Abstract
Filamentous bacteria of the genus Streptomyces possess linear chromosomes and linear plasmids. Theoretically, linear replicons may not need a decatenase for post-replicational separation of daughter molecules. Yet, Streptomyces contain parC and parE that encode the subunits for the decatenase topoisomerase IV. The linear replicons of Streptomyces adopt a circular configuration in vivo through telomere–telomere interaction, which would require decatenation, if the circular configuration persists through replication. We investigated whether topoisomerase IV is required for separation of the linear replicons in Streptomyces. Deletion of parE from the Streptomyces coelicolor chromosome was achieved, when parE was provided on a plasmid. Subsequently, the plasmid was eliminated at high temperature, and ΔparE mutants were obtained. These results indicated that topoisomerase IV was not essential for Streptomyces. Presumably, the telomere–telomere association may be resolved during or after replication to separate the daughter chromosomes. Nevertheless, the mutants exhibited retarded growth, defective sporulation and temperature sensitivity. In the mutants, circular plasmids could not replicate, and spontaneous circularization of the chromosome was not observed, indicating that topoisomerase IV was required for decatenation of circular replicons. Moreover, site-specific integration of a plasmid is impaired in the mutants, suggesting the formation of DNA knots during integration, which must be resolved by topoisomerase IV.
INTRODUCTION
Most bacterial chromosomes consist of covalently closed circular DNA with negative superhelicity. Two counteracting topoisomerases, gyrase and topoisomerase I (Topo I), are responsible for the maintenance of balanced negative superhelicity of these circular DNA molecules. Gyrase, a GyrA2GyrB2 heterotetramer, cuts and reseals two strands of DNA simultaneously using energy supplied by ATP to create negative supercoiling (Type II topoisomerase). In contrast, Topo I relaxes the negatively supercoiling by cutting and resealing one strand of DNA at a time (Type I topoisomerase).
The gyrase–Topo I pair also acts in concert to relieve the superhelicity generated during replication and transcription, i.e. the local positive supercoiling ahead of the replication forks and transcription bubbles is relaxed by gyrase, and the local negative supercoiling behind the transcription bubbles is compensated by Topo I. Because of these important physiological roles, gyrase and Topo I are basically essential for viability of bacterial cells, although some defects in one of these proteins may be tolerable or suppressed by mutation in the other.
Another topological issue arises at the termination of replication of circular chromosomes and plasmids, i.e. the resolution of the interlocking catenane daughter molecules. Playing this role is another Type II topoisomerase, topoisomerase IV (Topo IV), which is a homolog of gyrase (1,2). Mutations in parC or parE result in defective decatenation of the circular chromosomes and plasmids and are generally lethal (3–5), although they may be partially suppressed by simultaneous overexpression of both gyrase subunits in Escherichia coli (6).
Most bacteria possess both gyrase and Topo IV. A few exceptions are Corynebacteria, Campylobacter jejuni, Deinococcus radiodurans, Treponema pallidum and some Mycobacteria (such as Mycobacterium leprae, Mycobacterium smegmatis and Mycobacterium tuberculosis) (7,8), which lack parC and parE. Presumably, decatenation of the circular chromosomes in these bacteria is carried out by gyrase. This notion was supported by the demonstration that the gyrase of M. smegmatis indeed possesses a strong decatenation activity as well as supercoiling activity in vitro (9).
An interesting question arose when linear chromosomes were discovered in some bacteria such as Borrelia burgdorferi and Streptomyces spp., i.e. do these linear chromosomes require a Type II topoisomerase for decatenation? In theory, replication of linear DNA does not result in catenated molecules, and therefore may not require a decatenase for resolution. However, parC and parE homologs are present in the chromosomal sequences of these bacteria. In Gram-positive bacteria, gyrA and gyrB usually form an operon near oriC, and parC and parE lie separately in opposite orientations distally from oriC. This is also true for Streptomyces. For example, in S. coelicolor, the gyrAB operon (SCO3873-SCO3874) is near oriC, whereas the parC and parE homologs (SCO5836 and SCO5822) lie in opposite orientations separated by 13 kb on the right arm of the chromosome (Figure 1). Phylogenetic analysis shows that the parC and parE homologs are grouped in the ParC and ParE branches with those of other bacteria, distinct from the GyrA and GyrB branches, respectively (Supplementary Figure S1). That SCO5822 and SCO5836 of S. coelicolor encode the Topo IV subunits was confirmed in vitro by Schmutz et al. (8) using purified and assembled heterotetrameric topoisomerases. They showed that (SCO5822)2(SCO5836)2, like other Topo IV (6), possessed both decatenation and relaxation activity, but not supercoiling activity.
Figure 1.
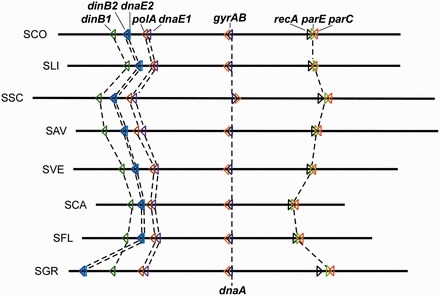
Synteny of parC and parE genes in Streptomyces genomes. Locations and direction of transcription (colored arrowheads) of parC, parE, gyrAB, dnaA, recA, and the five DNA polymerase genes are indicated on eight sequenced Streptomyces chromosomes (oriented according to the S. coelicolor chromosome). The chromosomes are centered and aligned at dnaA. Chromosome abbreviations: SCO, S. coelicolor; SLI, S. lividans; SSC, S. scabiei; SAV, S. avermitilis; SVE, S. venezuelae; SCA, S. cattleya; SFL, S. flavogriseus; SGR, S. griseus. The accession numbers and other details of the sequences used are in Supplementary Table S1.
Whereas gyrase is essential in Streptomyces as well as other bacteria, the role of Topo IV is not clear in Streptomyces. It is likely that Topo IV is required for post-replicational decatenation of circular plasmids in Streptomyces. In addition, spontaneous circularization of the chromosomes through fusion of the two arms occurs at relatively high frequencies (about 5 × 10−3 per sporulation cycle) in Streptomyces [reviewed in (10)]. One would expect that these circular chromosomes would require a decatenase for post-replicational segregation.
A more interesting question is whether the linear chromosomes and linear plasmids require Topo IV for decatenation in Streptomyces. These linear replicons are capped by terminal proteins (TPs) covalently bound at the 5′-ends of the DNA (11). It was shown recently that these TP-capped telomeres interact in vivo, resulting in the formation of a circular configuration with negative superhelicity, despite the linearity of these replicons (12). If the telomere–telomere interactions persist throughout and after the completion of replication, the requirement of decatenation would seem imperative.
Moreover, in eukaryotes, despite the linearity of their chromosomes, a type II topoisomerase, Topo II, appears to be required for untangling of the intertwined daughter chromosomes after replication. Mutations that inactivate the decatenation activity of Topo II in yeast result in interlocked chromosomes in S phase (13,14). Topo IV may also perform a similar role for the linear chromosomes in Streptomyces.
In this study, we addressed the question whether Topo IV was essential for the linear or circular DNA in Streptomyces. We investigated this issue by attempts to delete a Topo IV gene. Our results showed that deletion of parE could be achieved on a linear chromosome but not on a circular chromosome, indicating that Topo IV was essential for circular DNA but not for linear DNA in Streptomyces. This was confirmed by the ability of the parE deletion mutants to support replication of linear plasmids but not circular plasmids. In the parE deletion mutants, the linear replicons presumably bypass the requirement of Topo IV through dissociation and reestablishment of the telomere–telomere complex to achieve segregation. Nevertheless, Topo IV was likely important for efficient untangling of the linear daughter chromosomes, because the parE deletion mutants exhibited retarded growth and sporulation and temperature sensitivity. Moreover, it was also discovered that Topo IV was involved in resolution of DNA knots formed during site-specific integration of circular plasmids.
MATERIALS AND METHODS
Bacterial strains and plasmids
Strains and plasmids used in this study are listed in Table 1. Microbiological and genetic manipulations in E. coli and Streptomyces were according to Sambrook et al. (27) and Kieser et al. (26). Streptomyces strains were cultured on six solid media, LB (Difco), low-salt LB (LB Lennox, Difco), protoplast regeneration medium R5 (26), SFM (0.2% mannitol, 0.2% soya flour and 0.2% agar) (26), PYM (0.5% peptone, 0.3% yeast extract, 0.3% malt extract, 1% glucose and 2% agar) (28) and DNA (Difco Nutrient Agar), and two liquid media, TSB (0.3% tryptone soya broth powder) and YEME (0.3% yeast extract, 0.5% peptone, 0.3% malt extract, 1% glucose, 34% sucrose and 5 mM MgCl2) (26). pLUS355 was constructed from pLUS970 (21) by removing a 3.3-kb BclI–BsmI fragment containing the rlr locus required for replication in linear form and replacing a 0.6-kb BclI–SphI fragment downstream of tsr with multiple cloning site sequence.
Table 1.
Bacterial strains and plasmids used in this study
| Culture/plasmid | Genotype/description | Source/reference |
|---|---|---|
| S. coelicolor | ||
| M145 | Wild type, SCP1- SCP2- | (15) |
| M145/pLUS379 | pLUS379 integrated into the chromosome via single crossover | This study |
| M145ΔparE | M145 containing ΔparE::aac(3)IV mutation | This study |
| M145ΔparE/pLUS385 | M145ΔparE harboring pLUS385 | This study |
| 3456 | Pgl SCP1NF SCP2- | (16) |
| 3456/pLUS379 | pLUS379 integrated into the chromosome via single crossover | This study |
| 3456ΔparE | 3456 containing ΔparE::aac(3)IV mutation | Figure 2, this study |
| 3456ΔparE/pLUS385 | 3456ΔparE harboring pLUS385 | This study |
| E. coli | ||
| BW25113/pIJ790 | K12 derivative; araBAD rhaBAD/λ-RED (gam bet exo) cat araC rep101ts | (17) |
| ET12567/pUZ8002 | dam-13::Tn9 dcm cat tet hsdM hsdR zjj-201::Tn10/tra neo RP4 | (18) |
| Plasmids | ||
| pIJ773 | E. coli plasmid, aac(3)IV oriT | (17) |
| St5B8 | S. coelicolor cosmid containing spanning recA and parE | (19) |
| pLUS379 | St5B8 derivative in which parE is replaced by the Aprr cassette | This study |
| pHZ132 | E. coli–Streptomyces temperature-sensitive shuttle plasmid containing pSG5 ARS, cos, oriT, tsr and bla | (20) |
| pLUS355 | Derivative of pLUS970 (21), in which the rlr locus is removed and a multiple cloning site is inserted. | This study |
| pLUS356 | Derivative of pLUS970 (21), in which the HindIII–SfiI fragment is replaced by a multiple cloning site | Figure 5A, this study |
| pLUS383 | Derivative of pHZ132 (20) containing tsr and bla and a deletion of the 1.6-kb HPaI–HindIII fragment containing cos | This study |
| pLUS385 | pLUS383 derivative containing parE | Figure 2A, this study |
| pIJ702-117 | Derivative of pIJ702, tsr, melC (with a up-regulating promoter) | (22) |
| pLUS891 | Plasmid containing pSLA2 ARS (23) and tsr flanked by a pair of 320-bp telomere sequences of SCP1 | (24) |
| pIJ82 | pSET152 (25) derivative containing hyg replacing aac(3)IV | (26) |
| pIJ82–parE | pIJ82 containing parE | Figure 4A, this study |
Construction of parE mutants in Streptomyces
The gene replacement method based on Gust et al. (17) was used to generate the deletion mutants in this study. Basically, the parE-disrupting cassette was generated by polymerase chain reaction (PCR) (primers H4-5′ and H4-3′; Supplementary Table S2), which contained a unique priming site annealed to the apramycin-resistant (Aprr) cassette from pIJ773 and 36-bp sequences flanking each side of parE on the chromosome. This amplified fragment was introduced by transformation into E. coli BW25113/pIJ790 harboring a cosmid clone—St5B8 of S. coelicolor (19) containing parE, and Aprr transformants were selected, which harbored the cosmid with a ΔparE::aac(3)IV allele (designated pLUS379). pLUS379 was transferred by conjugation from E. coli ET12567/pUZ8002 (18) to S. coelicolor M145 and 3456 for gene replacement. Aprr exoconjugants were selected. These exoconjugants were kanamycin-resistant (Kamr), indicating that they contained integrated pLUS379. Further attempts to isolate kanamycin-sensitive (Kams) Aprr segregants among the exoconjugants to obtain ΔparE::aac(3)IV mutants failed. For complementation, a 2.6-kb sequence spanning parE and 200 bp upstream of it from S. coelicolor was inserted into pLUS383, a derivative of the temperature-sensitive plasmid pHZ132 (20), which conferred thiostrepton and viomycin resistance. The resulting plasmid, designated pLUS385, was introduced into S. coelicolor by transformation. From thiostrepton-resistant (Thior) exoconjugants, Kams segregants were obtained that contained the ΔparE deletion. Subsequently, loss of pLUS385 was achieved by screening at 40°C.
Complementation of parE mutants
To complement the parE mutation in 3456ΔparE and M145ΔparE, the parE coding sequence with upstream promoter region (761 bp) was generated by PCR (primers C4-XbaI-5’ and C4-EcoRV-3’; Supplementary Table S2). The amplified fragment was cloned into an integrative plasmid, pIJ82, giving rise to pIJ82–parE. The resulting plasmid was introduced into E. coli ET12567/pUZ8002 and further integrated into S. coelicolor 3456 and M145 ϕC31 attB site via E. coli–Streptomyces conjugal transfer. Hygromycin-resistant transconjugants were selected and verified by Southern blotting.
Microscopy
Aerial mycelium and spore chains were collected on sterile coverslips, inserted in minimal medium containing mannitol for 13 days, according to the methods of Kim et al. (8). The coverslips were stained with 5 µg/ml DAPI (4′,6′-diamino-2-phenylindole) in phosphate-buffered saline containing 50% glycerol, and then examined with a fluorescence microscope (Leica DMLB) with 360-nm excitation light and a 425-nm emission filter.
Phylogenetic analysis
Sixteen bacteria were selected to represent Streptomyces, actinobacteria and other bacteria (see Supplementary Table S1 for genomic source information). The orthologs of gyrase and Topo IV from each bacterium were extracted from the Kyoto Encyclopedia of Genes and Genomes database (29), and used for the construction of a phylogenetic tree using the Neighbor-Joining method in Molecular Evolutionary Genetics Analysis (MEGA) software version 5 (30).
RESULTS
The Topo IV component, parE, was deleted in two steps
We first attempted to delete parE (SCO5822) in wild-type S. coelicolor M145 using the REDIRECT procedure of Gust et al. (17). Cosmid pLUS379 contained a kanamycin resistance gene (aph) and a segment of S. coelicolor DNA, in which parE was replaced by an apramycin-resistance gene [aac(3)IV] cassette. Conjugal transfer of the cosmid from E. coli to M145 produced Aprr exoconjugants. The insertion of the cosmid in the parE region by homologous recombination in these exoconjugants was confirmed by PCR analysis (data not shown). Subsequent attempts to isolate kanamycin-sensitive (Kans) segregants from the M145/pLUS379 exoconjugants, which would have undergone a second crossover and deleted parE, failed among more than 600 colonies screened.
We have previously experienced similar difficulties in attempts to delete polA (DNA polymerase I) and recA from M145, but succeeded with relative ease in 3456 (a strain containing an integrated plasmid SCP1NF) (31,32). Thus, we attempted to delete parE in 3456 using the same procedure. Aprr 3456/pLUS379 exoconjugants were similarly isolated using pLUS379. However, attempts to isolate Kans segregants also failed among 450 exoconjugants screened.
To check the possibility that parE was essential for viability, we introduced a temperature-sensitive plasmid, pLUS385, which contained a viomycin-resistance gene (vph), a thiostrepton-resistance gene (tsr) and parE (Figure 2A), into M145/pLUS379 and 3456/pLUS379. From the Thior transformants, Kans segregants were readily isolated at frequencies of approximately 10−1 in M145/pLUS379 and 10−2 in 3456/pLUS379. That these Thior Kans segregants had suffered deletion of parE through double crossovers was confirmed by restriction and hybridization (Figure 2B and 2C). These ΔparE mutants still possessed pLUS385 (being Thior), and were designated M145ΔparE/pLUS385 and 3456ΔparE/pLUS385, respectively.
Figure 2.
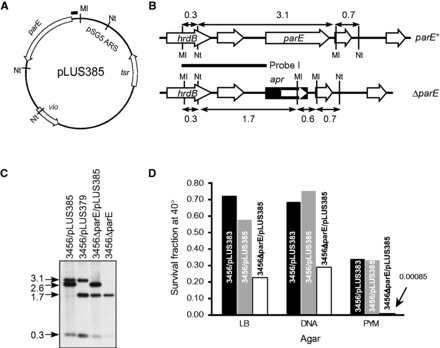
Creation and characterization of temperature-sensitive (ts) mutants of parE in M145 and 3456. (A) Temperature-sensitive plasmid pLUS385 containing parE (SCO5822) on the 2.6-kb MluI (Ml)-NotI (Nt) fragment for complementation. tsr, thiostrepton resistance gene. The bar on the top indicates the 268-bp sequence that may be hybridized by Probe I (see B). (B) Restriction maps of the hrdB-parE region on the chromosome of M145 and 3456 (parE+) and the ΔparE mutants. Probe I used in Southern blotting (below) is indicated by the horizontal bar. The MluI and NotI cutting sites are marked, and the sizes of the restriction fragments are indicated in kb. apr, apramycin resistance gene. (C) Genomic DNA was isolated from the constructed strains, digested with MluI and NotI, and subjected to Southern hybridization using Probe I. The sizes (kb) of the hybridizing fragments are indicated on the left. Representative data of the 3456 series are shown: 3456/pLUS385, 3456/pLUS379, 3456ΔparE/pLUS385, and 3456ΔparE. (D) Temperature sensitivity of the mutant strains. The relative plating efficiencies of 3456/pLUS383, 3456/pLUS385, and 3456ΔparE/pLUS385 at 40°C versus 30°C on LB, DNA and PYM agars are represented by the bars. The datum for 3456ΔparE/pLUS385 on PYM is too low to be visible in the chart, and is given as a number.
If parE was essential for viability of Streptomyces, it was expected that M145ΔparE/pLUS385 and 3456ΔparE/pLUS385 would exhibit temperature sensitivity, because replication of the vector (pHZ132) that carried parE was defective in replication at elevated temperature (20). Indeed, compared with the control cultures (M145/pLUS385 and 3456/pLUS385), the plating efficiencies of M145ΔparE/pLUS385 and 3456ΔparE/pLUS385 at 40°C were reduced by 60–70% on LB and DNA agar, and by more than two orders of magnitude on PYM agar (Figure 2D).
The colonies that survived the elevated temperature from plating of the M145ΔparE/pLUS385 and 3456ΔparE/pLUS385 spores were analyzed for the presence of pLUS385. If parE was essential, it was expected that these surviving cultures would have retained pLUS385. Instead, all these surviving cultures were plasmid-less and Thios (data not shown). Moreover, these cultures were all Aprr, and restriction and Southern hybridization confirmed deletion of the parE sequence (data not shown). Therefore, these results indicated that parE was not essential for either of these strains, which were designated M145ΔparE and 3456ΔparE, respectively. The initial failure to isolate these deletion mutants directly was most likely due to their retarded growth and sporulation (see following text).
ΔparE exhibited defective growth, and temperature sensitivity
Compared with the wild type, M145ΔparE and 3456ΔparE also grew more slowly at the normal temperature (30°C) on several solid media, particularly R5 and LB agars (Table 2). They grew also very slowly in YEME broth, but normally in TSB broth. We suspected that the poor growth might be correlated to higher osmolality of these media (Table 2 and Supplementary Table S3). This notion was supported by the comparison of LB (containing 10 g/l NaCl) and low-salt (Lennox) LB (containing 5 g/l NaCl), in which the lower salt appeared to benefit the growth of the mutants.
Table 2.
Growth defect of ΔparE mutants
| Medium | Solid medium |
Liquid medium |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| R5 | LB | Low-salt LB | SFM | PYM | DNA | YEME | TSB | ||
| Osmolality (mOsm/kg) | 815 | 415 | 243 | 147 | 122 | 65 | 1833 | 312 | |
| Growth at 30°C | parE+(a) | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| ΔparE(b) | + | + | ++ | +++* | ++ | +++ | + | +++ | |
| Growth at 42°C | parE+ | + | +++ | +++ | ND | +++ | +++ | ND | ND |
| ΔparE | + | − | + | ND | + | ++ | ND | ND | |
aM145, 3456, M145ΔparE/pIJ82–parE and 3456ΔparE/pIJ82–parE.
bM145ΔparE and 3456ΔparE.
+++, normal growth; ++, slightly reduced growth; +, poor growth; −, no growth; *, retarded sporulation; ND, not determined.
On the medium (SFM) used for conjugation during the construction of the mutants, the vegetative growth of M145ΔparE and 3456ΔparE was comparable with that of the wild-type strains. However, sporulation of the mutants was significantly retarded on this medium. Whereas white aerial hyphae formed in the mutant colonies at about the same time (about 4 days after plating of the spores) as in the wild type, gray spores appeared only sparsely even 10 days after plating (Figure 3A).
Figure 3.
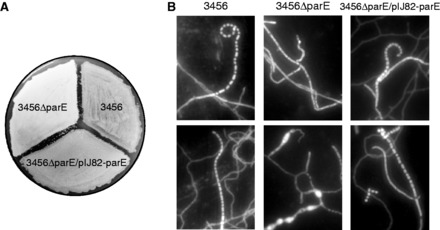
Growth characteristics of the parE mutant and the complementation strain. (A) 3456, 3456ΔparE, and 3456ΔparE/pIJ82–parE were grown on SFM agar for 4 days at 30°C. 3456ΔparE remained white with aerial hyphae, while the other two strains had produced gray spores. (B) 3456, 3456ΔparE, and 3456ΔparE/pIJ82–parE were grown over coverslips on MM containing mannitol for 13 days, and the spores were collected from the coverslips, stained with DAPI and imaged under a fluorescence microscope. Image contrast has been increased for better clarity. More sample photos are in Supplementary Figure S2.
The poor sporulation of the mutants was probably due to deficiency in decatenation of the chromosomes during spore formation, where proper partitioning is critical (33). When the 3456ΔparE colonies were examined by DAPI staining under the microscope, the aerial hyphae contained relatively few spores of normal shapes. In the rare spore chains, anucleate spaces (lacking spore-like structure) were often observed (Figure 3B upper panel). The frequency of these anucleate spaces was about 18%. In comparison, the frequencies of anucleate spores in M145 and M145ΔparE/pIJ82–parE were below 1%. In addition, the aerial hyphae of 3456ΔparE often contained very large bulges and extrusions that were packed with excess of DNA (lower panel). This was in contrast to the regularly distributed nucleoids in the aerial mycelial segments and the spore chains in 3456 and 3456ΔparE/pIJ82–parE.
ΔparE mutants were complemented by integrated parE
To complement the ΔparE mutation, the parE coding sequence with its upstream promoter region (761 bp; from the termination codon of the upstream gene to the initiation codon of parE) was inserted into an integrative plasmid, pIJ82 (26), and the resulting plasmid, pIJ82–parE (Figure 4A), was introduced into M145ΔparE and 3456ΔparE. Hygromycin-resistant transformants were isolated, and integration of the plasmid into the ϕC31 attB site was confirmed by restriction and hybridization (Figure 4B). These complementation strains, designated M145ΔparE/pIJ82–parE and 3456ΔparE/pIJ82–parE, grew as well as wild type on solid media and in liquid media tested (Table 2). The retarded sporulation on SFM and abnormal nucleoid distribution displayed by M145ΔparE and 3456ΔparE were also eliminated (Figure 3B). These results confirmed that the observed growth defects of M145ΔparE and 3456ΔparE were due to deletion of parE.
Figure 4.
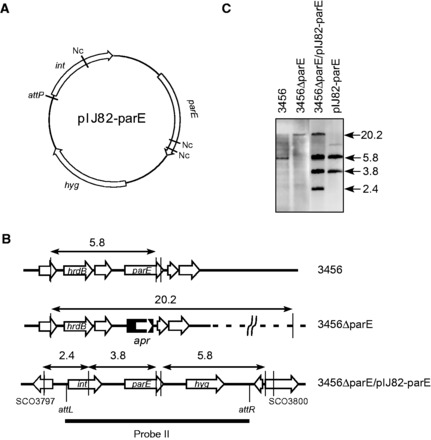
Complementation of the parE mutants of 3456 and M145. (A) The integrative plasmid pIJ82–parE containing the parE coding sequence and 761-bp upstream promoter region. hyg, hygromycin resistance gene. int, integrase gene of ϕC31 phage. attP, ϕC31 attachment site. Nc, NcoI site. (B) The restriction maps of the hrdB-parE region on the chromosome of 3456, 3456ΔparE, and 3456ΔparE/pIJ82–parE (harboring the integrated pIJ82–parE). Probe II used in Southern blotting (below) is indicated by the horizontal bar. The NcoI cutting sites are indicated by the vertical lines, and the sizes of the restriction fragments are indicated in kb. (C) Confirmation of integration of parE complementation. Genomic DNA was digested with NcoI, and subjected to Southern hybridization using the Probe II. The sizes (kb) of the hybridizing fragments are indicated on the right.
ΔparE mutants cannot maintain circular plasmids
The ability to tolerate the ΔparE mutation in M145 and 3456 indicated that these linear chromosomes in Streptomyces do not require Topo IV for resolution. This is intriguing in view of the fact that the linear chromosomes and plasmids form a circular configuration (herein termed ‘pseudo-circle’) in vivo through telomere–telomere association in Streptomyces (12). There are two possible bypass mechanisms for the post-replicational untangling of the daughter replicons of these pseudo-circles: (i) gyrase may substitute for Topo IV for the untangling function, and (ii) the interacting telomeres of these linear replicons may transiently dissociate from each other for the untangling. These hypotheses may be tested with a circular plasmid. The first bypass mechanism would allow a circular plasmid to propagate in the deletion mutants, whereas the second mechanism would not.
First, we tested pIJ702-117, which contained tsr and the melC operon (22). This plasmid transformed M145 and 3456 at ‘normal’ frequencies, but failed to produce any transformants in M145ΔparE and 3456ΔparE. Next, we tested two circular plasmids, pLUS356 and pLUS891, both of which included a linear plasmid sequence (Figure 5A) (24). These plasmids may appear and replicate as free linear molecules (with TP-capped telomeres) in the transformants under certain conditions. M145 and 3456 were transformed by these two plasmids at ‘normal’ frequencies, but M145ΔparE and 3456ΔparE were transformed at frequencies of about two orders of magnitude lower. The transformants were examined for the presence of plasmids. Interestingly, all of the 21 pLUS356 transformants (9 in 3456ΔparE and 12 in M145ΔparE) and 26 pLUS891 transformants of 3456ΔparE examined contained only the linear versions of these plasmids (i.e. ‘pLUS356L’ and ‘pLUS891L’; Figure 5B), and no circular plasmids. In contrast, the M145 and 3456 transformants contained only circular but no linear plasmids. For comparison, we tested pLUS355, a variant of pLUS970 that lacked the rlr locus required for replication in linear form and thus can replicate only in circular form in Streptomyces. No Thior transformants could be produced in M145ΔparE or 3456ΔparE.
Figure 5.
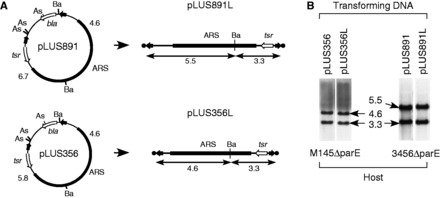
Inability of circular plasmids to replicate in the ΔparE mutants, M145ΔparE and 3456ΔparE. (A) Circular plasmids pLUS356 and pLUS891 (left) used for transformation and the linear versions, pLUS356L and pLUS891L, generated in the transformants (right). bla, beta-lactamase gene; tsr, thiostrepton resistance gene; ARS, autonomously replicating sequence of pSLA2; filled arrows, telomeres of the S. lividans chromosome (on pLUS356) or SCP1 plasmid (on pLUS891); filled circles, TPs. As, AseI site; Ba, BamHI site. The sizes (kb) of the BamHI fragments of the linear DNA are indicated. (B) M145ΔparE and 3456ΔparE were transformed by pLUS356 or pLUS891. Genomic DNA was isolated from the Thior transformants, digested with BamHI, and subjected to Southern blotting using the transforming DNA as the respective probes. Representative transformants are shown. The sizes (kb) of the hybridizing fragments are indicated.
Finally, another transformation was performed using a mixture of 3456 and 3456ΔparE protoplasts at comparable concentrations of colony forming units (Table 3). AseI-linearized pLUS356 DNA produced 221 transformants of 3456 and 120 transformants of 3456ΔparE, representing transformation frequencies of 9.6 × 10−5 and 2.3 × 10−5, respectively. The plasmids present in these transformants were all linear (pLUS356L; not shown). In contrast, pLUS355 DNA produced 594 Thior transformants of 3456, but none of 3456ΔparE.
Table 3.
The parE knockout mutant can maintain the linear plasmid (pLUS356L) but not circular plasmid (pLUS355)
| Treatment | 3456a | 3456ΔparEa | Totala |
|---|---|---|---|
| No. cfu regenerated | 2.3 × 106 | 5.2 × 106 | 7.5 × 106 |
| No. pLUS355 transformants | 594 | 0 | 594 |
| No. pLUS356/AseI transformants | 221 | 120b | 341 |
aTotal transformants were scored by thiostrepton resistance; 3456ΔparE was scored by the number of Aprr transformants; 3456 was scored by subtracting the total number of transformants by that of Aprr transformants.
bTwenty-two transformants were checked by Southern hybridization, and all showed the existence of the linear plasmids.
These results showed that Topo IV was required for the maintenance of circular plasmids, but not linear plasmids, in Streptomyces, and that gyrase could not functionally substitute for Topo IV in the decatenation of the circular plasmids.
ΔparE mutant cannot maintain a circular chromosome
Based on these findings, it was likely that Topo IV was also required for maintenance of circular chromosomes in Streptomyces. This could be tested by investigating spontaneous chromosome circularization in the ΔparE mutants. The linear chromosomes of Streptomyces are highly unstable, undergoing spontaneous deletions and fusions of terminal sequences at relatively high frequencies (11). These terminal deletions are often very long, up to 1 Mb (34), and are readily detected by the loss of chloramphenicol resistance genes located in the terminal region—e.g. cmlR1 (SCO7526) and cmlR2 (SCO7662) located 322 and 179 kb, respectively, from the right end of the S. coelicolor chromosome. Such chloramphenicol-sensitive (Cmls) mutants arise spontaneously at frequencies in the range of 10−3 to 10−2 after a sporulation cycle.
We reasoned that the lack of Topo IV in the ΔparE mutants should eliminate the appearance of circularized chromosomes in them, and thus reduce the appearance of Cmls mutants. This was confirmed by comparing the spontaneous Cmls mutation rates of 3456 and 3456ΔparE. In one sporulation cycle, 3456 produced Cmls mutants at a frequency of 0.28% (9/3200), and 3456ΔparE produced no Cmls mutants among 3200 colonies examined (<0.03%). Complementation of the parE gene in 3456ΔparE restored the spontaneous Cmls mutation rate to 0.36% (8/2100). The results supported the notion that Topo IV was essential for the viability of circular chromosomes (Fisher’s exact test, P-value = 0.00388).
Topo IV is involved in plasmid integration
During the introduction of pIJ82–parE into M145ΔparE and 3456ΔparE for complementation, parallel experiments were performed using pIJ82 as controls. Whereas pIJ82–parE transformed 3456ΔparE mutants at about the same efficiency as it did 3456, unexpectedly, pIJ82 transformed 3456ΔparE at frequencies about two orders of magnitude lower than it did 3456. The pIJ82 transformants of 3456ΔparE appeared very late. Whereas the pIJ82 transformants of 3456 (or the pIJ82–parE transformants of either strains) appeared within 4 days, the appearance of the pIJ82 transformants of 3456ΔparE was delayed by several days, and the sizes of the colonies varied widely (Figure 6A). Nevertheless, these transformants contained the integrated pIJ82, as shown by restriction and Southern hybridization (Figure 6B and C), and, on subsequent subculturing, they grew at about the same rate as 3456ΔparE, and did not exhibit heterogeneous colony size. Thus, it appeared that the obstacle lies within the initial transformed cells, which, once resolved, did not pose any hindrance in the progeny cells. We interpreted the obstacle to be DNA knots produced during site-specific recombination, which could not be resolved by Topo IV in 3456ΔparE, and caused lethality.
Figure 6.
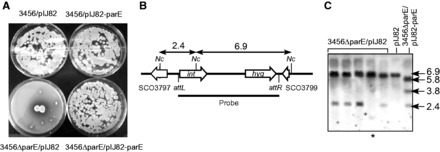
Faulty site-specific plasmid integration in the ΔparE mutants. (A) Transformation of 3456ΔparE with pIJ82 is defective. 3456 and 3456ΔparE were transformed with pIJ82 and pIJ82–parE, and scored for Hygr transformants after 10 days of incubation. (B) Restriction maps of the integration site. The phage attachment sites (attL, attR), the NcoI restriction sites, and the sizes (kb) of the expected restriction fragments are indicated. (C) Integration of pIJ82 DNA in 3456ΔparE. Genomic DNA was isolated from several Hygr transformants of 3456ΔparE, digested with NcoI, and subjected to electrophoresis and Southern hybridization using pIJ82 DNA as the probe. Representative samples are shown. The sizes (kb) of the hybridizing fragments are indicated. In one transformant (marked with the asterisk), the pattern of the hybridizing fragments was atypical, suggesting that the plasmid might have integrated into a secondary site.
DISCUSSION
Topo IV is essential for segregation of circular daughter chromosomes and plasmids in bacteria. Mutations in the coding genes have been found to cause either lethality or thermosensitivity in bacteria with circular chromosomes (4,35). Initially, we also failed to delete parE directly in M145 and 3456, and the preliminary results were reported previously (36). However, in this study, we showed that in Streptomyces cultures with a linear chromosome, the chromosomal parE could be deleted in the presence of a complementing copy on a plasmid, and the removal of the plasmid produced ΔparE mutants that exhibited growth and sporulation deficiencies. The deficiencies explain the earlier difficulties in isolating the mutants directly by screening for segregants through double crossovers.
The successful isolation of the ΔparE mutants demonstrated that Topo IV is not essential for wild-type Streptomyces with a linear chromosome. This is interesting because the linear chromosomes and linear plasmids have been demonstrated to exit in circular configuration through telomere–telomere interactions (12). If the telomere–telomere interactions persist through replication, ‘pseudo-catenanes’ would be produced, which would have to be resolved for proper segregation. Thus, in the ΔparE mutants, it is likely that dissociation of the telomere–telomere complex occurs during or after replication to allow the decatenation of the pseudo-catenane.
Such dissociation of the telomere–telomere complex has been proposed as a possible mechanism for solving another post-replicational segregation problem for linear replicons posed by intramolecular telomere–telomere associations. It was pointed out that if the ‘old’ TPs on the parental strains of the linear plasmid or chromosome remain associated with each other through replication, and the new TPs capping the daughter DNA become associated with each other, a pseudo-dimeric (Möbius strip-like) structure would result (12,36). These pseudo-dimers may be resolved by exchanging the interacting TPs, i.e. the ‘old’ TPs switching to associate with the ‘new’ ones at the opposing telomere. It is likely that this partner changing mechanism is also used to resolve the pseudo-catenanes at least in the ΔparE mutants.
In some actinobacteria (such as Corynebacteria and M. smegmatis), which lack parC and parE in their genomes, the role of decatenation of their circular replicons must fall on gyrase. However, in Streptomyces, gyrase is unlikely to perform decatenation, because circular plasmids and circular chromosomes cannot be maintained in the ΔparE mutants.
Why did the ΔparE mutants exhibit poor growth and sporulation? There are two plausible non-mutually exclusive possible reasons. First, it may be due to low efficiency of decatenation of the pseudo-catenanes by dissociation of the telomere–telomere complexes. The dissociated telomeres must reconnect in correct topology to achieve separation. Second, it could be that Topo IV is also important in untangling daughter linear chromosomes, as shown in yeasts (37–40). The Streptomyces chromosomes are typically 6–10 Mb, significantly larger than the chromosomes of Saccharomyces cerevisiae (2.2–0.2 Mb) and Schizosaccharomyces pombe (5.7–3.5 Mb). The anomaly of nucleoid size and distribution in the hyphae of the parE mutants (Figure 3B) supports these notions.
The poor growth of the ΔparE mutants was more severe on rich media and at high temperature. Presumably, the faster chromosomal replication in the cultures under these conditions demand timely post-replicational resolution and partitioning of the chromosomes before the next round of replication is completed. Alternatively, the higher osmotic pressure in the richer media (Supplementary Data and Supplementary Table S3) may be an important factor. Such elevated sensitivities to rich media, osmotic pressure and/or thermosensitivity have been often observed in topoisomerase mutants of bacteria (41–43).
It is intriguing that some actinobacteria with circular chromosomes lack Topo IV, whereas Streptomyces spp. with linear chromosomes possess Topo IV, which is not absolutely required. The linear chromosomes of B. burgdorferi also possess Topo IV. In this case, the telomeres are hairpinned instead, and there is no evidence for telomere–telomere interactions in this bacterium.
Why do Streptomyces spp. possess a decatenase that is not absolutely required? There are at least three possible advantages: (i) it decatenates better than the proposed transient dissociation of the telomere–telomere complex, (ii) it allows harboring of circular plasmids and (iii) it allows the survival of mutants in which the chromosomes have circularized. The last point is intimately related to the extremely high occurrence of chromosome circularization in Streptomyces, a puzzle that is poorly understood in terms of molecular mechanism and evolutional significances. As we have shown here, without Topo IV, no circular chromosomes can survive.
The ΔparE mutants also exhibited low transformation efficiencies of pIJ82, which integrates into the Streptomyces chromosomes at a specific attB site. Site-specific recombination involving a circular DNA molecule may produce knots, which are harmful for cells if not efficiently removed. Zechiedrich et al. (44) have shown that the products of site-specific recombination by λ Int or Tn3 resolvase were decatenated by Topo IV, not gyrase. López et al. (45) also showed Topo IV to be the topoisomerase that unknots DNA during replication. Presumably, Topo IV is also important for unknotting in Streptomyces.
The pIJ82 transformants of 3456ΔparE appeared in very few numbers very slowly and sporadically. In these transformants, the knots had presumably been removed by fortuitous events at various times before cell death, which allowed the hyphae to resume growth. One possibility was the occurrence of a double-strand break in the knot region followed by repair, which removed the knots by chance. In any case, on replating, these transformants grew like 3456ΔparE without wide variations in colony size. This supports the notion that the Topo IV was important only during the site-specific recombination stage when knots were formed.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Science Council [101-2311-B-010-009, 101-2321-B-010-019]; and a National Professorship from the Ministry of Education, R. O. C., (to C. W. C.). Funding for open access charge: National Science Council, R. O. C. [101-2311-B-010-009, 101-2321-B-010-019].
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Professor David Hopwood for critical reading of the manuscript and suggestions for improvement, Dr. Hsiu-Hui Tsai for osmolality determination and Professor Zixin Deng for plasmid pHZ132.
REFERENCES
- 1.Adams DE, Shekhtman EM, Zechiedrich EL, Schmid MB, Cozzarelli NR. The role of topoisomerase IV in partitioning bacterial replicons and the structure of catenated intermediates in DNA replication. Cell. 1992;71:277–288. doi: 10.1016/0092-8674(92)90356-h. [DOI] [PubMed] [Google Scholar]
- 2.Zechiedrich EL, Cozzarelli NR. Roles of topoisomerase IV and DNA gyrase in DNA unlinking during replication in Escherichia coli. Genes Dev. 1995;9:2859–2869. doi: 10.1101/gad.9.22.2859. [DOI] [PubMed] [Google Scholar]
- 3.Hirota Y, Ryter A, Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb. Symp. Quant. Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- 4.Kato J, Nishimura Y, Yamada M, Suzuki H, Hirota Y. Gene organization in the region containing a new gene involved in chromosome partition in Escherichia coli. J. Bacteriol. 1988;170:3967–3977. doi: 10.1128/jb.170.9.3967-3977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreuzer KN, Cozzarelli NR. Escherichia coli mutants thermosensitive for deoxyribonucleic acid gyrase subunit A: effects on deoxyribonucleic acid replication, transcription, and bacteriophage growth. J. Bacteriol. 1979;140:424–435. doi: 10.1128/jb.140.2.424-435.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato J, Suzuki H, Ikeda H. Purification and characterization of DNA topoisomerase IV in Escherichia coli. J. Biol. Chem. 1992;267:25676–25684. [PubMed] [Google Scholar]
- 7.Champoux JJ. DNA topoisomerases: structure, function, and mechanism. Annu. Rev. Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- 8.Schmutz E, Hennig S, Li SM, Heide L. Identification of a topoisomerase IV in actinobacteria: purification and characterization of ParYR and GyrBR from the coumermycin A1 producer Streptomyces rishiriensis DSM 40489. Microbiology. 2004;150:641–647. doi: 10.1099/mic.0.26867-0. [DOI] [PubMed] [Google Scholar]
- 9.Manjunatha UH, Dalal M, Chatterji M, Radha DR, Visweswariah SS, Nagaraja V. Functional characterisation of mycobacterial DNA gyrase: an efficient decatenase. Nucleic Acids Res. 2002;30:2144–2153. doi: 10.1093/nar/30.10.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CW, Huang CH, Lee HH, Tsai HH, Kirby R. Once the circle has been broken: dynamics and evolution of Streptomyces chromosomes. Trends Genet. 2002;18:522–529. doi: 10.1016/s0168-9525(02)02752-x. [DOI] [PubMed] [Google Scholar]
- 11.Lin YS, Kieser HM, Hopwood DA, Chen CW. The chromosomal DNA of Streptomyces lividans 66 is linear. Mol. Microbiol. 1993;10:923–933. doi: 10.1111/j.1365-2958.1993.tb00964.x. [DOI] [PubMed] [Google Scholar]
- 12.Tsai HH, Huang CH, Tessmer I, Erie DA, Chen CW. Linear Streptomyces plasmids form superhelical circles through interactions between their terminal proteins. Nucleic Acids Res. 2011;39:2165–2174. doi: 10.1093/nar/gkq1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiNardo S, Voelkel K, Sternglanz R. DNA topoisomerase II mutant of Saccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication. Proc. Natl Acad. Sci. USA. 1984;81:2616–2620. doi: 10.1073/pnas.81.9.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uemura T, Ohkura H, Adachi Y, Morino K, Shiozaki K, Yanagida M. DNA topoisomerase II is required for condensation and separation of mitotic chromosomes in S. pombe. Cell. 1987;50:917–925. doi: 10.1016/0092-8674(87)90518-6. [DOI] [PubMed] [Google Scholar]
- 15.Hopwood DA, Kieser T, Wright HM, Bibb MJ. Plasmids, recombination, and chromosomal mapping in Streptomyces lividans 66. J. Gen. Microbiol. 1983;129:2257–2269. doi: 10.1099/00221287-129-7-2257. [DOI] [PubMed] [Google Scholar]
- 16.Weaver D, Karoonuthaisiri N, Tsai HH, Huang CH, Ho ML, Gai S, Patel KG, Huang J, Cohen SN, Hopwood DA, et al. Genome plasticity in Streptomyces: Identification of 1 Mb TIRs in the S. coelicolor A3(2) chromosome. Mol. Microbiol. 2004;51:1530–1550. doi: 10.1111/j.1365-2958.2003.03920.x. [DOI] [PubMed] [Google Scholar]
- 17.Gust B, Challis GL, Fowler K, Kieser T, Chater KF. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl Acad. Sci. USA. 2003;100:1541–1546. doi: 10.1073/pnas.0337542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paget MS, Chamberlin L, Atrih A, Foster SJ, Buttner MJ. Evidence that the extracytoplasmic function sigma factor sigmaE is required for normal cell wall structure in Streptomyces coelicolor A3(2) J. Bacteriol. 1999;181:204–211. doi: 10.1128/jb.181.1.204-211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redenbach M, Kieser HM, Denapaite D, Eichner A, Cullum J, Kinashi H, Hopwood DA. A set of ordered cosmids and a detailed genetic and physical map for the 8 Mb Streptomyces coelicolor A3(2) chromosome. Mol. Microbiol. 1996;21:77–96. doi: 10.1046/j.1365-2958.1996.6191336.x. [DOI] [PubMed] [Google Scholar]
- 20.Hu Z, Bao K, Zhou X, Zhou Q, Hopwood DA, Kieser T, Deng Z. Repeated polyketide synthase modules involved in the biosynthesis of a heptaene macrolide by Streptomyces sp. FR-008. Mol. Microbiol. 1994;14:163–172. doi: 10.1111/j.1365-2958.1994.tb01276.x. [DOI] [PubMed] [Google Scholar]
- 21.Huang CH, Chen CY, Tsai HH, Chen C, Lin YS, Chen CW. Linear plasmid SLP2 of Streptomyces lividans is a composite replicon. Mol. Microbiol. 2003;47:1563–1576. doi: 10.1046/j.1365-2958.2003.03403.x. [DOI] [PubMed] [Google Scholar]
- 22.Leu WM, Wu SY, Lin JJ, Lo SJ, Lee YH. Analysis of the promoter region of the melanin locus from Streptomyces antibioticus. Gene. 1989;84:267–277. doi: 10.1016/0378-1119(89)90500-3. [DOI] [PubMed] [Google Scholar]
- 23.Qin Z, Cohen SN. Replication at the telomeres of the Streptomyces linear plasmid pSLA2. Mol. Microbiol. 1998;28:893–904. doi: 10.1046/j.1365-2958.1998.00838.x. [DOI] [PubMed] [Google Scholar]
- 24.Huang CH, Tsai HH, Tsay YG, Chien YN, Wang SL, Cheng MY, Ke CH, Chen CW. The telomere system of the Streptomyces linear plasmid SCP1 represents a novel class. Mol. Microbiol. 2007;63:1710–1718. doi: 10.1111/j.1365-2958.2007.05616.x. [DOI] [PubMed] [Google Scholar]
- 25.Bierman M, Logan R, O'Brien K, Seno ET, Rao RN, Schoner BE. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene. 1992;116:43–49. doi: 10.1016/0378-1119(92)90627-2. [DOI] [PubMed] [Google Scholar]
- 26.Kieser T, Bibb M, Buttner MJ, Chater KF, Hopwood DA. Practical Streptomyces Genetics. Norwich: The John Innes Foundation; 2000. [Google Scholar]
- 27.Sambrook J, MacCallum P, Russel D. Molecular Cloning. 3rd edn. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 28.Lee LF, Chen YJ, Kirby R, Chen C, Chen CW. A multidrug efflux system is involved in colony growth in Streptomyces lividans. Microbiology. 2007;153:924–934. doi: 10.1099/mic.0.2006/000018-0. [DOI] [PubMed] [Google Scholar]
- 29.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40:D109–D114. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang TW, Chen CW. A recA null mutation may be generated in Streptomyces coelicolor. J. Bacteriol. 2006;188:6771–6779. doi: 10.1128/JB.00951-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang TW, Chen CW. DNA polymerase I is not required for replication of linear chromosomes in Streptomyces. J. Bacteriol. 2008;190:755–758. doi: 10.1128/JB.01335-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu CC, Chen CW. Linear plasmid SLP2 is maintained by partitioning, intrahyphal spread, and conjugal transfer in Streptomyces. J. Bacteriol. 2010;192:307–315. doi: 10.1128/JB.01192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redenbach M, Flett F, Piendl W, Glocker I, Rauland U, Wafzig O, Leblond P, Cullum J. The Streptomyces lividans 66 chromosome contains a 1 Mb deletogenic region flanked by two amplifiable regions. Mol. Gen. Genet. 1993;241:255–262. doi: 10.1007/BF00284676. [DOI] [PubMed] [Google Scholar]
- 35.Kato JI, Nishimura Y, Imamura R, Niki H, Hiraga S, Suzuki H. New topoisomerase essential for chromosome segregation in E. coli. Cell. 1990;63:393–404. doi: 10.1016/0092-8674(90)90172-b. [DOI] [PubMed] [Google Scholar]
- 36.Chen CW. In: Microbial Linear Plasmids. Meinhardt F, Klassen R, editors. Berlin, Heidelberg: Springer-Verlag; 2007. pp. 33–61. [Google Scholar]
- 37.Holm C, Goto T, Wang JC, Botstein D. DNA topoisomerase II is required at the time of mitosis in yeast. Cell. 1985;41:553–563. doi: 10.1016/s0092-8674(85)80028-3. [DOI] [PubMed] [Google Scholar]
- 38.Holm C, Stearns T, Botstein D. DNA topoisomerase II must act at mitosis to prevent nondisjunction and chromosome breakage. Mol. Cel. Biol. 1989;9:159–168. doi: 10.1128/mcb.9.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Funabiki H, Hagan I, Uzawa S, Yanagida M. Cell cycle-dependent specific positioning and clustering of centromeres and telomeres in fission yeast. J. Cell Biol. 1993;121:961–976. doi: 10.1083/jcb.121.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spell RM, Holm C. Nature and distribution of chromosomal intertwinings in Saccharomyces cerevisiae. Mol. Cell. Biol. 1994;14:1465–1476. doi: 10.1128/mcb.14.2.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graeme-Cook KA, May G, Bremer E, Higgins CF. Osmotic regulation of porin expression: a role for DNA supercoiling. Mol. Microbiol. 1989;3:1287–1294. doi: 10.1111/j.1365-2958.1989.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 42.Higgins CF, Dorman CJ, Stirling DA, Waddell L, Booth IR, May G, Bremer E. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell. 1988;52:569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- 43.Bhriain NN, Dorman CJ. Isolation and characterization of a topA mutant of Shigella flexneri. Mol. Microbiol. 1993;7:351–358. doi: 10.1111/j.1365-2958.1993.tb01127.x. [DOI] [PubMed] [Google Scholar]
- 44.Zechiedrich EL, Khodursky AB, Cozzarelli NR. Topoisomerase IV, not gyrase, decatenates products of site-specific recombination in Escherichia coli. Genes Dev. 1997;11:2580–2592. doi: 10.1101/gad.11.19.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.López V, Martínez-Robles ML, Hernández P, Krimer DB, Schvartzman JB. Topo IV is the topoisomerase that knots and unknots sister duplexes during DNA replication. Nucleic Acids Res. 2012;40:3563–3573. doi: 10.1093/nar/gkr1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


