Abstract
FXTAS, a neurodegenerative disorder, affects Fragile X (FMR1) gene premutation carriers in late-life. Studies have shown cognitive impairments in FXTAS including executive dysfunction, working memory, and visuospatial deficits. However, less is known about cognition in females with FXTAS. Thus, we examined semantic processing and verbal memory in female FXTAS patients with event-related potentials (ERPs) and neuropsychological testing. 61 females (34 FXTAS Mage = 62.7, 27 controls Mage = 60.4) were studied with 32-channel ERPs during a category judgment task in which semantically congruous (50%) and incongruous items were repeated ~10–140s later. N400 and P600 amplitude data were submitted to ANCOVA. Neuropsychological testing demonstrated lower performance in verbal learning and executive function in females with FXTAS. ERP analyses revealed a significant reduction of the N400 congruity effect (incongruous–congruous) in the FXTAS group. The N400 congruity effect reduction in females with FXTAS was mainly due to increased N400 amplitude to congruous new words. No significant abnormalities of the N400 repetition effect or the P600 repetition effect were found, indicating preserved implicit memory and verbal memory, respectively, in females with FXTAS. The decreased N400 congruity effect suggests abnormal semantic expectancy and/or semantic network disorganization in female FXTAS patients. The enhanced N400 amplitude to congruous new words may reflect decreased cognitive flexibility among FXTAS women, making access to less typical category exemplar words more difficult.
Keywords: Fragile X premutation, female, FXTAS, N400, P600, semantic, memory
Introduction
Approximately 40% of older male and 8–17% of female FMR1 premutation carriers (with 55–200 CGG repeats) develop fragile X-associated tremor/ataxia syndrome (FXTAS), a neurodegenerative disorder characterized by intention tremor, cerebellar ataxia, peripheral neuropathy, and cognitive deficits in executive function, attention, memory, and visuospatial processing (Grigsby et al. 2008; Hagerman et al. 2001; Hagerman & Hagerman, 2013; Jacquemont et al. 2003).
Female carriers are generally believed to have milder impairments. However, mounting evidence suggests more variable phenotypes among female carriers, including possibly gender-specific neuromotor decline (as reviewed by Kraan et al. 2013), and a greater prevalence of psychiatric and autoimmune disorders than their male counterparts (Coffey et al. 2008; Hunter et al. 2011; Seritan et al. 2013). Cognitively, female carriers without FXTAS have demonstrated visuospatial deficits (e.g., Goodrich et al. 2011), attention problems (Hunter et al. 2008), and language dysfluencies progressing with age (Sterling et al. 2013). In a study examining older female carriers with and without FXTAS, Yang and colleagues (2013a) reported neuropsychological and electrophysiological phenotypes of hypofrontality in both carrier groups, with overall frontal/executive dysfunction more prominent in the FXTAS group, but more significant working memory decrement among female carriers without FXTAS. A volumetric MRI study found mild brain atrophy and white matter disease in women with FXTAS (Adams et al. 2007). Absent cerebellar inhibition over primary motor cortex, as well as reduced GABA-mediated intracortical and afferent inhibition, have been revealed in female premutation carriers in a transcranial magnetic stimulation (TMS) study (Conde et al. 2013). A recent clinic-neuropathological case series documented dementia in 4 of the 8 autopsied female premutation carriers, and found FXTAS-characteristic intranuclear inclusions in all of them, including two women without a FXTAS diagnosis before death (Tassone et al. 2012). Thus, female premutation carriers display a higher prevalence of clinical involvement than previously thought. Nonetheless, the nature of these neurodevelopmental and neurodegenerative (mainly FXTAS) impairments, as well as the distinctions and interactions between them, are not well understood.
Event-related potentials (ERP) provide an excellent non-invasive tool for measuring the precise timing of neural events that mediate a variety of cognitive processes, because of its high temporal resolution (Nunez & Srinivasan, 2006). In the first ERP study in FXTAS, Olichney and colleagues (2010) demonstrated that male-predominant FXTAS individuals have a significant reduced N400 word repetition effect –– an electrophysiological index of semantic processing and implicit memory –– which has also been shown highly sensitive to early-stage Alzheimer’s disease (AD) (Olichney et al. 2006, 2008). On the other hand, unlike AD, FXTAS patients had relatively normal verbal memory scores and the associated P600 word repetition effects (Olichney et al. 2010). In our recent placebo-controlled, double-blind, randomized clinical trial study of memantine (an uncompetitive antagonist of N-methyl-D-aspartate (NMDA) glutamate receptor) in FXTAS (Yang et al. 2013b), we found that improvements in cued memory retrieval were associated with increases in the N400 repetition effect.
In the present study, neuropsychological testing and the ERP paradigm used in Olichney et al. (2010) were integrated to characterize semantic and memory processing in older female premutation carriers with FXTAS.
Methods
Subjects
Participants were 34 female patients with mild FXTAS symptoms (mean FXTAS clinical stage = 2.8, range: 2–4) and 27 female normal elderly controls (NC), recruited through the MIND Institute at the University of California Davis (UCD). All subjects are native English speakers and provided written informed consent for a study protocol approved by the UCD Institutional Review Board. FXTAS was diagnosed according to the criteria for probable or possible FXTAS (Jacquemont et al. 2003). FMR1 allele status was identified in all subjects by DNA testing using a combination of PCR and southern blot methods as described elsewhere (Tassone et al. 2008). As expected, FMR1 mRNA levels were significantly elevated in the patients with FXTAS (t34= 4.0, p < 0.001). There was no significant group difference in age (t59= 0.98, p = 0.33). The NC group had a slightly higher education level (t57= 2.85, p = 0.006). CGG data were missing for 8 controls, none of whom had any history of neurologic diseases or indication of FXS in their family (see Table 1 for demographics and genetic-molecular data).
Table 1.
Demographics and genetic-molecular measures (mean ± SD)
| NC (N = 27) | FXTAS (N = 34) | |
|---|---|---|
| Age | 60.4 ± 6.8 | 62.7 ± 10.7 |
| Education | 16.5 ± 2.6 | 14.7 ± 2.1* |
| CGG repeats | 31.4 ± 6.7 | 85.5 ± 18.3* |
| FMR1 mRNA | 1.6 ± 0.3 | 2.3 ± 0.6* |
| FXTAS stage | – | 2.8 ± 0.7 |
Note.
p < 0.01.
NC = normal control.
Neuropsychological Testing
Each subject underwent extensive neuropsychological evaluations. The Mini-Mental State Examination (MMSE; Folstein et al. 1978) was used as a measure of global cognitive abilities. Executive function was evaluated using the Stroop Color and Word Test (Stroop, 1935), Behavioral Dyscontrol Scale 2nd edition (BDS-2; Grigsby & Kaye, 1996) and the Controlled Oral Word Association Test (COWAT; DesRosiers & Kavanaugh, 1987). The color-word component of the Stroop Test is a measure of the capacity for cognitive inhibition. The BDS-2 provides a valid and reliable measure of the capacity for behavioral self-regulation involving intentional control of voluntary motor behavior. The COWAT is a measure of verbal fluency, widely considered to be a component of executive function, as it involves the active generation of information. Verbal learning and memory were assessed with the California Verbal Learning Test (CVLT) (Delis et al. 1987).
EEG/ERP experiment
Electroencephalogram (EEG) recordings were performed during a semantic category decision task, in which auditory category statements were followed by semantically congruous (50%) or incongruous (50%) visual target words.
Procedure
Participants were seated 100 cm in front of a computer monitor. Category statements were read aloud, each followed approximately one second later by a visually-presented target word. Target words were presented within a rectangle in the center of the monitor (visual angle 0.4°). Subjects were instructed to wait three seconds following the appearance of the target, then say the perceived word aloud, followed by “yes/no” to indicate whether it was an exemplar of the defined category or not. Three blocks of EEG data were recorded, each lasting slightly over 20 minutes.
After the EEG recordings were completed, three unanticipated paper and pencil memory tests (free recall, cued recall, and multiple-choice recognition) were administered in that order. The free recall task instructed subjects to recall as many target words as possible, regardless of whether they were congruous or incongruous. In the cued recall task, subjects were provided with a list of 40 categories (22 of which had preceded congruous target words, 18 had preceded incongruous words) and subjects were asked to fill in the word that had been presented during the experiment. The final task, multiple-choice recognition, consisted of 40 category statements, each followed by six possible target word completions (four congruous category exemplars and two incongruous nouns).
Stimuli
The stimuli were 216 category-target pairs (e.g., ‘a type of meat’─‘chicken’). Categories and targets were selected with the aid of published norms and locally administered normative questionnaires (Olichney et al. 2000). Half of the target words (‘congruous’ words) were medium-typicality category exemplars. The other half of the targets were concrete nouns ‘incongruous’ with their associated category, but matched to the congruous target words on length and frequency of usage (Francis & Kucera, 1982; Olichney et al. 2010).
Subjects were randomly assigned to one of three counterbalanced stimulus lists, which included 36 congruous targets presented once, 36 presented twice, 36 presented three times and equal numbers of incongruous targets in the same pseudo-randomized repetition conditions, with a total of 432 trials evenly divided into three blocks. Therefore, half of the stimuli were congruous and half were incongruous; half were new and half were repeated. Repeated target words always appeared paired with the same category as on first presentation. For words repeated once, the lag between first and second presentations was 0–3 intervening trials (spanning approximately 10–40 s). For doubly repeated words, the lag for both second and third presentations was 10–13 intervening trials (~110–140 s).
Electrophysiological Recording
Thirty-two (32)-channel EEG was recorded with a Nicolet-SM-2000 digital amplifier (bandpass =0.016–100 Hz, sampling rate =250 Hz). Interelectrode impedance was maintained at ≤ 5 kΩ. EEG was recorded from midline (Fz, Cz, Pz, POz), lateral frontal (F3, F4, F7, F8, FC1, FC2, Fp1, Fp2), temporal (T5, T6), parietal (P3, P4, CP1, CP2) and occipital sites (O1, O2, PO7, PO8) defined by the International 10–20 system. Additional lateral sites included approximations of Broca’s area (Bl), Wernicke’s area (Wl) and their right hemisphere homologues (Br, Wr), and an electrode pair 33% of the interaural distance lateral to Cz over the superior temporal lobe (41L, 41R). All scalp electrodes were referenced online to the left mastoid and subsequently re-referenced offline to the average of the left and right mastoids. Electro-oculogram (EOG) from vertical and horizontal eye movements was recorded with 4 electrodes, one directly beneath and one at the outer canthus of each eye.
Data Analysis
EEG trials contaminated by eye blinks or movements, excessive muscle activity, or amplifier blocking were rejected. By averaging artifact-free epochs (length = 1024 ms, pre-stimulus baseline = 100 ms) of each condition, separate ERP waveforms were obtained for new congruous and incongruous target words, as well as for repeated congruous and incongruous target words. ERP dependent variables included the N400 congruity effect (a larger negative deflection to incongruous than congruous words in normal subjects because the N400 is a sensitive index of semantic processing load (Chwilla et al. 1995)), the N400 repetition effect (an index of priming/implicit memory), and the P600 repetition effect (a larger positive deflection to congruous new than old words in normal subjects, reflecting verbal memory) (Olichney et al. 2000, 2010).
ERP data from 26 scalp electrodes (excluding Fp1 and Fp2 due to their vulnerability to muscle activity) were submitted to repeated-measures analyses of covariance (ANCOVA; SPSS 21, IBM) with years of education controlled for as a covariate. N400 measures were obtained from 300–550 ms time window. Mean amplitude of the N400 congruity effect (new congruous versus incongruous words) was analyzed with the between-subjects factor of Group and two within-subjects factors of Congruity (congruous/incongruous) and Electrode. Analysis on the N400 word repetition effect mean amplitude (new versus old incongruous words) had the between-subjects factor of Group and two within-subjects factors of Repetition (New/Old) and Electrode. The late positive components (LPC/P600) word repetition effect mean amplitude (new versus old congruous words) was analyzed with between-subjects factor of Group and three within-subjects factors: Repetition (New/Old), Latency (300–550 ms, 550–800 ms) and Electrode. The Greenhouse-Geisser correction was used whenever the assumption of sphericity was violated.
Partial correlations between neuropsychological test scores and a composite N400 ERP measure (derived from the mean of 6 posterior electrodes: Pz, P4, POz, Wr, T6, O2) were examined with years of education controlled for as a covariate, as were correlations between the P600 repetition effect amplitude at Pz and neuropsychological test scores. These electrode sites were chosen to conform with where the largest ERP effects are normally present. It has been well established that the N400 component has a right hemisphere bias, and is maximal at the parietal and right posterior channels (Kutas & Federmeier, 2011), while the P600 component is most prominent in midline parietal channels (Olichney et al. 2000, 2010).
To control for multiple comparisons, only p-values less than 0.01 were considered as significant in all statistical analyses.
Results
Behavioral Results
Table 2 summarizes the neuropsychological test scores and group comparisons with years of education controlled. Global abilities, as measured by the MMSE, demonstrated no significant difference between groups.
Table 2.
Neuropsychological test scores (mean ± SD) in normal controls (NC) and female FXTAS patients (with education controlled for as a covariate).
| NC (N = 27) | FXTAS (N = 34) | F | p | |
|---|---|---|---|---|
| Global Abilities | ||||
| MMSE | 28.2 ± 2.2 | 27.7 ± 1.6 | 1.39 | 0.24 |
| Executive Function | ||||
| COWAT | 50.0 ± 15.2 | 41.2 ± 11.9 | 4.34 | 0.04# |
| BDS-2 | 21.7 ± 3.6 | 16.2 ± 3.5 | 20.85 | < 0.0001*** |
| Stroop | 43.2 ± 8.0 | 32.3 ± 10.0 | 14.53 | < 0.001** |
| Verbal memory | ||||
| CVLT List A, trials 1–5 | 53.6 ± 8.6 | 45.1 ± 9.5 | 7.46 | 0.009* |
| CVLT SD FR | 11.2 ± 3.1 | 9.33 ± 3.4 | 3.68 | 0.06# |
| CVLT SD CR | 12.0 ± 2.6 | 10.6 ± 2.7 | 2.19 | 0.15 |
| CVLD LD FR | 11.1 ± 2.8 | 9.6 ± 3.6 | 2.23 | 0.14 |
| CVLT LD CR | 11.8 ± 2.5 | 10.7 ± 2.7 | 1.51 | 0.23 |
| Subsequent Memory (for ERP Stimuli) | ||||
| Free Recall | 21.6 ± 9.3 | 18.9 ± 9.7 | 1.86 | 0.18 |
| Cued Recall | 18.7 ± 3.1 | 17.0 ± 4.1 | 3.43 | 0.07# |
| MC Recognition | 30.0 ± 8.0 | 30.2 ± 7.1 | 0.004 | 0.95 |
Note.
Marginal significance.
COWAT= Controlled Oral Word Association Test; BDS-2= Behavioral Dyscontrol Scale 2nd edition; CVLT= California Verbal Learning Test, SD FR= Short-delay free recall, SD CR= Short-delay cued recall, LD FR= Long-delay free recall, LD CR= Long-delay cued recall; MC= Multiple-choice.
The FXTAS group demonstrated executive function deficits, with lower performance on the BDS-2 and Stroop Test than the NC group. Verbal learning, as measured by the CVLT List A trials 1–5, was also mildly impaired in FXTAS patients. No group differences were found for the subsequent memory tests of free recall and multiple-choice recognition. However, FXTAS patients showed a trend for lower scores on the cued recall task.
ERP Results
Semantic congruity
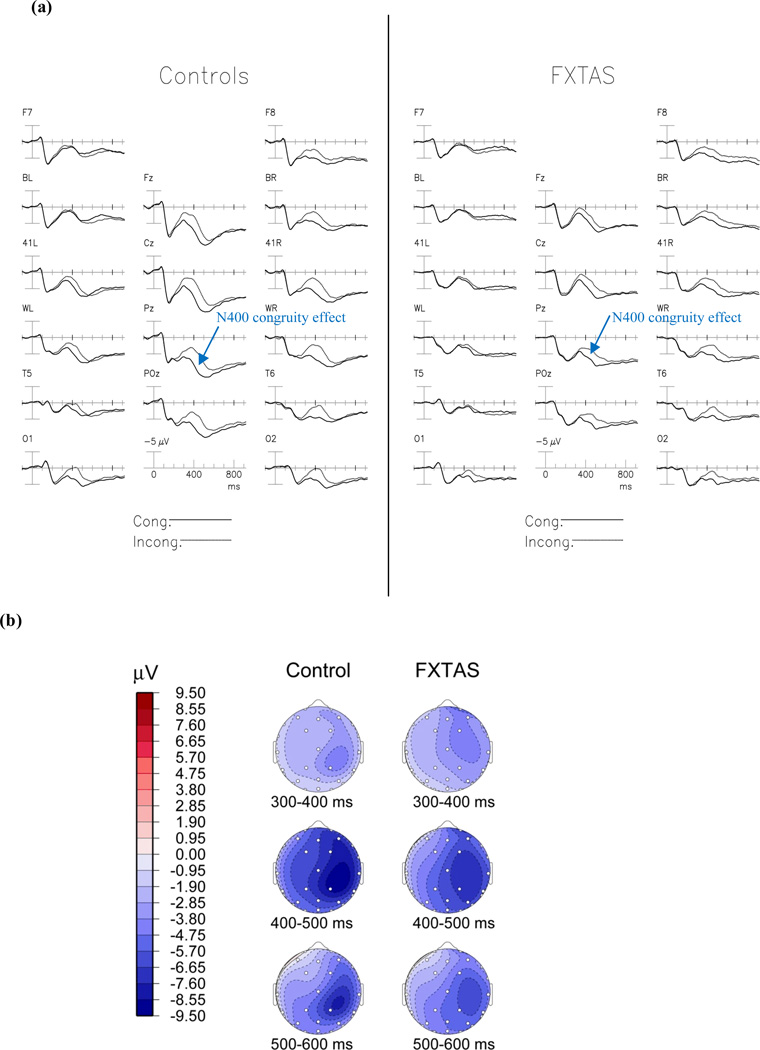
Figure 1a shows the grand average ERPs elicited by the initial presentations of congruous and incongruous target words within two groups. The N400 elicited by incongruous words is most prominent in the right posterior channels. The congruity effect (the difference between the new congruous and new incongruous words) began around 300 ms after stimulus onset and peaked at about 450 ms (Figure 1b).
Figure 1.
The N400 Congruity Effect. (A) Grand average ERPs to initial presentations of congruous (solid line) and incongruous (dotted line) words in normal controls (left) and participants with FXTAS (right). (B) Topographical maps of the N400 congruity effect (incongruous-congruous words). FXTAS group showed reduced N400 congruity effect compared to the normal controls (p = 0.007).
ANCOVA revealed a marginally significant main effect of congruity [F(1,56) = 4.95, p = 0.03], with initial incongruous words producing larger negativities (N400s) than congruous words. There was a significant group × congruity interaction [F(1,56) = 7.97, p = 0.007], due to reduced N400 congruity effect in the FXTAS group [N400 Congruity Effect: NC Mean = −2.85 µV, SE = 0.29; FXTAS Mean = −1.65 µV, SE = 0.26]. Further analysis revealed that the FXTAS group had larger (more negative) N400 amplitude to initial congruous words [NC Mean = 6.6 µV, SE = 0.66; FXTAS Mean = 4.16 µV, SE = 0.56; F(1,56) = 7.42, p = 0.009]. There were no group differences on initial incongruous words [F(1,56) = 1.76, p = 0.19].
Repetition of incongruous words
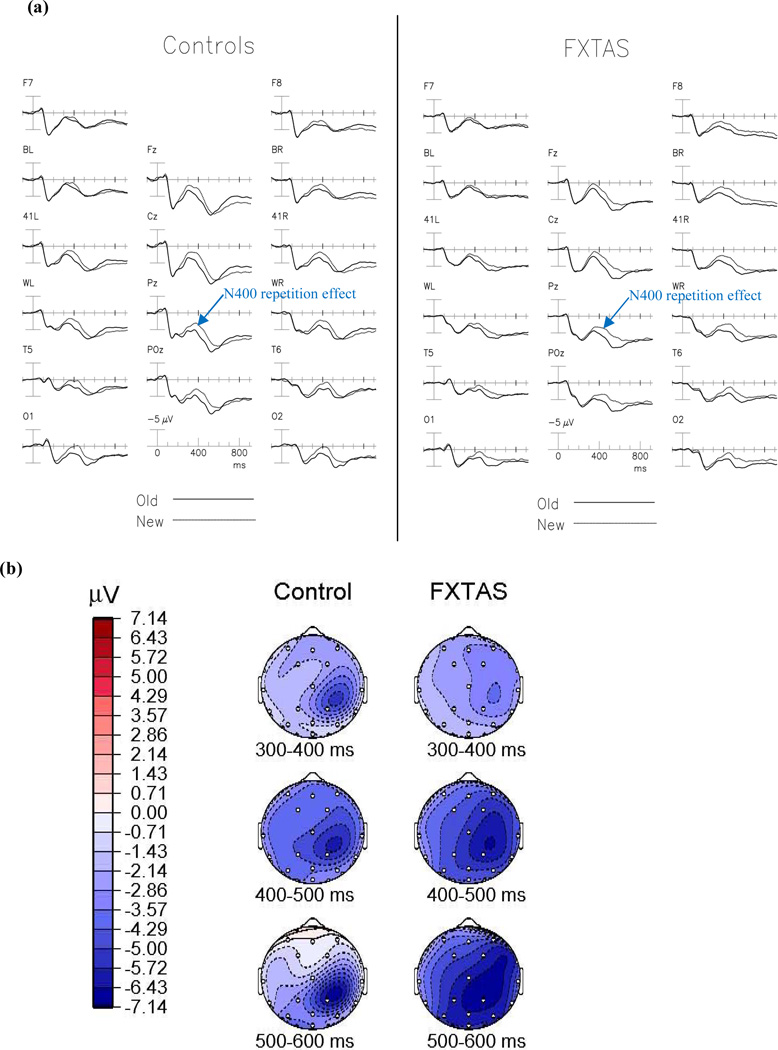
Figure 2a shows the grand average ERPs elicited by the initial and repeated presentations of incongruous words.
Figure 2.
The N400 Repetition Effect. (A) Grand average ERPs to initial (dotted line) and repeated (solid line) presentations of incongruous words, collapsed across repetition lag in normal controls (left) and participants with FXTAS (right). (B) Topographical maps of the N400 repetition effect (new-old incongruous words).
The ANCOVA did not find a significant main effect for repetition [F(1,56) = 1.71, p = 0.20] across all electrode sites. There was also not a significant group × repetition interaction [F(1,56) = .18, p = 0.67], nor a main effect of group [F(1,56) = 2.24, p = 0.14] or a group × repetition × electrode interaction [F= 1.47, p = 0.22] after Greenhouse-Geisser correction.
Repetition of congruous words
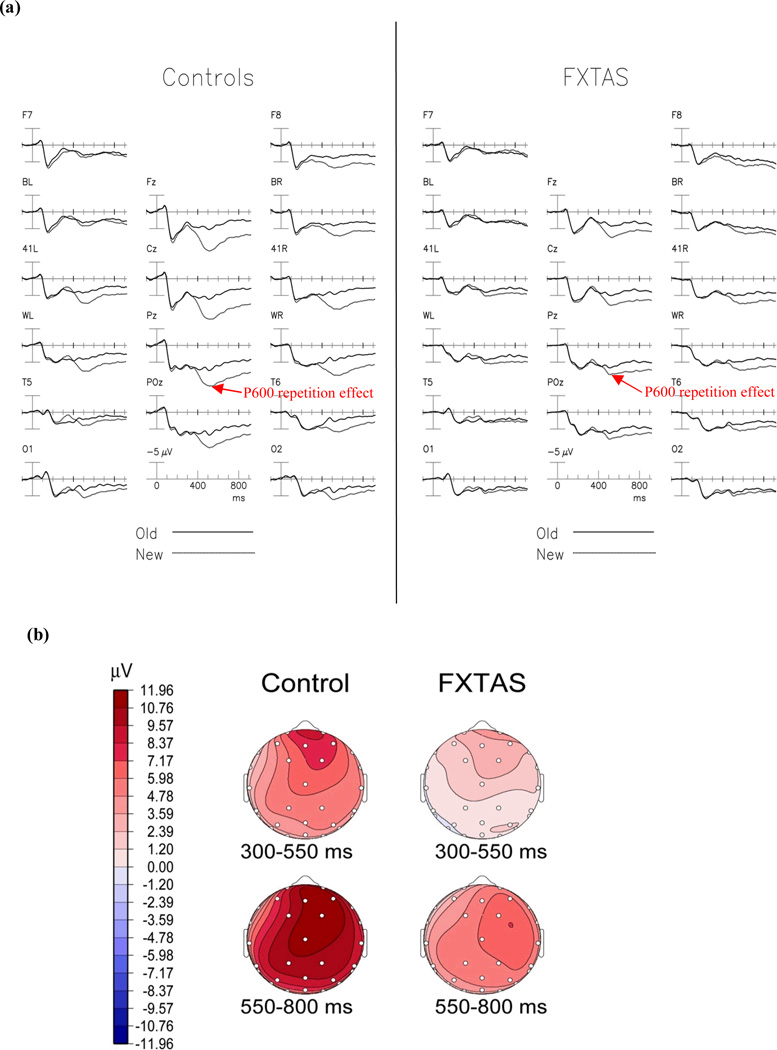
Figure 3a shows the P600 repetition effect (difference between new and old congruous words) with a larger positive voltage to new than old congruous words. This difference was more pronounced in the NC group. This effect was most prominent in the midline channels (Fz, Cz, Pz), began around 400 ms, peaked near 550 ms, and persisted beyond 800 ms post-stimulus onset.
Figure 3.
The P600 Repetition Effect. (A) Grand average ERPs to initial (dotted line) and repeated (solid line) presentations of congruous words, collapsed across repetition lag in normal controls (left) and participants with FXTAS (right). (B) Topographical maps of the LPC repetition effect (new-old congruous words). FXTAS group showed reduced P600 repetition effect compared to the normal controls (p = 0.006).
There was a marginally significant main effect for repetition [F(1,56) = 4.22, p = 0.045] across all electrode sites. There was also a significant group × repetition interaction [F(1,56) = 8.19, p = 0.006] indicating reduced P600 repetition effect amplitude in the FXTAS group. There was borderline significance of a group × latency interaction [F(1,56) = 3.66, p = 0.061]. Because of this, we performed further analyses within two time windows separately (Early: 300–550 ms, Late: 550–800 ms). There was a marginally significant main effect of repetition [F(1,56) = 4.57, p = 0.037] in the late time window. However, in the early time window, the repetition effect did not achieve significance [F(1,56) = 3.16, p = 0.081] across all subjects. Both time windows elicited a significant group × repetition interaction [Early: F(1,56) = 7.48, p = 0.008; Late: F(1,56) = 7.40, p = 0.009] indicating a reduced P600 repetition effect in the FXTAS group in the 300–800 ms time window across all electrodes [P600 Repetition Effect: NC Mean = 5.34 µV, SE =0.51; FXTAS Mean = 4.25 µV, SE = 0.43]. Whereas, the group difference in the P600 repetition effect became non-significant after controlling for the covariate of the CVLT List A Trials 1–5 total score in an ANCOVA [group × repetition interaction: F(1,50) = 1.95, p = 0.17], suggesting that this intergroup difference was mainly due to the mildly reduced verbal learning and memory abilities in FXTAS.
Correlation Results
As expected, FMR1 mRNA levels and number of CGG repeats were significantly correlated across all subjects (r = 0.71, p < 0.001). Within FXTAS patients, this correlation was moderately strong and of marginal significance (r = 0.43, p = 0.05). CGG repeat length correlated with the CVLT long-delay free recall measure (r = −0.50, p < 0.01) among the FXTAS group, and showed a correlation with the P600 repetition effect amplitude at channel Pz which narrowly missed significance (r = 0.42, p = 0.03). No significant correlations with FMR1 mRNA levels were observed.
Partial correlational analyses, with years of education controlled for, were conducted across all subjects (NC and FXTAS) to test if the main ERP amplitude measures correlated with neuropsychological test scores (COWAT, BDS-2, and Stroop). The N400 congruity effect in correlational analysis was defined as the mean voltage for new incongruous minus new congruous words between 300–550 ms, averaged across 6 posterior sites (Pz, P4, POz, Wr, T6, O2). This effect, like the N400 repetition effect, normally has a negative polarity/value. The N400 congruity effect amplitude was significantly associated with the BDS-2 scores (p < 0.01) and showed a trend of correlation with scores on the COWAT in the FXTAS group, but did not significantly correlate with any of the three executive function tests in the NC group (Table 3).
Table 3.
Partial correlation coefficients between N400 congruity effect and frontal-executive test scores (with education controlled for as a covariate).
Note.
p < 0.01;
0.01 ≤ p ≤ 0.07.
The N400 Congruity Effect here is a composite score based on the mean amplitude across 6 posterior electrodes (Pz, P4, POz, Wr, T6, O2).
NC = normal control.
The N400 repetition effect in correlational analysis was defined as the mean voltage difference between 300–550 ms at the posterior sites (Pz, P4, POz, Wr, T6, O2) for new minus old incongruous words. The N400 repetition effect amplitude did not display any significant correlations with any of the memory test scores, neither for the congruous words, nor for the incongruous words (|r|’s < 0.21, p’s ≥ 0.15; Table 4).
Table 4.
Partial correlation coefficients between word repetition effects and memory test scores (across all subjects; education was controlled for as a covariate).
| N400 Repetition Effect | P600 Repetition Effect | |
|---|---|---|
| CVLTlist A, trials 1–5 | −0.17 | 0.32# |
| Free Recall | −0.17 | 0.36* |
| Cued Recall | 0.05 | 0.30# |
| Multiple Choice | 0.01 | 0.21 |
Note.
p = 0.006;
0.024 ≤ p ≤ 0.025.
The N400 repetition effect here is a composite score equal to the mean amplitude across 6 posterior electrodes (Pz, P4, POz, Wr, T6, O2). The P600 repetition effect is the mean amplitude of the posterior midline electrode (Pz). Memory scores (Free Recall, Cued Recall and Multiple Choice) for incongruous words were tested for correlations with the N400 repetition effect, and memory scores for congruous words were correlated with the P600 repetition effect amplitude.
The P600 repetition effect in correlational analysis was defined as the mean voltage difference in the 550–800 ms window at Pz for new minus old congruous words. Amplitude of the P600 repetition effect correlated significantly with free recall (p = 0.006) for congruous items across all subjects (Table 4). Correlations with the CVLT learning measure and multiple choice recognition for the experimental stimuli showed marginal significance.
Discussion
The current study examined semantic processing and verbal memory in older female FXTAS patients by combining an ERP word repetition experiment and neuropsychological testing. The female FXTAS group displayed reductions in the ERP N400 congruity effect (reflecting semantic processing) compared to normal controls, with a normal N400 repetition effect (a measure of priming/implicit memory) and a relatively spared P600 repetition effect (indexing verbal memory). The smaller N400 congruity effect among these female FXTAS patients was associated with poorer performance on executive function tests.
The current study did not observe a reduced N400 repetition effect as Olichney et al. (2010) previously demonstrated in a male-predominant group of FXTAS patients (75% males in both the FXTAS and control groups) using the same ERP word repetition paradigm. The normal N400 repetition effect indicates more intact implicit verbal memory in our female FXTAS patients. The current study and Olichney et al. (2010) suggest that verbal memory is relatively preserved in FXTAS patients regardless of gender, as the group difference on the P600 repetition effect was not present in our female patients after controlling for the CVLT performance. Also, except for decreased verbal learning (on the CVLT List A, trials 1–5), the other intergroup differences on verbal memory scores were only of marginal significance. There were similar correlations between CGG repeat length and P600 measures in both ERP word repetition studies, but female patients in the present study did not exhibit associations between FMR1 mRNA and N400 measures, implicating different FMR1 mRNA mediated mechanisms may underlie gender specific electrophysiological phenotypes. The spared implicit memory processes in FXTAS females, compared to their male counterparts, coincide with the general observations of milder clinical involvement and cognitive impairment in female premutation carriers. Differences in the N400 repetition effect of males and females with FXTAS might be due to the protection by the expression of FMR1 from their unaffected X allele and/or estrogen effects (Hagerman, 2004).
The novel finding of a reduced N400 congruity effect (ERPs to incongruous new words minus congruous new words) in our FXTAS female group appears inconsistent with the prior ERP word repetition study (Olichney et al. 2010). However, it is important to note that, these authors actually showed a trend for an intergroup difference on the N400 congruity effect (p = 0.11), with qualitatively similar results to the present study (see topographic maps in Figure 1B of Olichney et al. 2010). The uneven and smaller sample sizes (32 FXTAS vs. 16 controls) in that study may have reduced the power to detect a group difference in the N400 congruity effect.
The N400 congruity effect reduction observed in the current study was due to a larger N400 response to congruous new words in our FXTAS females compared to controls. It has been well-established that the N400 component is larger to semantic violation or low cloze probabilities (i.e., relative lower probability of words appearing in a given semantic context (Kutas & Hillyard, 1984). The larger responses to congruous new words could represent that the FXTAS female group processed these words in a somewhat similar manner to which they processed incongruous words, i.e., as a relative violation of their semantic expectancy. The congruous target words used in the present study are medium-typicality exemplars. Therefore, following a preceding category statement, if an individual has expectancy and semantic activation for only high typicality exemplars (e.g., ‘King’ for ‘A male member of royalty’), but restricted expectancy/activation for exemplars of medium/low typicality (e.g. ‘Botany’ for ‘A science’), relatively larger N400 would be present for the latter due to extra activation needed to integrate these less activated target words into the semantic context (Kutas & Federmeier, 2011).
Interestingly, the N400 congruity effect amplitude was correlated with performance on executive function tests within the female FXTAS group, but not in controls, suggesting to some extent, executive function deficits likely affect semantic processing in FXTAS women. Executive dysfunction has been consistently reported both in FXTAS males (Berry-Kravis et al. 2007; Grigsby et al. 2008; Yang et al. 2013c) and females (Yang et al. 2013a). Using the Hayling Sentence Completion test (Burgess & Shallice, 1997), Cornish et al. (2008, 2011) have discovered impaired executive inhibition to congruous yet inappropriate semantic responses in male premutation carriers with and without FXTAS. This finding, like the present study, suggests abnormalities in semantic processing and/or inhibitory processes. Brega and colleagues (2008) examined the influence of executive dysfunction on other cognitive symptoms in FXTAS and concluded that executive dysfunction is the primary deficit mediating the other “secondary” deficits in FXTAS involving cognitive domains such as learning, memory, and visuospatial processing. The correlation observed between executive dysfunction and the N400 congruity effect amplitude in the current study supports this view, suggesting executive dysfunction may cause secondary impairment in language processing. The N400 has been shown to be significantly modulated by selective attention (as reviewed in Deacon & Shelley-Tremblay, 2000; Kutas & Federmeier, 2011), a key component of executive function. Therefore, executive function decrements in FXTAS could affect semantic processing by decreasing cognitive flexibility and restricting attention and/or semantic activation. The impact of executive dysfunction on the N400 has also been observed in a group of non-demented Parkinson disease (PD) patients who showed larger N400 congruity effects relative to age-matched normal controls (Kutas et al. 2013). Specifically, PD individuals showed increased N400 to incongruous stimuli in highly-constrained semantic tasks (e.g., antonym judgment), using different linguistic stimuli than the present study. The authors suggested that the enhanced N400 amplitude in PD could be linked to insufficient inhibition of irrelevant semantic information and/or abnormally heavy reliance on external cues (e.g., the preceding semantic prime). Unlike females with FXTAS, the PD patients did not have increased N400 activity to semantically congruous words.
The pathophysiological basis of the abnormal N400 congruity effect in FXTAS females could be the FMR1 mRNA mediated glutamatergic abnormalities found both in fragile X premutation mouse models (Cao et al. 2012; Hunsaker et al. 2012) and in induced pluripotent stem cell-derived human neurons harboring the premutation expansion (Liu et al. 2012). Using a picture recognition paradigm, depth recording studies in the anteromedial temporal lobe found that the N400 amplitude was modulated/reduced by the NMDA glutamate receptor antagonist ketamine (Grunwald et al. 1999). Also, it is worth noting that dopamine circuits may modulate the N400 potential, as abnormal N400 amplitude has been documented in two disorders characterized by abnormal dopaminergic transmission and frontal executive dysfunction, i.e., PD and schizophrenia patients on psychotropic medications (e.g., Nestor et al. 1997; Salisbury et al. 2002; Hokama et al. 2003; Kutas et al. in press). Furthermore, dopamine modulates cortical glutamatergic activity via multiple mechanisms, including D1 receptors on dendritic spines which modulate excitatory inputs to temporal association cortex (Smiley et al. 1992, 1994; Erickson et al. 2000; Sesack et al. 2003). Thus, both glutamatergic and/or dopaminergic neural transmission abnormalities may underlie the altered N400 congruity effect in these women affected by FXTAS. One study limitation is that levels of the fragile X protein (FMRP) were not obtained in most of these subjects. Thus, we can not rule out a possible pathogenic role of FMRP, perhaps mediating the N400 abnormalities observed. FMRP is thought to act as a master regulator of many synaptic proteins (e.g., Liu-Yesucevitz et al. 2011), and it is fascinating to note that the characteristic cognitive deficits in FXTAS include abnormal regulation of behavior and controlled cognitive processes.
This study represents the first examination of the language and memory phenotypes using cognitive ERPs and neuropsychological measures in female FXTAS patients. The results demonstrate relatively preserved memory abilities, but abnormal semantic processing (as indexed by the N400 congruity effect reduction likely related to decreased cognitive flexibility), in women with FXTAS. The current study and our prior ERP studies in FXTAS (Olichney et al. 2010; Yang et al. 2013a,b,c) have demonstrated synaptic dysfunction in this neurogeneti disorder manifesting fronto-cerebellar grey matter loss and white matter disease (Hagerman & Hagerman, 2013). These findings may enlighten our choice of interventions for this disease. For example, targeted treatment trials which aim to improve glutamatergic and/or dopaminergic transmission appear rational and justified for future efforts to improve cognition in this neurodegenerative disorder.
Acknowledgements
This work was supported by a National Institute of Health Roadmap Interdisciplinary Research Consortium Grant (UL1DE019583 and RL1AG032119 to P.J.H., RL1AG032115 to R.J.H.), and by a National Institute on Aging grant (R01 AG18442 to J.M.O.). Dr. Randi J. Hagerman received research support from Seaside therapeutics, Novartis, Forest and Roche and consultation with Novartis, Genentech and Roche for fragile X syndrome treatment studies. We would like to thank all of the participants in the study and their families. We also thank Flora Tassone Lab for genetic testing, Louise Gane for genetic counseling, Jennifer Cogswell for part of the neuropsychological assessments, Yi (Lisa) Mu, Danh Nguyen and Kylee Cook for help with data retrieval.
References
- Adams JS, Adams PE, Nguyen D, Brunberg JA, Tassone F, Zhang W, Koldewyn K, Rivera SM, Grigsby J, Zhang L, DeCarli C, Hagerman PJ, Hagerman RJ. Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS) Neurology. 2007;69:851–859. doi: 10.1212/01.wnl.0000269781.10417.7b. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Abrams L, Coffey SM, Hall DA, Greco C, Gane LW, Grigsby J, Bourgeois JA, Finucane B, Jacquemont S, Brunberg JA, Zhang L, Lin J, Tassone F, Hagerman PJ, Hagerman RJ, Leehey MA. Fragile X-associated tremor/ataxia syndrome: Clinical features, genetics, and testing guidelines. Mov Disord. 2007;22:2018–2030. doi: 10.1002/mds.21493. [DOI] [PubMed] [Google Scholar]
- Brega AG, Goodrich G, Bennett RE, Hessl D, Engle K, Leehey MA, Bounds LS, Paulich MJ, Hagerman RJ, Hagerman PJ, Cogswell JB, Tassone F, Reynolds A, Kooken R, Kenny M, Grigsby J. The primary cognitive deficit among males with fragile X-associated tremor/ataxia syndrome (FXTAS) is a dysexecutive syndrome. J Clin Exp Neuropsychol. 2008;30:853–869. doi: 10.1080/13803390701819044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P, Shallice T. The Hayling and Brixton Tests. England: Thames Valley Test Company; 1997. [Google Scholar]
- Cao Z, Hulsizer S, Tassone F, Tang HT, Hagerman RJ, Rogawski MA, Hagerman PJ, Pessah IN. Clustered burst firing in FMR1 premutation hippocampal neurons: amelioration with allopregnanolone. Hum Mol Genet. 2012;21:2923–2935. doi: 10.1093/hmg/dds118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwilla DJ, Brown CM, Hagoort P. The N400 as a function of the level of processing. Psychophysiology. 1995;32:274–285. doi: 10.1111/j.1469-8986.1995.tb02956.x. [DOI] [PubMed] [Google Scholar]
- Coffey SM, Cook K, Tartaglia N, Tassone F, Nguyen DV, Pan RQ, Bronsky HE, Yuhas J, Borodyanskaya M, Grigsby J, Doerflinger M, Hagerman PJ, Hagerman RJ. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet A. 2008;146A:1009–1016. doi: 10.1002/ajmg.a.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde V, Palomar FJ, Lama MJ, Martínez R, Carrillo F, Pintado E, Mir P. Abnormal GABA-mediated and cerebellar inhibition in women with the fragile X premutation. J Neurophysiol. 2013;109:1315–1322. doi: 10.1152/jn.00730.2012. [DOI] [PubMed] [Google Scholar]
- Deacon D, Shelley-Tremblay J. How automatically is meaning accessed: a review of the effects of attention on semantic processing. Front Biosci. 2000;5:E82–E94. doi: 10.2741/deacon. [DOI] [PubMed] [Google Scholar]
- Debruille JB. The N400 potential could index a semantic inhibition. Brain Res Rev. 2007;56:472–477. doi: 10.1016/j.brainresrev.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test. San Antonio: The Psychological Corporation; 1987. [Google Scholar]
- DesRosiers G, Kavanagh D. Cognitive assessment in closed head injury: Stability, validity and parallel forms for two neuropsychological measures of recovery. Int J Clin Neuropsyc. 1987;9:162–173. [Google Scholar]
- Erickson SL, Sesack SR, Lewis DA. Dopamine innervation of monkey entorhinal cortex: postsynaptic targets of tyrosine hydroxylase-immunoreactive terminals. Synapse. 2000;36:47–56. doi: 10.1002/(SICI)1098-2396(200004)36:1<47::AID-SYN5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Francis WN, Kucera H. Frequency analysis of English usage: lexicon and grammar. Boston: Houghton Mifflin; 1982. [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reaction. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- Goodrich-Hunsaker NJ, Wong LM, McLennan Y, Srivastava S, Tassone F, Harvey D, Rivera SM, Simon TJ. Young adult female fragile X premutation carriers show age- and genetically-modulated cognitive impairments. Brain Cogn. 2011;75:255–260. doi: 10.1016/j.bandc.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish KM, Li L, Kogan CS, Jacquemont S, Turk J, Dalton A, Hagerman RJ, Hagerman PJ. Age-dependent cognitive changes in carriers of the fragile X syndrome. Cortex. 2008;44:628–636. doi: 10.1016/j.cortex.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish KM, Hocking DR, Moss SA, Kogan CS. Selective executive markers of at-risk profiles associated with the fragile X premutation. Neurology. 2011;77:618–622. doi: 10.1212/WNL.0b013e3182299e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Engle K, Leehey MA, Hagerman RJ, Tassone F, Hessl D, Hagerman PJ, Cogswell JB, Bennett RE, Cook K, Hall DA, Bounds LS, Paulich MJ, Cook K, Reynolds A. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008;22:48–60. doi: 10.1037/0894-4105.22.1.48. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Kaye J. The Behavioral Dyscontrol Scale: manual. 2nd ed. Denver: BDS; 1996. [Google Scholar]
- Grunwald T, Beck H, Lehnertz K, Blumcke I, Pezer N, Kurthen M, Fernandez G, Van Roost D, Heinze HJ, Kutas M, Elger CE. Evidence relating human verbal memory to hippocampal N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 1999;96:12085–12089. doi: 10.1073/pnas.96.21.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman PJ, Hagerman RJ. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013;12:786–798. doi: 10.1016/S1474-4422(13)70125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Greco C, Chudley A, Leehey M, Tassone F, Grigsby J, Hills J, Wilson R, Harris SW, Hagerman PJ. Neuropathology and neurodegenerative features in some older male premutation carriers of fragile X syndrome. Am J Hum Genet. 2001;69:177–177. [Google Scholar]
- Hagerman RJ, Leavitt BR, Farzin F, Jacquemont S, Greco CM, Brunberg JA, Tassone F, Hessl D, Harris SW, Zhang L, Jardini T, Gane LW, Ferranti J, Ruiz L, Leehey MA, Grigsby J, Hagerman PJ. Fragile-X-associated tremor/ataxia syndrome (FXTAS) in females with the FMR1 premutation. Am J Hum Genet. 2004;74:1051–1056. doi: 10.1086/420700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokama H, Hiramatsu KI, Wang J, O'Donnell BF, Ogura C. N400 abnormalities in unmedicated patients with schizophrenia during a lexical decision task. Int J Psychophysiol. 2003;48:1–10. doi: 10.1016/s0167-8760(02)00156-3. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Arque G, Berman RF, Willemsen R, Hukema RK. Mouse models of the fragile x premutation and the fragile X associated tremor/ataxia syndrome. Results Probl Cell Differ. 2012;54:255–269. doi: 10.1007/978-3-642-21649-7_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JE, Allen EG, Abramowitz A, Rusin M, Leslie M, Novak G, Hamilton D, Shubeck L, Charen K, Sherman SL. Investigation of phenotypes associated with mood and anxiety among male and female fragile X premutation carriers. Behav Genet. 2008;38:493–502. doi: 10.1007/s10519-008-9214-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JE, Epstein MP, Tinker SW, Abramowitz A, Sherman SL. The FMR1 premutation and attention-deficit hyperactivity disorder (ADHD): evidence for a complex inheritance. Behav Genet. 2012;42:415–422. doi: 10.1007/s10519-011-9520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Leehey M, Grigsby J, Zhang L, Brunberg JA, Greco C, Des Portes V, Jardini T, Levine R, Berry-Kravis E, Brown WT, Schaeffer S, Kissel J, Tassone F, Hagerman PJ. Fragile X premutation tremor/ataxia syndrome: Molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72:869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraan CM, Hocking DR, Bradshaw JL, Fielding J, Cohen J, Georgiou-Karistianis N, Cornish KM. Neurobehavioural evidence for the involvement of the FMR1 gene in female carriers of fragile X syndrome. Neurosci Biobehav Rev. 2013;37:522–547. doi: 10.1016/j.neubiorev.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP) Annu Rev Psychol. 2011;62:621–647. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Brain potentials during reading reflect word expectancy and semantic association. Nature. 1984;307:161–163. doi: 10.1038/307161a0. [DOI] [PubMed] [Google Scholar]
- Kutas M, Iragui V, Niu Y-Q, D'Avanzo T, Yang J-C, Salmon D, Olichney J. Altered N400 congruity effects in Parkinson's disease without dementia. In: Mangun R, editor. Cognitive Electrophysiology of Attention: Signals of the Mind. Academic Press; 2013. pp. 254–267. [Google Scholar]
- Liu J, Koscielska KA, Cao Z, Hulsizer S, Grace N, Mitchell G, Nacey C, Githinji J, McGee J, Garcia-Arocena D, Hagerman RJ, Nolta J, Pessah IN, Hagerman PJ. Signaling defects in iPSC-derived fragile X premutation neurons. Hum Mol Genet. 2012;21:3795–3805. doi: 10.1093/hmg/dds207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Yesucevitz L, Bassell GJ, Gitler AD, Hart AC, Klann E, Richter JD, Warren ST, Wolozin B. Local RNA translation at the synapse and in disease. J Neurosci. 2011;31:16086–16093. doi: 10.1523/JNEUROSCI.4105-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric fields of the brain: the neurophysics of EEG. 2nd Ed. New York: Oxford University Press; 2006. pp. 163–166. [Google Scholar]
- Nestor PG, Kimble MO, O'Donnell BF, Smith L, Niznikiewicz M, Shenton ME, McCarley RW. Aberrant semantic activation in schizophrenia: a neurophysiological study. Am J Psychiatry. 1997;154:640–646. doi: 10.1176/ajp.154.5.640. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Chan S, Wong LM, Schneider A, Seritan A, Niese A, Yang JC, Laird K, Teichholtz S, Khan S, Tassone F, Hagerman R. Abnormal N400 word repetition effects in fragile X-associated tremor/ataxia syndrome. Brain. 2010;133:1438–1450. doi: 10.1093/brain/awq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Iragui VJ, Salmon DP, Riggins BR, Morris SK, Kutas M. Absent event-related potential (ERP) word repetition effects in mild Alzheimer's disease. Clin Neurophysiol. 2006;117:1319–1330. doi: 10.1016/j.clinph.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Taylor JR, Gatherwright J, Salmon DP, Bressler AJ, Kutas M, Iragui-Madoz VJ. Patients with MCI and N400 or P600 abnormalities are at very high risk for conversion to dementia. Neurology. 2008;70:1763–1770. doi: 10.1212/01.wnl.0000281689.28759.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Van Petten C, Paller KA, Salmon DP, Iragui VJ, Kutas M. Word repetition in amnesia. Electrophysiological measures of impaired and spared memory. Brain. 2000;123:1948–1963. doi: 10.1093/brain/123.9.1948. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, Nestor PG, McCarley RW. Semantic bias, homograph comprehension, and event-related potentials in schizophrenia. Clin Neurophysiol. 2002;113:383–395. doi: 10.1016/s1388-2457(02)00003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seritan AL, Bourgeois JA, Schneider A, Mu Y, Hagerman RJ, Nguyen DV. Ages of Onset of Mood and Anxiety Disorders in Fragile X Premutation Carriers. Curr Psychiatry Rev. 2013;9:65–71. doi: 10.2174/157340013805289662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci U S A. 1994;91:5720–5724. doi: 10.1073/pnas.91.12.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Williams SM, Szigeti K, Goldman-Rakic PS. Light and electron microscopic characterization of dopamine-immunoreactive axons in human cerebral cortex. J Comp Neurol. 1992;321:325–335. doi: 10.1002/cne.903210302. [DOI] [PubMed] [Google Scholar]
- Sterling AM, Mailick M, Greenberg J, Warren SF, Brady N. Language dysfluencies in females with the FMR1 premutation. Brain Cogn. 2013;82:84–89. doi: 10.1016/j.bandc.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Greco CM, Hunsaker MR, Seritan AL, Berman RF, Gane LW, Jacquemont S, Basuta K, Jin L-W, Hagerman PJ, Hagerman RJ. Neuropathological, clinical and molecular pathology in female fragile X premutation carriers with and without FXTAS. Genes Brain Behav. 2012;11:577–585. doi: 10.1111/j.1601-183X.2012.00779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Pan R, Amiri K, Taylor AK, Hagerman PJ. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile x (FMR1) gene in newborn and high-risk populations. Journal of Molecular Diagnostics. 2008;10:43–49. doi: 10.2353/jmoldx.2008.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J-C, Simon C, Niu Y-Q, Bogost M, Schneider A, Tassone F, Seritan A, Grigsby J, Hagerman P, Hagerman R, Olichney JM. Phenotypes of hypofrontality in older female fragile X premutation carriers. Ann Neurol. 2013a;74:275–283. doi: 10.1002/ana.23933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J-C, Niu Y-Q, Simon C, Chen L, Seritan A, Schneider A, Torabi Moghaddam S, Tassone F, Hagerman P, Hagerman R, Olichney JM. Effects of Memantine on Language/Memory-related Brain Potentials in Fragile X-associated Tremor/Ataxia Syndrome (FXTAS). Paper presented at: 1st International Conference on FMR1 Premutation: Basic Mechanisms and Clinical Involvement; June 23–26, 2013; Perugia, Italy. 2013b. [Google Scholar]
- Yang J-C, Chan S-H, Khan S, Schneider A, Nanakul R, Teichholtz S, Niu Y-Q, Seritan A, Tassone F, Grigsby J, Hagerman P, Hagerman R, Olichney J. Neural substrates of executive dysfunction in fragile x-associated tremor/ataxia syndrome (FXTAS): a brain potential study. Cereb Cortex. 2013c;23:2657–2666. doi: 10.1093/cercor/bhs251. [Epub 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]