Abstract
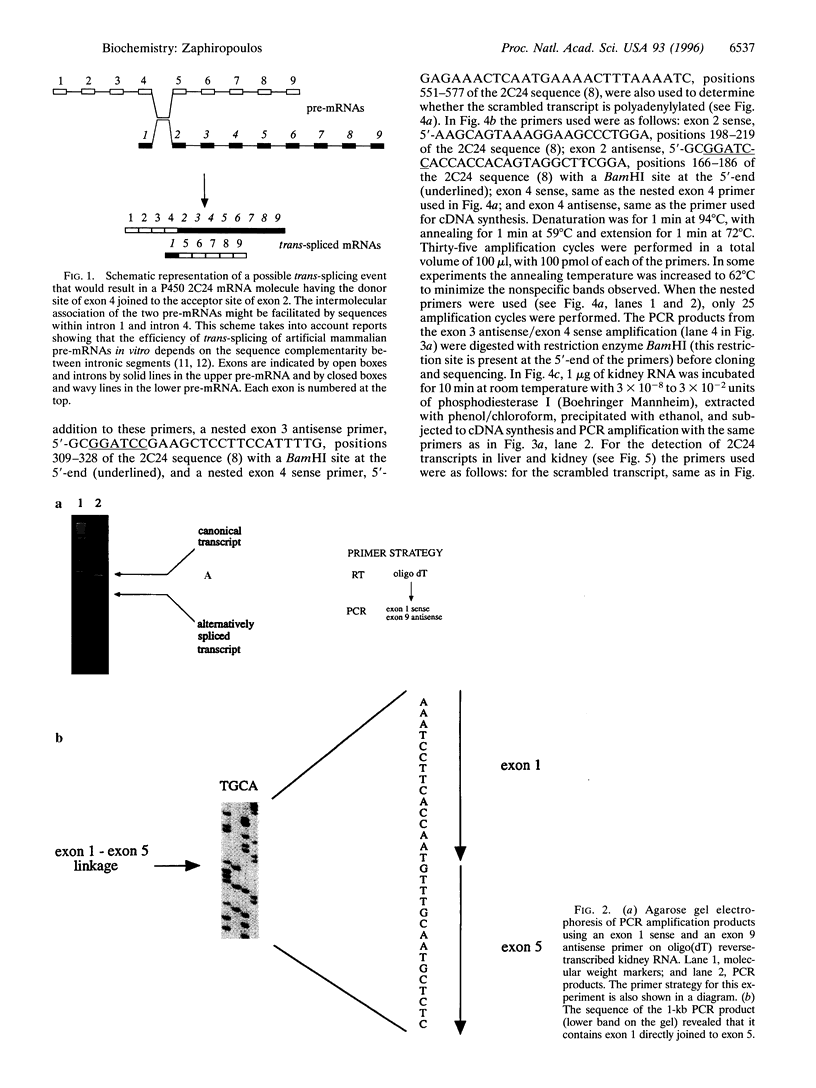
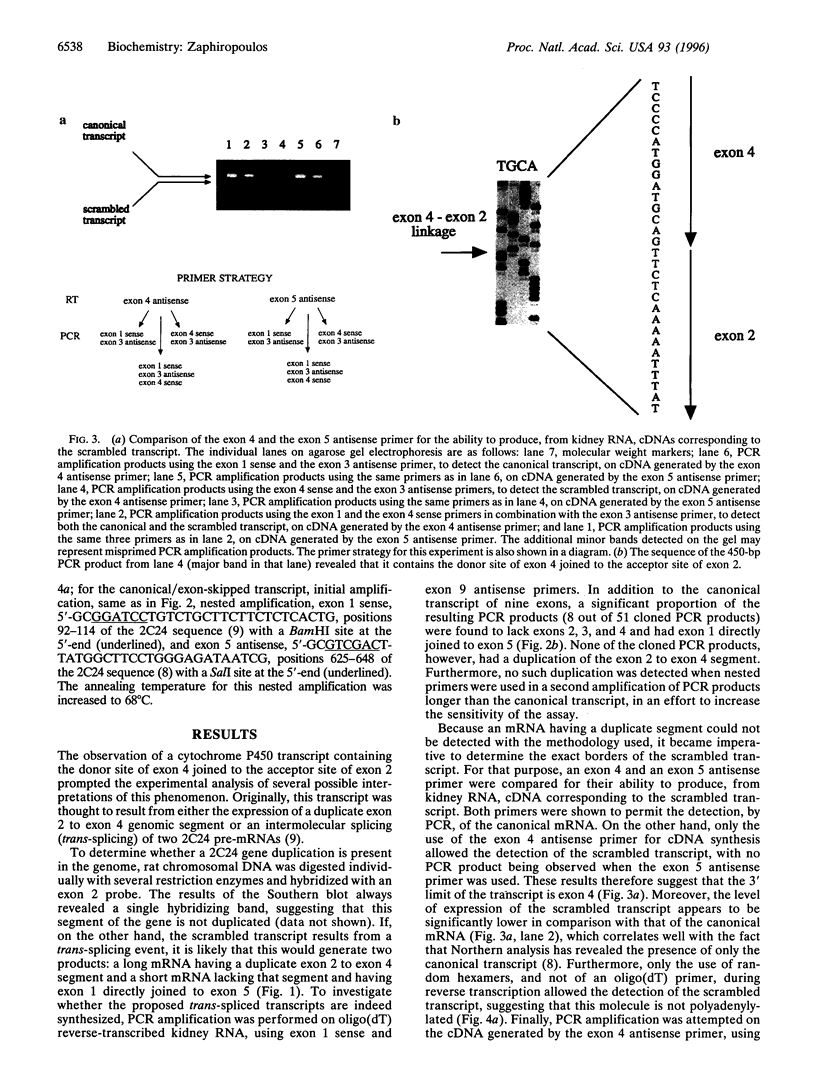
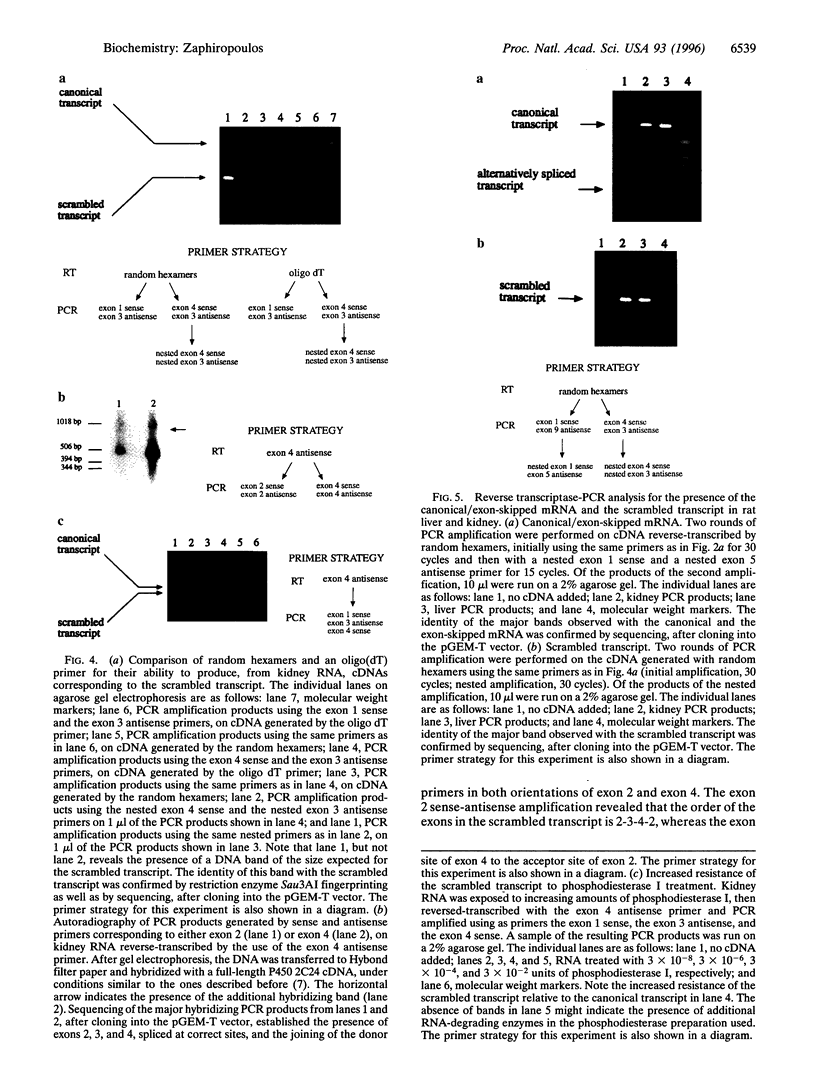
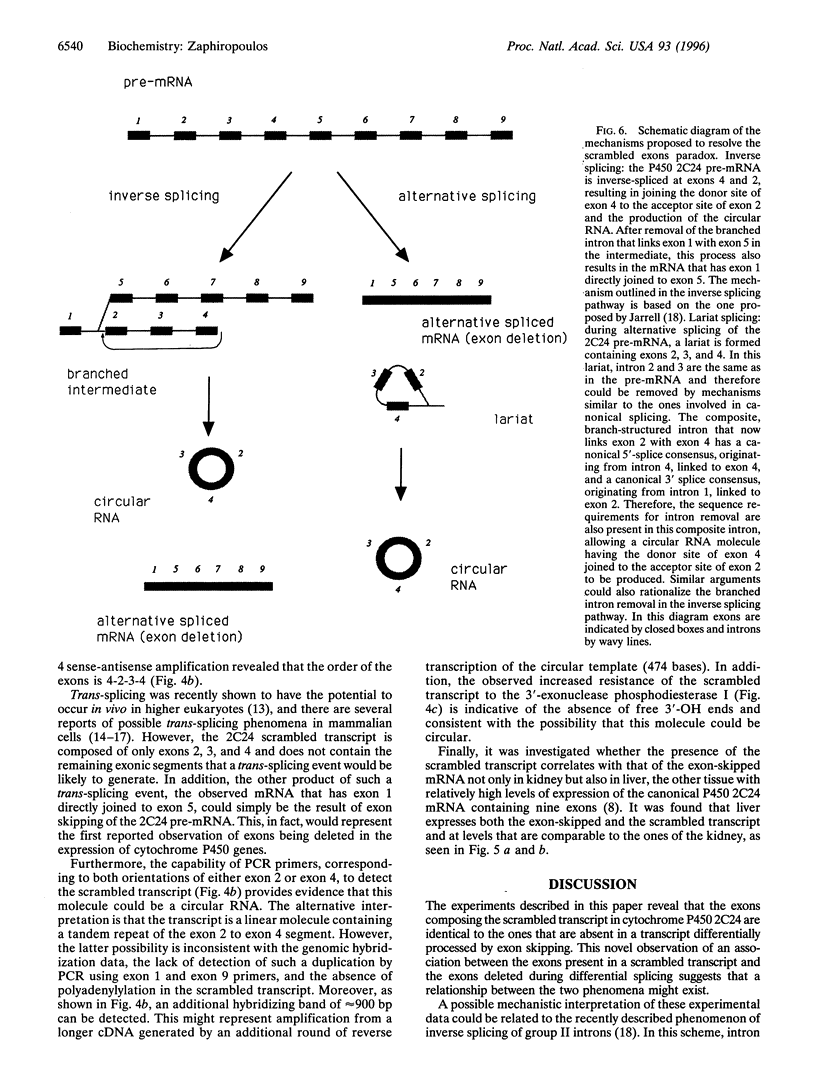
The cytochrome P450 2C24 gene is characterized by the capability to generate, in rat kidney, a transcript containing exons 2 and 4 spliced at correct sites but having the donor site of exon 4 directly joined to the acceptor site of exon 2 (exon scrambling). By reverse transcriptase-PCR analysis, it is now shown that the only exons present in the scrambled transcript are exons 2, 3, and 4 and that this molecule lacks a poly(A)+ tail. Furthermore, the use of PCR primers in both orientations of either exon 2 or exon 4 revealed that the orders of the exons in the scrambled transcript are 2-3-4-2 and 4-2-3-4, respectively. These results, combined with the observation that P450 2C24 is a single-copy gene, with no duplication of the exon 2 to exon 4 segment, suggest that the scrambled transcript has properties consistent with that of a circular molecule. In line with this is the observation of an increased resistance of the transcript to phosphodiesterase I, a 3'-exonuclease. Moreover, an alternatively processed cytochrome P450 2C24 mRNA, lacking the three scrambled exons and having exon 1 directly joined to exon 5, has been identified in kidney and liver, tissues that express the scrambled transcript. This complete identity of the exons that are absent in the alternatively processed mRNA but present in the scrambled transcript is interpreted as indicative of the possibility that exon scrambling and exon skipping might be interrelated phenomena. It is therefore proposed that alternative pre-mRNA processing has the potential to generate not only mRNAs lacking one or more exons but also circular RNA molecules.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breitbart R. E., Andreadis A., Nadal-Ginard B. Alternative splicing: a ubiquitous mechanism for the generation of multiple protein isoforms from single genes. Annu Rev Biochem. 1987;56:467–495. doi: 10.1146/annurev.bi.56.070187.002343. [DOI] [PubMed] [Google Scholar]
- Bruzik J. P., Maniatis T. Spliced leader RNAs from lower eukaryotes are trans-spliced in mammalian cells. Nature. 1992 Dec 17;360(6405):692–695. doi: 10.1038/360692a0. [DOI] [PubMed] [Google Scholar]
- Capel B., Swain A., Nicolis S., Hacker A., Walter M., Koopman P., Goodfellow P., Lovell-Badge R. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell. 1993 Jun 4;73(5):1019–1030. doi: 10.1016/0092-8674(93)90279-y. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cocquerelle C., Daubersies P., Majérus M. A., Kerckaert J. P., Bailleul B. Splicing with inverted order of exons occurs proximal to large introns. EMBO J. 1992 Mar;11(3):1095–1098. doi: 10.1002/j.1460-2075.1992.tb05148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocquerelle C., Mascrez B., Hétuin D., Bailleul B. Mis-splicing yields circular RNA molecules. FASEB J. 1993 Jan;7(1):155–160. doi: 10.1096/fasebj.7.1.7678559. [DOI] [PubMed] [Google Scholar]
- Coon M. J., Ding X. X., Pernecky S. J., Vaz A. D. Cytochrome P450: progress and predictions. FASEB J. 1992 Jan 6;6(2):669–673. doi: 10.1096/fasebj.6.2.1537454. [DOI] [PubMed] [Google Scholar]
- Eguchi H., Westin S., Ström A., Gustafsson J. A., Zaphiropoulos P. G. Gene structure and expression of the rat cytochrome P450IIC13, a polymorphic, male-specific cytochrome in the P450IIC subfamily. Biochemistry. 1991 Nov 12;30(45):10844–10849. doi: 10.1021/bi00109a006. [DOI] [PubMed] [Google Scholar]
- Green M. R. Pre-mRNA splicing. Annu Rev Genet. 1986;20:671–708. doi: 10.1146/annurev.ge.20.120186.003323. [DOI] [PubMed] [Google Scholar]
- Jarrell K. A. Inverse splicing of a group II intron. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8624–8627. doi: 10.1073/pnas.90.18.8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M. M., Padgett R. A., Sharp P. A. Trans splicing of mRNA precursors in vitro. Cell. 1985 Aug;42(1):165–171. doi: 10.1016/s0092-8674(85)80112-4. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Kamataki T., Waxman D. J., Guengerich F. P., Estabrook R. W., Feyereisen R., Gonzalez F. J., Coon M. J., Gunsalus I. C., Gotoh O. The P450 superfamily: update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cell Biol. 1993 Jan-Feb;12(1):1–51. doi: 10.1089/dna.1993.12.1. [DOI] [PubMed] [Google Scholar]
- Nigro J. M., Cho K. R., Fearon E. R., Kern S. E., Ruppert J. M., Oliner J. D., Kinzler K. W., Vogelstein B. Scrambled exons. Cell. 1991 Feb 8;64(3):607–613. doi: 10.1016/0092-8674(91)90244-s. [DOI] [PubMed] [Google Scholar]
- Romkes M., Faletto M. B., Blaisdell J. A., Raucy J. L., Goldstein J. A. Cloning and expression of complementary DNAs for multiple members of the human cytochrome PH50IIC subfamily. Biochemistry. 1993 Feb 9;32(5):1390–1390. doi: 10.1021/bi00056a025. [DOI] [PubMed] [Google Scholar]
- Schindewolf C. A., Domdey H. Splicing of a circular yeast pre-mRNA in vitro. Nucleic Acids Res. 1995 Apr 11;23(7):1133–1139. doi: 10.1093/nar/23.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu A., Honjo T. Synthesis and regulation of trans-mRNA encoding the immunoglobulin epsilon heavy chain. FASEB J. 1993 Jan;7(1):149–154. doi: 10.1096/fasebj.7.1.7916698. [DOI] [PubMed] [Google Scholar]
- Solnick D. Alternative splicing caused by RNA secondary structure. Cell. 1985 Dec;43(3 Pt 2):667–676. doi: 10.1016/0092-8674(85)90239-9. [DOI] [PubMed] [Google Scholar]
- Solnick D. Trans splicing of mRNA precursors. Cell. 1985 Aug;42(1):157–164. doi: 10.1016/s0092-8674(85)80111-2. [DOI] [PubMed] [Google Scholar]
- Sullivan P. M., Petrusz P., Szpirer C., Joseph D. R. Alternative processing of androgen-binding protein RNA transcripts in fetal rat liver. Identification of a transcript formed by trans splicing. J Biol Chem. 1991 Jan 5;266(1):143–154. [PubMed] [Google Scholar]
- Vellard M., Sureau A., Soret J., Martinerie C., Perbal B. A potential splicing factor is encoded by the opposite strand of the trans-spliced c-myb exon. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2511–2515. doi: 10.1073/pnas.89.7.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaphiropoulos P. G. Differential expression of cytochrome P450 2C24 transcripts in rat kidney and prostate: evidence indicative of alternative and possibly trans splicing events. Biochem Biophys Res Commun. 1993 Apr 30;192(2):778–786. doi: 10.1006/bbrc.1993.1482. [DOI] [PubMed] [Google Scholar]
- Zaphiropoulos P. G. cDNA cloning and regulation of a novel rat cytochrome P450 of the 2C gene subfamily (P450IIC24). Biochem Biophys Res Commun. 1991 Oct 31;180(2):645–651. doi: 10.1016/s0006-291x(05)81114-3. [DOI] [PubMed] [Google Scholar]