Oct, Nov) |
6 (10%) 17 (28%) 19 (32%) 18 (30%) |
4 (19%) 4 (19%) 5 (24%) 8 (38%) |
2 (5%) 13 (33%) 14 (36%) 10 (26%) |
0.19c |
| Current Smoker [n(%)] | 24 (40%) | 8 (38%) | 16 (41%) | 0.83d |
T-Test based on pooled method for equal variances
T-test based on Satterthwaite method for unequal variances
Exact Fisher’s Test for equal proportions
Chi-Square lest
e number of participants with at least 1 medication in each category
PARC2= Physical Activity and Function in Adults with Bipolar Disorder
NOS/SA = Not Otherwise Specified/Schizoaffective Disorder
HRSD17= Hamilton Rating Scale for Depression, 17-item
HRSD25= Hamilton Rating Scale for Depression, 25-item
YMRS= Young Mania Rating Scale
Generally, participants were female, middle-age, and overweight or obese (Table 1). No statistically or clinically significant gender differences were noted for demographic characteristics or psychiatric symptoms (Table 1). None of the participants had mobility limitations that required a wheelchair.
On average, adults with BP wore actigraphs over 17 hours/day, 7 days/ week averaging almost 166,000 counts per day or 159 counts/min (Table 2). The majority of the monitoring time was classified as sedentary (approximately 13.5 hours/day or 78% of the monitoring time). On average, light physical activity accounted only for 21% (215 mins/day) of the monitoring time/day. Only moderate/vigorous activity (mins and %/day) approached a statistically significant difference by gender (p=0.05) (data not shown). On average, males engaged in 9 more minutes of moderate/vigorous activity than females (20 vs 11 mins/day and 2 vs. 1% of the monitoring time for males and females, respectively). Otherwise, no significant associations were noted for the objective measures of physical activity or sedentary behavior and age (p>0.20), gender (p≥0.12), or method of diagnosis (SCID vs. MINI, p≥0.09) (data not shown).
Table 2.
Adults with BP matched by gender, age and closest BMI to MHS users and non-users.
| Variable | Adults with BP 1 (n=60) |
Users of MHS (NHANES) (n=60) |
Non-Users of MHS (NHANES) (n=60) |
|---|---|---|---|
| BMI (kg/m2) [Mean ± STD] Odds Ratio (95% Wald CI) p-value |
28.9 ± 6.9 | 29.9 ± 6.4 0.95 (0.88, 1.03) 0.23a |
28.8 ± 6.5 1.04 (0.79,1.37) 0.78b |
| Current Smoker [N (%)] Odds Ratio (95% Wald CI) p-value |
24 (40) | 21 (36) d
1.27 (0.58, 2.80) 0.55a,c |
21 (37) e
1.17 (0.54, 2.52) 0.70b |
| Monitoring [Mean ± STD] Days Odds Ratio (95% Wald CI) p-value Min/day Odds Ratio (95% Wald CI) p-value |
7.4 ± 2.2 1042 ± 179 |
6.1 ± 1.0 1.65 (1.22, 2.24) < 0.01 a 967 ± 170 1.002 (1.000, 1.005) 0.03a |
6.0 ± 1.1 1.67 (1.24, 2.25) <0.0001 918 ± 113 1.005 (1.002, 1.007) <0.0001 |
| Sedentary [Mean ± STD] Min/day Odds Ratio (95% Wald CI) p-value % of wear time Odds Ratio (95% Wald CI) p-value |
812 ± 168 77.9 ±8.2 |
639 ±168 1.006 (1.003, 1.008) <0.0001 a 65.8 ± 11.5 1.16 (1.08, 1.25) 0.0001a |
539 ± 129 1.010 (1.005, 1.015) 0.0001b 58.6 ± 11.2 1.20 (1.09, 1.31) <0.0001 b |
| Light Activity [Mean ± STD] Min/day Odds Ratio (95% Wald CI) p-value % of wear time Odds Ratio (95% Wald CI) p-value |
215 ± 80 20.7 ± 7.4 |
310 ± 108 0.99 (0.98, 0.99) 0.01a 32.3 ± 11.0 0.86 (0.79, 0.93) 0.0001 |
356 ± 100 0.98 (0.97, 0.99) <0.0001 38.9 ± 10.4 0.83 (0.75, 0.91) 0.0001 b |
| Moderate/Vigorous Activity [Mean ± STD] Min/day Odds Ratio (95% Wald CI) p-value % of wear time Odds Ratio (95% Wald CI) p-value |
14 ± 15 1.4 ± 1.6 |
19 ± 18 0.98 (0.96, 1.01) 0.13 a 1.9 ± 1.7 0.80 (0.62, 1.03) 0.08 a |
23 ± 19 0.96 (0.93, 0.99) 0.01 b 2.5 ± 2.1 0.62 (0.45, 0.85) < 0.01b |
| Total Activity [Mean ± STD] Min/day Odds Ratio (95% Wald CI) p-value % of wear time Odds Ratio (95% Wald CI) |
229 ± 87 22.1 ± 8.2 |
328 ± 115 0.988 (0.981, 0.995) <0.01 34.2 ± 11.5 0.86 (0.80, 0.93) 0.0001 a |
379 ± 107 0.98 (0.97, 0.99) 0.0001b 41.4 ± 11.2 0.84 (0.77, 0.92) <0.0001 b |
| Activity [Mean ± STD] Counts/day Odds Ratio (95% Wald CI) p-value Counts/min Odds Ratio (95% Wald CI) p-value |
165861 ± 87643 159.3 ± 87.2 |
230982 ± 121453 0.992 (0.988, 0.997) < 0.01 a 238.4 ± 114.1 0.990 (0.984, 0.996) <0.01a |
269661 ± 111569 0.99 (0.98, 0.99) <0.0001 b 295.9 ± 121.0 0.99 (0.98, 0.99) <0.0001 b |
BP = bipolar disorder
BMI= Body Mass Index
MHS= Mental Health Services
NHANES= National Health and Nutrition Examination Survey 2003-2004
CI= confidence interval
Based on conditional logistic regression model comparing PARC2 Study participants matched by gender, age and closest BMI to users of MHS in NHANES 2003-2004.
Based on conditional logistic regression model comparing PARC2 Study participants matched by gender, age and closest BMI to non-users of MHS in NHANES 2003-2004.
Missing category excluded from analyses
n=1 missing smoking information from NHANES 2003-2004
n=3 missing smoking information from NHANES 2003-2004
f to counts/day scaled as counts/(days x 1000) in model
Matched comparison of PARC2 participants (n=60) with MHS users and non-users (NHANES)
No statistical or clinical significant differences for BMI or smoking status (p≥0.23, Table 2) were noted after PARC2 participants were matched by gender, age and closest BMI to users and non-users of MHS. Actigraphs were worn by adults with BP approximately 1 day more than MHS users and non-users (Table 2, p<0.01). In addition, adults with BP wore the actigraphs approximately 1 to 2 hours more per day than the MHS users and non-users (p<0.05). However, the results did not differ regardless of whether mins/day or percentage of wear time was used in the analyses for activity and sedentary behaviors.
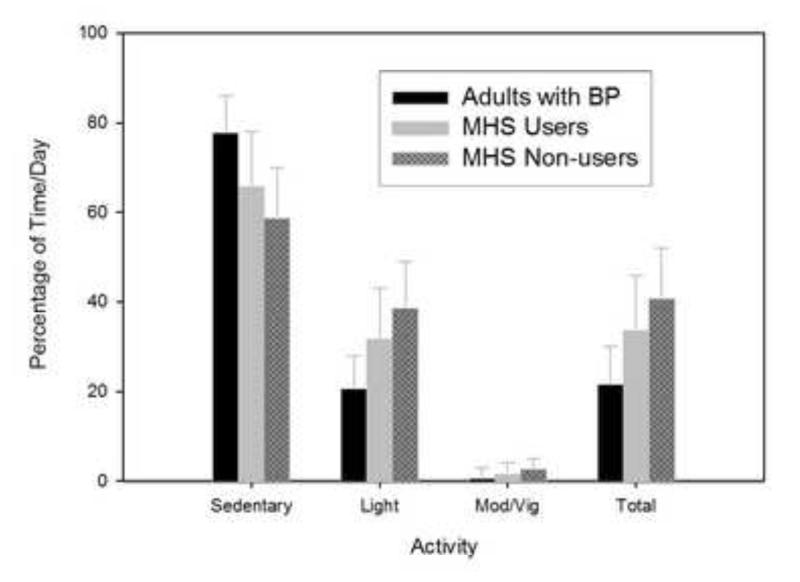
Overall, adults with BP were significantly less active and more sedentary (mins/day or percentage of wear time) than users (p≤0.01) and non-users (p<0.01) of MHS (Table 2). Although the comparison only approached statistical significance (p≥0.08), adults with BP engaged in less moderate/vigorous activity (14 mins/day and 1% of time/day) than users of MHS (19 mins/day and 2% of time/day). Consistent trends in physical activity and sedentary behavior were observed between the groups (p<0.01) (Figure 1). Specifically, adults with BP were less active than users of MHS who were less active than non-users of MHS, regardless of the intensity of the activity. Conversely, adults with BP were more sedentary than users of MHS who were more sedentary than non-users of MHS. On average, adults with BP exhibited approximately one-half the activity levels of non-users of MHS. None of the participants (adults with BP, users and non-users of MHS) achieved 150 mins/wk of moderate/vigorous activity as recommended by national physical activity guidelines.
Figure 1.
Percentage of time/day engaged in sedentary and physical activity behaviors.
Discussion
The PARC2 Study provides the first profile of objectively measured physical activity and sedentary behavior in adult outpatients with BP. Adult outpatients with BP primarily engaged in sedentary behavior (78% of the wear time). On average, moderate/vigorous and light physical activity accounted for 1.4% (14 min/day) and 21% (215 mins/day) of wear time/day. None of the participants achieved national guidelines for physical activity. As hypothesized, adults with BP were significantly less active and more sedentary than users and non-users of MHS (NHAMES) matched by gender, closest BMI, and age. Overall, adults with BP had approximately two thirds of the activity levels of the users of MHS (NHANES) matched by gender, closest BMI and age. Although causality cannot be established given the current study design, these novel findings imply that lack of physical activity may be one factor increasing the risk of common medical comorbidities in adults with BP and suggest that physical activity interventions in this high-risk population are indicated.
Our findings compare favorably to studies conducted among inpatients at clinical research wards; lower average daily activity in recovered, euthymic patients with BP compared to age-matched controls (Salvatore et al., 2008) and lower activity among depressed inpatients diagnosed with BP (n=4) versus those diagnosed with major depression disorder (n=7) (Kupfer et al., 1974). In previous studies, no differences in subjectively measured physical activity were observed among individuals with BP (n=831), major depression (n=4713) or the general population(n=31,834) (Cairney J et al., 2009), or in adolescents diagnosed with any BP (n=39) compared to those with no mental disorder(n=1589)(Strohle et al., 2007).
Interestingly, adults with BP (n=88) self-reported significantly less activity than age- and gender-matched reference subjects (n=445) when occupational as well as leisure physical activities were assessed subjectively (Elmslie et al., 2001). Although speculative, it is plausible that the difference in self-reported physical activity reported by Elmslie and associates may be due to the inclusion of occupational physical activities since the employment rate among adults with BP is significantly lower than the general population (Shippee et al., 2011).
In future studies examining physical activity and BP, the objective measurement of physical activity may be important. Studies relying only on subjective measures of physical activity may not be able to detect clinically significant differences in physical activity among adults with BP resulting in misleading conclusions. Objective monitoring that is collected on a minute-by-minute basis may provide the opportunity to examine physical activity patterns and how these patterns align with dynamic constructs of mood, function, motivation, energy, and sleep. These objective assessments of physical activity may also be useful in studying the complex relationship between physical activity, weight gain, anti-psychotic medications and common chronic diseases such as obesity, diabetes, and heart disease observed in this population (Kupfer DJ, 2005, Soreca et al., 2008, Kilbourne et al., 2004, Fagiolini and Goracci, 2009).
Since the proper diagnosis and treatment of BP has been shown to be clinically challenging (Kupfer et al., 2009, Charney et al., 2003), a major strength of this study is the diagnosis of BP using established, structured, and standard research methods (SCID or MINI) administered by independent evaluators experienced working with individuals with BP. Since mood may influence physical activity and vice versa, assessments of mood for the week corresponding to the physical activity monitoring is another strength of this study. In addition, the objective assessment of physical activity permitted measuring 1) low-intensity, intermittent, and unstructured physical activities that may be difficult for participants to recall, 2) physical activity over extended time periods, and 3) eliminated overestimates of frequency, duration, and intensity of physical activity due to recall and social desirability bias (Sallis and Saelens, 2000, Bassett, 2000, Kriska A, 2000).
While accelerometry measures the intensity and duration of physical activity, it does not record the type of physical activities performed. Measuring the type of physical activities may be important for optimal clinical care as well as improved quality of life for adults with BP since the actual activities as well as the intensity and duration of these activities may significantly differ by mood states as suggested by monitoring of inpatients with BP (Kupfer and Foster, 1973, Weiss et al., 1974, Salvatore et al., 2008), especially if used prospectively. Other known limitations for accelerometry includes limited accuracy and precision for measuring upper body activity that involves little or no ambulation (Matthews et al., 2012) and the inability to measure water-based activities.
Finally, reliable and valid accelerometry data was provided by 95% of the adults with BP which exceeds the ~80% rate observed in various population-based studies (Janney CA et al., 2008, Hagstromer et al., 2007). Our high compliance rate may represent optimal clinical research conditions; participants were enrolled in ongoing studies, accustomed to visiting the clinic regularly, and received monetary compensation. It should be noted that these research participants may not be a representative sample of adult outpatients with BP due to the following reasons; better access to quality mental health care resulting in longer remissions, reduced symptoms, better function (Bauer et al., 2001, Nallamothu et al., 2008) and optimal treatment. Fortunately, the participants were recruited from research studies imposing few restrictions or exclusions (medical, pharmacological, or therapeutic) for study participation hence improving the generalizability of these findings.
It should be noted that the majority of the participants were relatively asymptomatic with most (87%) having no more than mild depressive symptoms and none experiencing severe manic symptoms. It is possible that research volunteers with BP may be more physically active and experience fewer depressive, hypomanic, or manic symptoms than a less highly selected BP outpatient population. Their illness may also be under better control because of the careful monitoring associated with participation in research protocols. Our findings may therefore over-estimate the physical activity levels of adults with BP in the general population, most of whom do not have access to clinics that specialize in the treatment of BP and who, therefore, may be more symptomatic than our sample. This potential bias should represent a ceiling effect and highlight not only the lack of activity in the daily lives of adult outpatients with BP but also the critical need for physical activity interventions in this high risk population.
Future studies may want to control for the sedating effects of medications which, in addition to the illness itself, may contribute to reduced physical activity. Also longitudinal data is necessary to establish a causal link between low physical activity and various mental and physical health outcomes including metabolic disease onset in adults with BP.
Conclusions
Adults with BP were significantly less active and more sedentary than MHS users and non-users in NHANES 2003-2004 matched by gender, age, and BMI. From public health and clinical perspectives, these findings justify physical activity interventions targeting adults with BP. Physical activity may also be an effective behavioral intervention for the treatment and/or management of mood episodes and the corresponding impairments in quality of life, and occupational and social functioning observed in adults with BP (Goodrich DE and Kilbourne AM, 2010, Wright KA et al., 2009, Revicki et al., 2005). Furthermore, physical activity may be an effective intervention for decreasing the elevated risk of common medical comorbidities observed in this high-risk population. Moreover, future research is warranted to determine the most effective intervention approaches for increasing physical activity in adults with BP.
Acknowledgements
We are grateful to Anne B. Newman, MD for the generous loan of the actigraphs, and the following PIs for referring study participants: David J. Kupfer, MD and the Bipolar Disorder Center of Pennsylvania(BDCP) Study 35 (ME 02385), and Reducing Medical Risks in Individuals with BP (Medrisk) Study (Kupfer, PI) (R01-MH081003); Holly A. Swartz, MD and Acute Psychotherapy for Bipolar II Depression Study (R01-MH084831); Edward S. Friedman, MD and the Comparative Effectiveness of a Newer Antipsychotic Mood Stabilizer and a Classic Mood Stabilizer for BP (CHOICE Study) (R01-HS19371); Ariel Gildengers, MD and The Effect of Bipolar Disorder and Its Comorbidities on Cognition in Older Adults (R01-MH084921); and Mary L. Phillips, MD and Toward the Identification of Biomarkers of BP (R01-MH076971). We would also like to thank those individuals who participated in this study.
Role of Funding Source
PARC2 study was partially funded by Charles F. Kline and Actigraph, Inc. The actigraphy programming for PARC2 and NHANES 2003-2004 study was partially supported by Career Development Awards from NHLBI (K23HL075098)(Richardson) and NIA (K01AG025962)(Strath).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Carol A Janney has received research support from Actigraph, Inc for this study.
Andrea Fagiolini is/has been a consultant and/or speaker and/or has received grants from: Angelini, Astra Zeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Pfizer, Eli Lilly, Janssen, Lundbeck, Novartis, Otsuka, Roche and Sigma Tau.
Holly A. Swartz has received honoraria from Servier, Astra Zeneca, SciMed, Bristol-Myers Squibb, Eli Lilly, and Sanofi. She has received royalties from UpToDate.
John M. Jakicic has received an honorarium for scientific presentations from the Nestle Nutrition Institute and JennyCraig. He has served on the scientific advisory board for Alere WellBeing. He has also served as the Principal Investigator on a research grant awarded to the University of Pittsburgh from BodyMedia, Inc..
Robert G. Holleman and Caroline R. Richardson have no financial disclosures.
References
- BASSETT DR., JR. Validity and reliability issues in objective monitoring of physical activity. Research Quarterly for Exercise & Sport. 2000;71:S30–6. [PubMed] [Google Scholar]
- BAUER MS, WILLIFORD WO, DAWSON EE, AKISKAL HS, ALTSHULER L, FRYE C, GELENBERG A, GLICK H, KINOSIAN B, SAJATOVIC M. Principles of effectiveness trials and their implementation in VA Cooperative Study #430: 'Reducing the Efficacy-Effectiveness Gap in Bipolar Disorder'. Journal of Affective Disorders. 2001;67:61–78. doi: 10.1016/s0165-0327(01)00440-2. [DOI] [PubMed] [Google Scholar]
- BRYANT-COMSTOCK L, STENDER M, DEVERCELLI G. Health care utilization and costs among privately insured patients with bipolar I disorder. Bipolar Disorders. 2002;4:398–405. doi: 10.1034/j.1399-5618.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- CAIRNEY J, VELDHUIZEN S, FAULKNER G, SCHAFFER A, RODRIGUEZ MC. Bipolar disorder and leisure-time physical activity: Results from a national survey of Canadians. Mental Health and Physical Activity. 2009;2:65–70. [Google Scholar]
- CHARNEY DS, REYNOLDS CF, 3RD, LEWIS L, LEBOWITZ BD, SUNDERLAND T, ALEXOPOULOS GS, BLAZER DG, KATZ IR, MEYERS BS, AREAN PA, BORSON S, BROWN C, BRUCE ML, CALLAHAN CM, CHARLSON ME, CONWELL Y, CUTHBERT BN, DEVANAND DP, GIBSON MJ, GOTTLIEB GL, KRISHNAN KR, LADEN SK, LYKETSOS CG, MULSANT BH, NIEDEREHE G, OLIN JT, OSLIN DW, PEARSON J, PERSKY T, POLLOCK BG, RAETZMAN S, REYNOLDS M, SALZMAN C, SCHULZ R, SCHWENK TL, SCOLNICK E, UNUTZER J, WEISSMAN MM, YOUNG RC. Depression and Bipolar Support Alliance consensus statement on the unmet needs in diagnosis and treatment of mood disorders in late life. Archives of General Psychiatry. 2003;60:664–72. doi: 10.1001/archpsyc.60.7.664. DEPRESSION AND BIPOLAR SUPPORT, A. [DOI] [PubMed] [Google Scholar]
- DEPARTMENT OF HEALTH AND HUMAN SERVICES CENTER FOR DISEASE CONTROL AND PREVENTION NHANES 2003-2004 Public Data General Release File Documentation. 2005 [Online]. Available: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/general_data_release_doc_03-04.pdf [Accessed 4/12/2007]
- DEPARTMENT OF HEALTH AND HUMAN SERVICES CENTER FOR DISEASE CONTROL AND PREVENTION National Health and Nutrition Examination Survey Documentation, Codebook, and Frequencies MEC Laboratory Component: Serum Cotinine. 2006 [Online]. Available: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l06cot_c.pdf [Accessed 4/5/2009] [Google Scholar]
- ELMSLIE JL, MANN JI, SILVERSTONE JT, WILLIAMS SM, ROMANS SE. Determinants of overweight and obesity in patients with bipolar disorder. Journal of Clinical Psychiatry. 2001;62:486–91. doi: 10.4088/jcp.v62n0614. quiz 492-3. [DOI] [PubMed] [Google Scholar]
- FAGIOLINI A, FRANK E, SCOTT J, TURKIN S, KUPFER D. Metabolic syndrome in bipolar disorder: Findings from the Bipolar Disorder Center for Pennsylvanians. Bipolar Disorders. 2005;7:424–430. doi: 10.1111/j.1399-5618.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- FAGIOLINI A, GORACCI A. The effects of undertreated chronic medical illnesses in patients with severe mental disorders. Journal of Clinical Psychiatry. 2009;3(70 Suppl):22–9. doi: 10.4088/JCP.7075su1c.04. [DOI] [PubMed] [Google Scholar]
- FAGIOLINI A, KUPFER DJ, HOUCK PR, NOVICK DM, FRANK E, FAGIOLINI A, KUPFER DJ, HOUCK PR, NOVICK DM, FRANK E. Obesity as a correlate of outcome in patients with bipolar I disorder. American Journal of Psychiatry. 2003;160:112–7. doi: 10.1176/appi.ajp.160.1.112. [DOI] [PubMed] [Google Scholar]
- FAGIOLINI AM, CHENGAPPA KNR, SORECA I, CHANG J. Bipolar Disorder and the Metabolic Syndrome: causal factors, psychiatric outcomes and economic burden. CNS Drugs. 2008;22:655–669. doi: 10.2165/00023210-200822080-00004. [DOI] [PubMed] [Google Scholar]
- FIRST MB, SPITZER RL, GIBBON M, WILLIAMS JBW. Structured clinical interview for DSM-IV Axis I Disorders, clinician version (SCID-CV) American Psychiatric Press, Inc.; Washington DC: 1996. [Google Scholar]
- FREEDSON PS, MELANSON E, SIRARD J. Calibration of the Computer Science and Applications, Inc. accelerometer. Medicine & Science in Sports & Exercise. 1998;30:777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- GOODRICH DE, KILBOURNE AM. A long-time coming - The creation of an evidence base for physical activiy prescription to improve health outcomes in bipolar disorder. Mental Health and Physical Activity. 2010;3:1–3. doi: 10.1016/j.mhpa.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGSTROMER M, OJA P, SJOSTROM M. Physical activity and inactivity in an adult population assessed by accelerometry. Medicine & Science in Sports & Exercise. 2007;39:1502–8. doi: 10.1249/mss.0b013e3180a76de5. [DOI] [PubMed] [Google Scholar]
- HAMILTON M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANNEY CA, RICHARDSON CR, HOLLEMAN RG, GLASHEEN C, STRATH SJ, CONROY MB, KRISKA AM. Gender, mental health service use and objectively measured physical activity: Data from the National Health and Nutrition Examination Survey (NHANES 2003-2004) Mental Health and Physical Activity. 2008;1:9–16. doi: 10.1016/j.mhpa.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOFFE R, MACQUEEN G, MARRIOTT M, TREVOR YOUNG L. A prospective, longitudinal study of percentage of time spent ill in patients with bipolar I or bipolar II disorders. Bipolar Disorders. 2004;6:62–66. doi: 10.1046/j.1399-5618.2003.00091.x. [DOI] [PubMed] [Google Scholar]
- JUDD LL, AKISKAL HS, SCHETTLER PJ, CORYELL W, ENDICOTT J, MASER JD, SOLOMON DA, LEON AC, KELLER MB. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Archives of General Psychiatry. 2003a;60:261–269. doi: 10.1001/archpsyc.60.3.261. [DOI] [PubMed] [Google Scholar]
- JUDD LL, SCHETTLER PJ, AKISKAL HS, MASER J, CORYELL W, SOLOMON D, ENDICOTT J, KELLER M. Long-term symptomatic status of bipolar I vs. bipolar II disorders. International Journal of Neuropsychopharmacology. 2003b;6:127–137. doi: 10.1017/S1461145703003341. [DOI] [PubMed] [Google Scholar]
- KILBOURNE AM, CORNELIUS JR, HAN X, PINCUS HA, SHAD M, SALLOUM I, CONIGLIARO J, HAAS GL. Burden of general medical conditions among individuals with bipolar disorder. Bipolar Disorders. 2004;6:368–73. doi: 10.1111/j.1399-5618.2004.00138.x. [DOI] [PubMed] [Google Scholar]
- KILBOURNE AM, ROFEY DL, MCCARTHY JF, POST EP, WELSH D, BLOW FC. Nutrition and exercise behavior among patients with bipolar disorder. Bipolar Disorders. 2007;9:443–52. doi: 10.1111/j.1399-5618.2007.00386.x. [DOI] [PubMed] [Google Scholar]
- KRISKA A. Ethnic and Cultural Issues in Assessing Physical Activity. Research Quarterly for Exercise & Sport. 2000;71:S47–53. [PubMed] [Google Scholar]
- KUPFER DJ. The increasing medical burden in bipolar disorder. JAMA. 2005;293:2528–30. doi: 10.1001/jama.293.20.2528. [DOI] [PubMed] [Google Scholar]
- KUPFER DJ, AXELSON DA, BIRMAHER B, BROWN C, CURET DE, FAGIOLINI A, FRANK E, FRIEDMAN ES, GROCHOCINSKI VJ, HOUCK PR, KILBOURNE AM, MULSANT BH, POLLOCK BG, REYNOLDS CF, STOFKO MG, SWARTZ HA, THASE ME, TURKIN SR, WHYTE EM. Bipolar disorder center for Pennsylvanians: implementing an effectiveness trial to improve treatment for at-risk patients. Psychiatric services. 2009;60:888–97. doi: 10.1176/appi.ps.60.7.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUPFER DJ, FOSTER FG. Sleep and activity in a psychotic depression. Journal of Nervous & Mental Disease. 1973;156:341–8. doi: 10.1097/00005053-197305000-00007. [DOI] [PubMed] [Google Scholar]
- KUPFER DJ, WEISS BL, FOSTER G, DETRE TP, MCPARTLAND R. Psychomotor activity in affective states. Archives of General Psychiatry. 1974;30:765–8. doi: 10.1001/archpsyc.1974.01760120029005. [DOI] [PubMed] [Google Scholar]
- MATTHEWS CE, CHEN KY, FREEDSON PS, BUCHOWSKI MS, BEECH BM, PATE RR, TROIANO RP. Amount of Time Spent in Sedentary Behaviors in the United States, 2003-2004. American Journal of Epidemiology. 2008;167:875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS CE, HAGSTROMER M, POBER DM, BOWLES HR. Best Practices for Using Physical Activity Monitors in Population-Based Research. Medicine & Science in Sports & Exercise. 2012;44:S68–S76. doi: 10.1249/MSS.0b013e3182399e5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY C, LOPEZ A. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. The Lancet. 1997;349:1436–42. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- NALLAMOTHU BK, HAYWARD RA, BATES ER. Beyond the randomized clinical trial: the role of effectiveness studies in evaluating cardiovascular therapies. Circulation. 2008;118:1294–1303. doi: 10.1161/CIRCULATIONAHA.107.703579. [DOI] [PubMed] [Google Scholar]
- PEELE BP, XU Y, DJ K. Insurance expenditures on bipolar disorder: clinical and parity implications. Am J Psychiatry. 2003;160:1286–1290. doi: 10.1176/appi.ajp.160.7.1286. [DOI] [PubMed] [Google Scholar]
- REVICKI DA, MATZA LS, FLOOD E, LLOYD A. Bipolar disorder and health-related quality of life : review of burden of disease and clinical trials. Pharmacoeconomics. 2005;23:583–94. doi: 10.2165/00019053-200523060-00005. [DOI] [PubMed] [Google Scholar]
- SALLIS JF, SAELENS BE. Assessment of physical activity by self-report: status, limitations, and future directions. Research Quarterly for Exercise & Sport. 2000;71:S1–14. [PubMed] [Google Scholar]
- SALVATORE P, GHIDINI S, ZITA G, DE PANFILIS C, LAMBERTINO S, MAGGINI C, BALDESSARINI RJ. Circadian activity rhythm abnormalities in ill and recovered bipolar I disorder patients. Bipolar Disorders. 2008;10:256–65. doi: 10.1111/j.1399-5618.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- SHEEHAN D, LECRUBIER Y, SHEEHAN K, AMORIM P, JANAVS J, WEILLER E, HERGUETA T, BAKER R, DUNBAR G. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- SHIPPEE ND, SHAH ND, WILLIAMS MD, MORIARTY JP, FRYE MA, ZIEGENFUSS JY. Differences in demographic composition and in work, social, and functional limitations among the populations with unipolar depression and bipolar disorder: results from a nationally representative sample. Health & Quality of Life Outcomes. 2011;9 doi: 10.1186/1477-7525-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLE B, BONNIN C, TORRENT C, MARTINEZ-ARAN A, POPOVIC D, TABARES-SEISDEDOS R, et al. Neurocognitive impairment across the bipolar spectrum. CNS Neurosci Ther. 2012;18:194–200. doi: 10.1111/j.1755-5949.2011.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SORECA I, FAGIOLINI A, FRANK E, HOUCK PR, THOMPSON WK, KUPFER DJ. Relationship of general medical burden, duration of illness and age in patients with bipolar I disorder. Journal of Psychiatric Research. 2008;42:956–61. doi: 10.1016/j.jpsychires.2007.10.009. [DOI] [PubMed] [Google Scholar]
- STROHLE A, HOFLER M, PFISTER H, MULLER A-G, HOYER J, WITTCHEN H-U, LIEB R. Physical activity and prevalence and incidence of mental disorders in adolescents and young adults. Psychological Medicine. 2007;37:1657–66. doi: 10.1017/S003329170700089X. [DOI] [PubMed] [Google Scholar]
- THASE ME, CARPENTER L, KUPFER DJ, FRANK EF. Clinical significance of reversed vegetative subtypes of recurrent major depression. Psychopharmacology Bulletin. 1991;27:17–22. [PubMed] [Google Scholar]
- TROST SG, MCIVER KL, PATE RR. Conducting Accelerometer-Based Activity Assessments in Field-Based Research. Medicine & Science in Sports & Exercise. 2005;37(11):S531–S543. doi: 10.1249/01.mss.0000185657.86065.98. [DOI] [PubMed] [Google Scholar]
- WEISS BL, FOSTER FG, REYNOLDS CF, 3RD, KUPFER DJ. Psychomotor activity in mania. Archives of General Psychiatry. 1974;31:379–83. doi: 10.1001/archpsyc.1974.01760150083012. [DOI] [PubMed] [Google Scholar]
- WRIGHT KA, EVERSON-HOCK ES, TAYLOR AH. The effects of physical activity on physical and mental health among individuals with bipolar disorder: A systematic review. Mental Health and Physical Activity. 2009;2:86–94. [Google Scholar]
- YOUNG RC, BIGGS JT, ZIEGLER VE, MEYER DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]