Abstract
The genus Astraeus is shown to be even more complex than recent studies have found. There are problems defining what the molecular fingerprint is of the generic type species. The present article, based on molecular and morphological information and the classical literature, attempts to throw further light on these important ectomycorrhizal fungi. Our studies go part way in an endeavour to unravel the taxonomy and systematics of this genus, necessitating the recognition of at least three new species. Potential nomenclatural problems are also outlined.
Keywords: Ectomycorrhizal fungi, gasteromycetes, phylogeny, taxonomy, nomenclature, ITS nrDNA
INTRODUCTION
Astraeus is a member of Boletales, although the morphology is superficially similar to the earthstars of Geastrum (syn. Geaster), such as G. multifidum (syn. G. coronatum), and into which Astraeus was originally placed. Geastrum hygrometricum is the type of Astraeus by original designation, and for many years the genus was considered monospecific (Morgan 1889). Similarities with some Geastrum species in gross morphology and hygroscopic physiology have led to some confusion with members of that genus from the earliest times. Thus, Coker & Couch (1928) showed that Woodward’s (1794) Lycoperdon recolligens, which was based on Schmidt’s plate defining G. recolligens (syn. G. coronilllum), is undoubtedly a mixture of a true earthstar (G. mammosum, a further synonym of G. corollinum) and Astraeus, a notion previously adopted by Persoon (1801) and Fries (1829), and an unnecessary ”β anglicum” was introduced for Woodward’s part of the concept. A further example is Bolton’s (1788) report of an Astraeus, later called G. boltonii Willd. 1795 and from Swaines Moor (Halifax West Yorkshire, UK) a most unlikely locality, which R.W. has examined; the figure on which Bolton’s record is based is rather poor, but and most probably represents G. rufescens, according to J. Palmer (pers. comm., 11 Feb. 1958). Accordingly, any previously published interpretations and associated distributional records of A. hygrometricus must be considered with caution. With its so-called ease of identification, Astraeus has been recorded from many sites without adequate examination and was said to exhibit a wide host range and distribution. Although a common species, juvenile enclosed gasterothecia have been occasionally confused with entirely hypogeous taxa, or as Lloyd (1902) indicates, macroscopically, when young basidiomes resemble an undeveloped Scleroderma.
Dipterocarpaceae, Fagaceae, and Pinaceae are well-known to form ectomycorrhizal associations; but as the fungus occurs in sandy fields, the hosts are undoubtedly angiosperms. Unfortunately, until recently little attention has been paid to the host association (Wilson et al. 2012). The species was previously thought to be cosmopolitan and quite common in warm temperate to subtropical and tropical climates and in all the continents except Antarctica. It is not found in arctic-alpine communities and is less frequent in boreal areas, although it ranges from low to montane regions in the Himalayas. In contrast to North America, it is evidently rare in South America. Perhaps this all-embracing distributional concept is reflected in the epithet “vulgare” given to A. hygrometricus by Corda (1842). In Thailand, what had been considered to be A. hygrometricus had a long history of edibility, and is regularly found on sale in urban and rural markets; however, our previous studies have shown that in Thailand only two species of Astraeus are found: A. asiaticus (Phosri et al. 2007) and A. odoratus (Phosri et al. 2004).
Massee (1889) described Geaster lilacinus from India based on a collection of Gamble (no. 105 in K), differing it is said by the larger basidiospores. Ahmad (1950) examined the material and found the spore-size to fall in line with what was then considered to be the range for “hygrometricus”. Massee’s measurements of microscopic characters are notorious for being erroneous according to the late Derek A. Reid (formerly of the Herbarium, Royal Botanic Gardens, Kew). Ahmad, during his re-examination, indicated that A. hygrometricus was common in India, citing material from Dehra Dun, Mussories, and Saharanpur in Uttar Pradesh, and Simla, Dalhousie and the Kulu Hills in Punjab.
The fungus was the purported to have a wide distribution and broad host range correlated with slight differences in basidiospore morphology from one area to another that indicated to one of us (R.W.) that the picture presently accepted was too simple. The great range in the world exhibited by this fungus indicated that the recognition of a single species, A. hygrometricus, required re-investigation. Astraeus pteridis from North America and A. koreanus from Korea, however, have also been recognised but they appear rarely in the mycological literature.
The complexity of A. hygrometricus started to be revealed in some of our more recent studies (Phosri et al. 2007), but a wider ranging investigation was shown to be necessary to understand the full diversity of the fungi occurring under this name. This article aims to examine a wide sweep of specimens from a range of localities accross the world including Africa, South-East Asia, the New World, the Himalayas, as well as the classic areas of Europe. We also address issues surrounding the purported wide distribution of A. hygrometricus, the number of actual species constituting the genus, and their mycogeography. Some nomenclatorial problems are also considered, emanating from our study, and three new species are described.
MATERIAL AND METHODS
An in depth study of the genus based on cultured (Pibulsongkram Rajabhat University, Thailand) and herbarium material was undertaken by C.P., but a final analysis could only be made by the use of molecular techniques carried, out by M. M., in parallel with a study of the classical literature by R. W.
Morphological study
Additional herbarium specimens under the name Astraeus hygrometricus were examined as described in Phosri et al. (2004), and compared with data on 160 specimens studied in Martín (1988) and Phosri et al. (2004, 2007) located in BCN, E, K, L, OSC, MA-Fungi, and the A. D. Parker Herbarium (Wisconsin, USA).
Amongst the additional specimens examined, was collection E00159977, (Southern Colorado, USA) collected by C. F. Baker in 1899 and identified by Morgan as A. hygrometricus, which we are able to include in the molecular analyses (Table 1).
Table 1.
List of specimens included in morphological and molecular analyses, not studied in Phosri et al (2007).
| DNA isolation Code | Voucher, Herbarium | Country. Locality | Collection Date | Accession No. |
|---|---|---|---|---|
| A. hygrometricus | ||||
| ASTRAE122 | MJ1558 (E, MA-Fungi). Neotype. | France. Provence, Avignon. | 2 Jan. 1981 | HG000287 |
| ASTRAE129 | Isiloglu 2113 (E, MA-Fungi) | Turkey. Aydŷn, Cine, Kuruköy village. | 21 Nov. 2004 | HG000293 |
| ASTRAE131 | Isiloglu 8149 (E, MA-Fungi) | Turkey. Ýzmir, Bayindir, Sariyurt village. | 25 Nov. 2006 | HG000294 |
| ASTRAE132 | Isiloglu 8383 (E, MA-Fungi) | Turkey. Antalya, Gundogmus, Guneycik village. | 11-May-07 | HG000295 |
| A. morganii | ||||
| ASTRAE134* | C.F. Baker (E-00159977). Holotype | USA. Colorado, La Plata co. | 29 Mar. 1899 | HG000296, HG000296- HG000302** |
| A. pteridis | ||||
| ASTRAE124 | MJ9732 (E) | Portugal. Madeira, Valley of Nuns. | 21 Jan. 1990 | HG000288-HG000290** |
| ASTRAE126 | CORD2123 (E) | Argentina. Punilla valley of Córdoba. | 20 Jun. 1996 | HG000291 |
| ASTRAE127 | CORD2123 (MA-Fungi) | Argentina | - | HG000292 |
| A. telleriae | ||||
| ASTRAE121 | MJ4705 (GB) | Spain. Barcelona, Maresme. | 30 Oct. 1998 | HG000286 |
* Specimen identified by Morgan as Astraeus hygrometricus (Pers.) Morgan.
** Sequences obtained after cloning.
DNA isolation, amplification and sequencing
Samples for DNA extraction were excised from dry basidiomes. To avoid contamination by other fungi, tissues were taken from the inner part of the basidiome. DNA extraction, amplification and sequencing of the ITS regions, including the 5.8S ribosomal RNA gene cluster, followed the protocols in Phosri et al. (2009), with the primer pair ITS1F/ITS4 (White et al. 1990; Gardes & Bruns 1993), and the cycling protocol described in Martín & Winka (2000). Aliquots of the purified products were mixed separately with the direct and reverse primers before sending to Macrogen (South Korea) for sequencing. When weak PCR products were obtained, the products were cleaned from the gel and cloned; both strands were separately sequenced using vector specific primers T7 and M13. Sequences obtained in this study are included in Table 1.
Consensus sequences where assembled using Sequencher (Gene Codes Corporation, Ann Arbor, MI). Prior to the alignment, sequences were compared with homologous sequences in EMBL/GenBank/DDBJ (Cochrane et al. 2011) using the BLASTn algorithm (Altschul et al. 1997). Multiple sequence alignment of the consensus sequences obtained in this study and homologous sequences from EMBL/GenBank/DDBJ, mainly presented in Phosri et al. (2007) and Fangfuk et al. (2010), were performed using Se-Al v. 2.0a11 Carbon (Rambaut 2002). The alignment was optimized visually. Alignment gaps were indicated as “-” and ambiguous nucleotides were marked as “N”. Two sequences were included as outgroup: Pisolithus arhizus (AJ629887) and Scleroderma verrucosum (AJ629886).
Phylogenetic analyses
The alignment was analysed under a heuristic search, using PAUP v. 4.0b10 (Swofford 2003), and under a Bayesian approach (Huelsenbeck et al. 2000, Larget & Simon 1999) assuming a HKY+G model, using MrBAYES v. 3.0 (Huelsenbeck & Ronquist 2001), as described in Telleria et al. (2010). The phylogenetic tree was viewed with FigTree v. 1.3.1 (http://tree.bio.ed.ac.uk/sofware/figtree/) and edited with Adobe Illustrator CS3 v. 11.0.2 (Adobe Systems).
RESULTS
Seventeen new sequences from Astraeus specimens were generated, including those obtained from E00159977 (see above). From the Madeira collection (ASTRAE 124, MJ9732 in E), three sequences were obtained after cloning; these sequences show three variable positions (two in ITS1 and one in ITS2 due to transitions T/C). From the Morgan sample, one sequence was obtained after direct PCR and six after cloning; these sequences had six variable positions, five in ITS 1 and one in ITS2, due to one deletion and five transitions (both G/A and T/C).
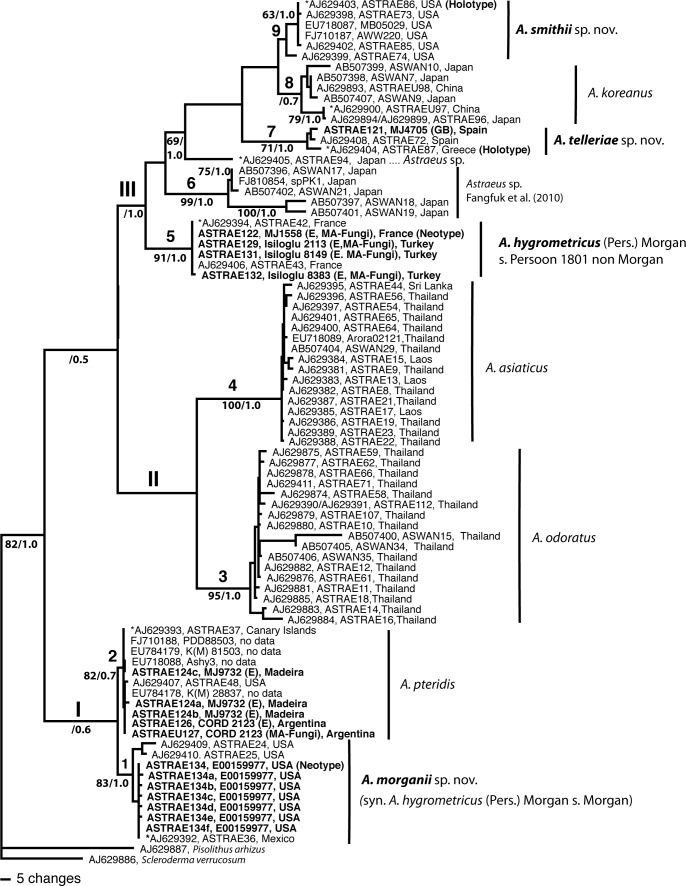
The ITS nrDNA dataset contained 83 sequences and 891 aligned positions, 468 of them were constant, 170 parsimony-uninformative, and 253 parsimony-informative. Maximum parsimony analysis yielded 100 most parsimonious trees with 779 steps, CI = 0.7125, HI = 0.3790, RI = 0.9276; the strict consensus MP tree (not shown) and the 50 % majority rule Bayesian tree (Fig. 1) had a similar topology.
Fig. 1.
One of the 100 most parsimonious trees inferred from a heuristic search of ITS nrDNA sequences of Astraeus spp. Clades I–III and subclades 1–9 as mentioned in the text. Numbers separated by “/” represent maximum parsimony bootstrap values (BS) and Bayesian posterior probabilities values (PP), respectively. Percentage of bootstrap values < 50 % and Bayesian posterior probability values < 0.5 are not included. Sequences from new specimens included in this study are marked and sequences from type specimens designated here are in bold. (*) before accession numbers from Phosri et al. (2007), indicates that SEM spores and basidiomes are illustrated in Figs 2 and 3.
Astraeus species formed a monophyletic group with high support; the bootstrap value (BS) was 82 %, and the posterior probability (PP) 1.0). At least three main lineages (I, II and III) and nine terminal clades were revealed and are described and related with spore size and morphology here (Fig. 2).
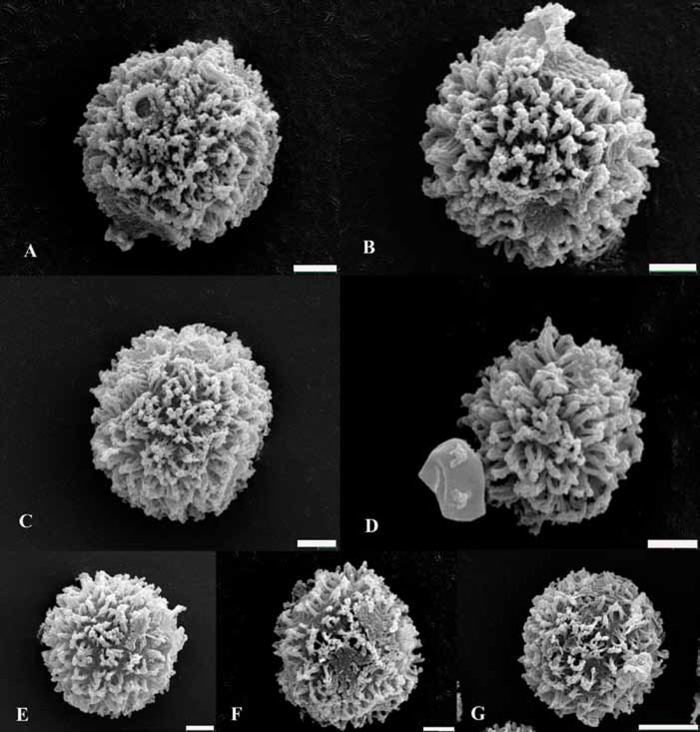
Fig. 2.
SEM image of basidiospore ornamentation. A. Astraeus morganii (AJ629392, ASTRAE36). B. A. pteridis (AJ629393, ASTRAE37). C. A. koreanus (AJ629900, ASTRAE97). D. Astraeus sp. (AJ629405, ASTRAE94). E. A. hygrometricus (AJ629394, ASTRAE42). F. A. telleriae (AJ629404, ASTRAE87). G. A. smithii (AJ629403, ASTRAE73). Bars: A–F = 2 μm, G = 5 μm. Numbers between brackets correspond with those in Fig. 1.
Lineage I consisted of clade 1 (BS = 83 %; PP = 1.0) and clade 2 (BS = 82 %; PP = 0.7). Clade 1 included sequences from Phosri et al. (2007) under the name A. pteridis and from Mexico and Wisconsin (USA), as well as the seven sequences from the Morgan specimens (E00159977, under A. hygrometricus). The spore size ranged from 7.5–10.0 μm, mean ± SD = 9.44 ± 0.86 (Fig. 2A). Clade 2 (BS = 82 %; PP = 0.7) grouped 11 sequences, among them the new sequences from Argentina and Madeira (3 clones), and sequences AJ629392 (Canary Islands) and AJ629407 (specimen OSC49749 from Oregon) under A. pteridis in Phosri et al. (2007); the spore size ranges from 7.5–12.5 μm, mean ± SD = 10.19 ± 1.24 (Fig. 2B). Sequence AJ629903 from Cornwall, UK (Phosri et al. 2007) was not included in the phylogenetic analyses because it was short (465 bp), but it is similar to sequences from clade 2; the Kimura-2-parameter pairwise distance among AJ629903 and nine sequences of this clade gave a value of 0.0000 (e.g. specimens from Argentina, Madeira) or 0.00215 (Canary Islands and Oregon).
Lineage II consisted of the strongly supported clade 3 (BS = 95 %; PP = 1.0) and clade 4 (BS = 100 %; PP = 1.0) from SE Asian specimens, mainly from Thailand. Both clades were reported on previously (Phosri et al. 2007) and resulted in the need to recognize two new species, A. odoratus and A. asiaticus, respectively.
Lineage III included specimens from all around the world grouped in at least five monophyletic clades and a single sequence from Japan (AJ629405; Phosri et al. 2007, collection E00159827). The remaining isolates from Asia are distributed in two clades: clade 6 (BS = 99 %; PP = 1.0) consisted of five specimens from Japan (Fangfuk et al. 2010, Japanese Astraeus group 2), and clade 8 (BS < 50 %; PP = 0.7) grouped six sequences, four from Japan and two from China. The specimens from clade 8 are associated with Pinus thunbergii; the basidiomes apparently key admirably to Stanek’s A. hygrometricus var. koreanus (in Pilàt 1958) considered by Kreisel (1969) as be an independent species on the basis of the morphology of both the basidiome and of the basidiospores; the spore size ranges from 5.5–11.5 μm, mean ± SD = 10.15 ± 1.48 (Fig. 2C). The specimens from clade 6 are quite distinct on molecular and morphological grounds from A. koreanus, and clearly require formal recognition, although the “species” may even have to be split further in the future along the lines of host, one being associated with Fagaceae (AB507396, AB507402, FJ810854) and the other Pinaceae, P. densiflora (AB507397, AB507401). Also, more specimens should be analyzed to delimit the taxon of specimen E00159827 (AJ629405); the spore size ranges from 7.0–9.0 μm, mean ± SD = 7.92 ± 0.66 (Fig. 2D).
Many isolates considered to be A. hygrometricus were grouped in three clades in lineage III. Clade 5 (BS = 91 %; PP = 1.0) consisted of six specimens from France and Turkey; the spore size ranges from 8.5–12.5 μm, mean ± SD = 10.56 ± 1.11 (Fig. 2E). Clade 7 (BS = 71 %; PP = 1.0) grouped three from Spain and Greece; the spore size ranges from 7.5–12.5 μm, mean ± SD = 9.91 ± 1.11 (Fig. 2F). Clade 9 (BS = 65 %; PP = 1.0) comprised six mainly from USA (Wisconsin and Michigan); the spore size ranges from 7.5–12.5 μm, mean ± SD = 10.5 ± 1.24 (Fig. 2G).
DISCUSSION
Now that a division of this species complex is recognised, a major issue is the identity of the original Astraeus hygrometricus, first described under the generic name Geastrum by Persoon (1801) from material collected around Paris. The well-supported group (clade 5) incorporating collections from France and Turkey, appears, based on distributional data, to represent Persoon’s fungus. Unfortunately it has not been possible to extract DNA from any collection made by Persoon.
A second problem arises because none of the collections from the New World so far studied, fall within the circumscription of the European-biased material. Astraeus hygrometricus in its strictest sense has not been found in the United States, and collections agreeing with those from North America have only been found in the UK (sequence AJ629903). This is a rather different picture from the movement of the related Pisolithus species around the world with their mycorrhizal hosts, e.g. Australian taxa with Eucalyptus trees. in Africa (Phosri et al. 2012).
An issue then arises as to what Morgan (1889), who demonstrated the differences between Geastrum hygrometricum and true earth-stars, had to hand. He correctly felt it necessary to erect a separate genus based on his interpretation of G. hygrometricum. Morgan introduced the generic name Astraeus to accommodate the single species he had identified as Geastrum hygrometricum. On the basis of what we have revealed about the genus, we suggest that he actually had a different species related to, but not the same as, A. pteridis. Interestingly, although Morgan described the genus in a paper from Cincinnati he never collected or recorded the fungus from there. However, typification of generic names under the Code is based on the types of the names of the included species, whether or not the names were misapplied by the describing author. As Astraeus was based on Persoon’s specific name, the type of which must be that of, in this case Geastrum hygrometricum. Consequently, the generic name Astraeus is typified by the European species G. hygrometricum (e.g. French specimens in clade 5).
In our proposed scheme, the type of Astraeus hygrometricus must have lain in one of clades 1, 2 or 9. Clades 1 and 2 encompass what has been called A. pteridis (Shear) Zeller 1948, characterized by basidiomes that are generally larger than those of A. hygrometricus of most authors. Lloyd (1901) described Geastrum hygrometricum var. giganteum from California, and, almost at the same time, Shear (1902) described Scleroderma pteridis from material collected in 1899 from the western US as discussed by Guzmán (1970). Lloyd (1902) noted, in addition to California, material from Iowa and Washington. Zeller (1948), after studying the type specimens of S. pteridis in NYBG, made the new combination A. pteridis. Astraeus hygrometricus was also discussed at length by Zeller (1948), who drew attention not only to the differences from what was then considered the true A. hygrometricus (i.e. Morgan’s fungus), but to the great variability of the species, probably also having taken into account observations on European material deposited in the NYBG. This variability had been reflected over 100 years earlier in Desvaux’s (1809) treatment in which where four species were erected; see Pilát (1958). Such a resource was most certainly not available to Morgan when he conducted his anatomical observations. Thus, we adopt the name A. pteridis here for the larger Pacific Northwest American species characterising clade 2 (such as collection OSC49749 from Oregon, sequence AJ629407). This species has also been found in the Canary Islands and Madeira, both Atlantic archipelagos, and Argentina, which has strong links with Lusitania. This distribution may indicate either a translocation or that we have sampled extremes of a wide distribution. The remaining closely allied clade 1 brings together the collection made by Morgan and material from Mexico. This is not as surprising as might be first thought as the collecting site in Colorado is in the arid area close to the Texan border. The unique features of specimens analysed from clade 1, clade 7 and clade 9 indicated that it is necessary to recognize three new species: Astraeus morganii, A. telleriae, and A. smithii, respectively. On the other hand, additional studies are needed to resolve the taxonomic status of specimens from India named as Geaster lilacinus, and included in Ahmad (1950) under A. hygrometricus, and material from Africa presently under study.
TAXONOMY
Astraeus Morgan, J. Cincinnati Nat. Hist. Soc. 11: 20 (1889).
Type species: Astraeus hygrometricus (Pers.) Morgan 1889.
Astraeus hygrometricus (Pers.) Morgan, J. Cincinnati Nat Hist. Soc. 11: 20 (1889).
Fig. 3.
Basidiomes. A. Astraeus morganii (K(M) 19550; AJ629392, ASTRAE36). B. A. pteridis (AJ629393, ASTRAE37). C. A. koreanus (AJ629900, ASTRAE97). D. Astraeus. sp. (AJ629405, ASTRAE94). E. A. hygrometricus (AJ629394, ASTRAE42). F. A. telleriae (AJ629404, ASTRAE87). G. A. smithii (AJ629403, ASTRAE73). Bars = 2.0 cm. Numbers between brackets correspond with those in Fig. 1.
Basionym: Geastrum hygrometricum Pers., Syn. Meth. Fung. 1: 135 (1801).
Synonyms: Lycoperdon stellatus Scop., Fl. Carniol., edn 2: 63 (1760).
Geastrum stellatum (Scop.) Wettst., Verh. Zool.-bot. Ges. Wien 35: 576 (1885); as “Geaster stellatus”.
Astraeus stellatus (Scop.) E. Fisch., Nat. Pflanzenfam. 1 (1**): 341 (1900).
Type: France: Provence: Avignon, Tarascon-sur-Rhone, Abbaye de Frigolet, in dry sandy grassland, 2 Jan. 1981, M. Jeppson MJ1558 (E – neotype designated here, MBT176569; MA-Fungi isoneotype; ITS nrDNA sequence ex-neotype HG000287).
Description: Basidiome globular or slightly flattened laterally, sometimes with a hint of a basal dome, fully enveloping when dry an inner peridium revealed when mature and moist, 20–25 mm diam before opening, splitting into 12–14 distinct rays, with only 2–3 as minor splittings, rays becoming 36–38 mm broad; outermost layer of outer peridium fuscous- to umber-brown, darkening further when moist; inner surface concolorous, cracking deeply, when moist forming an irregular diamond-shaped pattern, areoles silvery grey; inner peridium 13–14 mm diam, a thin pale buff or pale clay-coloured sack when dry, darkening considerably when moist although retaining a pale, narrow zone around the irregular apical aperture; rhizoids lacking; capillitium very slightly ornamented with irregularly distributed low warts, hyaline to honey coloured, 4.5–6.5 μm broad, brown, formed of clearly branched formed of irregularly anastomosing hyphal elements. Spores minutely warted from low, small rounded prominences, globose, 10–12.5(–13.5) μm diameter, pale brown with larger ones generally paler and often with large central guttule.
Habitat: In dry sandy grassland.
Distribution: Southern France and Turkey.
Specimens confirmed by ITS nrDNA analyses: France: East of Orange and NE of Avignon, Mollans sur Ouveze, Aug. 1977, E. A. Ellis (K(M) 104969; ITS nrDNA sequence AJ629406); Gard, 23 Sept. 1997, B. W. Brown (K(M) 50616; ITS nrDNA sequence AJ629394). – Turkey: Aydŷn, Cine, Kuruköy village, in Quercus forest, 21 Nov. 2004, M. Isiloglu 2113 (E, MA-Fungi; ITS nrDNA sequence HG000293); Antalya, Gundogmus, Guneycik village, in Pinus brutia and Quercus forest, 11 May 2007, M. Isiloglu 8383 (E, MA-Fungi; ITS nrDNA sequence HG000295); Ýzmir, Bayindir, Sariyurt village, in Pinus brutia and Quercus sp. forest, 25 Nov. 2006, M. Isiloglu 8149 (E, MA-Fungi; ITS nrDNA sequence HG000294).
Observations: The earliest name for this species, Lycoperdon stellatus, is not to be taken up for this species since Persoon (1801) sanctioned the use of Geastrum hygrometricum (Art. 13.1(d)).
Astraeus morganii Phosri, Watling & M.P. Martín, sp. nov.
MycoBank MB803905
Etymology: Named after A. P. Morgan (1836—1907) who first demonstrated the significance of the development of Astraeus and how it fundamentally differed from the true earthstars.
Diagnosis: This new species is closely related to A. pteridis, but differs in the smaller basidiome, larger spores, and its unique ITS nrDNA sequence.
Type: USA: Colorado: La Plata Co., Hormosa, north of Durango, 29 Mar. 1899, C. F. Baker [Plants of Southern Colorado no. 13; identified by Morgan] (E-00159977 – holotype; direct ITS nrDNA sequence HG000296; and sequences HG000297-HG000302 after cloning).
Description: Basidiome depressed-globose, outermost layer and attached mycelium, deciduous, outer peridium splitting into 7–20 pointed rays, at least 5 or so narrower than the rest, expanding to 50–76 mm, strongly hygroscopic, inner layer of outer peridium cartilaginus-gelatinous, hard and rigid when dry, swelling greatly and flexible when wet, smooth to irregularly rugulose, then becoming increasingly cracked and fissured, retaining hygroscopic qualities, stellate, remaining on the soil surface, spreading out in moist weather and bending inward when dry; inner peridium 20–25 mm depressed-globose, sessile, reticulate, pitted, whitish becoming grey or brownish; capillitium ”rather thinner than spores fide Morgan”, hyaline. Spores globose, minutely warted, brown, 7.5–10 μm diam.
Habitat: In fields and woods in sandy soil.
Distribution: Central to Southern United States southwards to Mexico.
Observation: The above description represents Morgan’s understanding of A. hygrometricus on which his new genus was based but this differs from what we believe to be Persoon’s original concept (see above). The holotype collection was purchased for E in 1900, and the collection was dated the same year as the publication of Astraeus. Morgan apparently did not find Astraeus in Cincinnati, the state where he published his new observations from, but he considered A. hygrometricus, in parallel with many other classical and post-classical mycologists, to be a very common fungus with a worldwide distribution. This is now patently untrue. It is recorded by Morgan for several sites in the US but without molecular data it is impossible to say which might be one of the three groupings now recognized there. His records include: California (Harkness), Florida (Calkins), Kansas (Cragin), New England (Frost), New Mexico (Wright), New York (Peck), Pennsylvania (Schweinitz & Gentry), South Carolina (Aitken in Ravanel, Exs. no. 471), South Carolina (E), Texas (Drummond), and Wisconsin (Brown & Trelease). Unfortunately the material available from these collections, after several attempts, did not allow the reclamation of good DNA.
The type of Geastrum fibrillosum, a species described by Schweinitz (1822) from Carolina (Synops. Fungor. Carol. no. 330, FH not re-located), was examined by Coker & Couch (1928) and shown to be a weathered member of the A. hygrometricus consortium. The persistent basidiomes become fibrillose and ragged, especially in overwintered material; Lloyd (1908) concurred with this decision. However, from our observations above this cannot be assigned to A. hygrometricus as now defined, not to one of the newly described taxa from North America since we can not confirm morphological data and do molecular analyses.
Specimens confirmed by ITS nrDNA analyses: Mexico: sine loc., 10 Mar. 1991, W. C. Weightman (K(M) 19550; ITS nrDNA sequence AJ629392). – USA: Wisconsin: Adams County (Alan D. Parker Herbarium, Fungi of Wisconsin; ITS nrDNA sequence AJ629410); Walworth County, Young Rd. Steinke, A. D. Parker, 15 Oct. 1995 (Alan D. Parker Herbarium, Fungi of Wisconsin; ITS nrDNA sequence AJ629409).
Astraeus smithii Watling, M.P. Martín & Phosri, sp. nov.
MycoBank MB803906
Etymology: Named after the late Alexander H. Smith (1904–1986), formerly Ann Arbor, who published on Michigan puffballs and their allies amongst his many other North American macromycete monographs.
Diagnosis: This species is characterised by the inner peridium at maturity becoming matted-fibrillose to reticulate, the dark almost blackish rhizoids, and its unique ITS nrDNA.
Type: USA: Michigan: Chippewa County, Upper Penninsula, Whitefish Bay, Whitefish Point, on surface of sandy soil in sand-dune system, 25 Aug. 1965, R. Watling Wat. 874/2023 (E-00159828 – holotype; ITS nrDNA sequence AJ629403).
Description: Basidiome 10–20(–30) mm, almost globose or slightly ellipsoid, with dark almost blackish rhizoids some of which persist at the base, splitting into 7–15 rays; outer peridium distinctly layered, surface matted-fibrillose and intermixed or embedded with sand particles, overlying a thin, fugacious layer, which breaks up, although hard when dry, seated on a fibrous-rimose innermost and exposed layer; inner peridium a pale coloured, papery-thin sack, the surface at maturity matted-fibrillose to reticulate, opening by a single tear; gleba white when young, becoming cocoa-colour at maturity; capillitium buff to pale brown, encrusted, thick-walled, highly branched hyphal elements. Spores 7.5–12.5 μm, globose with a hyaline sheath overlying thickened warty layer composed of pegs.
Habitat: On soil surface margins of woodland, open areas.
Distribution: Central and Northern United States.
Specimens confirmed by ITS nrDNA analyses: USA: Michigan: Luce County, Upper Peninsula, Lake Superior, Crisp Point, on sandy soil in sand-dune system, 5 Aug. 1965, R. Watling 709/1137A (E-00159829; ITS nrDNA sequence AJ629402). Wisconsin: Adams County, Castle Rock, 21 Sept. 2001, A. D. Parker (Alan D. Parker Herbarium; ITS nrDNA sequence AJ629398); Portage County, Stevens Point, 29 Sept. 1989, A. D. Parker (Alan D. Parker Herbarium; ITS nrDNA sequence AJ629399).
Astraeus telleriae M.P. Martín, Phosri & Watling, sp. nov.
MycoBank MB803958
Etymology: Named after Maria Teresa Telleria, Director of the Real Jardín Botánico-CSIC 1994–2006, who has contributed extensively to Spanish mycology especially through the Flora Mycologica Iberica project.
Diagnosis: This new species differs from related species in the very pubescent, even minutely woolly, inner layer of the outer peridium, and its unique ITS nrDNA sequence.
Type: Greece: Ventuna, Nov. 1908, M. Wilson 167 (E-00159833 – holotype; ITS nrDNA sequence AJ629404).
Description: Basidiome 35–42 mm, globose at first, splitting into 12 small rays; outer peridium distinctly layered, pubescent s.l., without adhering soil particles; inner layer of outer peridium very pubescent even minutely woolly but then concentrically rimose; inner peridium thin, pale buff with central, irregular fissure at apex, cocoa-coloured within; capillitium buff to pale brown, some elements encrusted, thick-walled and strongly branched. Spores 7.5–12.5 μm, globose and ornamented with small warts.
Habitat: On soil surface, margins of woodland (Pinus spp. and Quercus spp.), open areas.
Distribution: Mediterranean – Southern Spain to Greece.
Specimens confirmed by ITS nrDNA analyses: Spain: Catalunya: Barcelona, Maresme, Santa Ischia, Arenys de Munt, 30 Oct. 1998, M. Jeppson MJ4705 (GB; ITS nrDNA sequence HG000286). Madrid: Majadahonda, 23 Feb. 2002, P. P. Däniels & C. Phosri (MA-Fungi; ITS nrDNA sequence AJ629408).
CONCLUSIONS
Our study indicates that the name Astraeus hygrometricus has previously covered several separate species. Further, in addition to the three new species recognized here, more taxa remain to be categorised. More fresh collections are required for a species originating in India, for which there may be a possible previous name available, and at least one noted from Japan. Much work is required on collections from Japan and North America, and more material is needed from South America where the collections may represent recent introductions. The names “Astraeus hygrometricus” and “A. pteridis” are both mentioned from Australia (May et al. 2003), so in any further study it would be essential to incorporate material from that country. Simple examination of material in E collected by A. Morrison in Western Australia shows great variation including a collection with “pygmy” basidiomes, little more than 15 mm across even when expanded, and spores 6.5–7.0 × 6.0–6.5 μm.
With the integration of studies of type material deposited in herbaria, with molecular data from fresh collections of known provenance, a fuller attempt can be made to resolve the taxonomy of this interesting genus. At least the branches of the tree so far developed clearly demonstrate that Astraeus hygrometricus, as previously circumscribed, to be not a single species but made up of a multitude of cryptic species. The present study is a partial solution to this ever-increasing conundrum.
Acknowledgments
We thank the herbaria BCN, K, L, MA-Fungi, OSC, and Alan D. Parker (Fungi of Wisconsin) for loan of specimens used in this and in our previous studies; especially to E for permission to perform molecular analyses of E-00159977 (our Morgan sample). Thanks are also due to Mikael Jeppson for providing us specimens of Astraeus from his private herbarium, to Michael J. Richardson and Marian Glenn for English grammatical correction and two anonymous reviewers for improving our manuscript. The research was financially supported primarily by national projects from the Royal Thai Government, National Research Council of Thailand and the Flora Mycologica Iberica project (CGL2006-12732-CO2-01/BOS). Work in the Molecular Systematics Laboratory of the Real Jardín Botánico-CSIC, Madrid, was supported by SYNTHESYS Project http://www.synthesys.info/.
REFERENCES
- Ahmad S. (1950) Studies in gasteromycetes. Sydowia 4: 124–129 [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research 25: 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton J. (1788) An History of Fungusses growing about Halifax. 4 vols. Huddersfield: printed by J. Brook, for the author, [German translation by K. L. Willdenow (1795–1820), Beschreibung der um Halifax wachsenden Pilze Berlin: Reimer.] [Google Scholar]
- Coker WC, Couch JN. (1928) The Gasteromycetes of the Eastern United States and Canada. Chapel Hill, NC: North Carolina University Press [Google Scholar]
- Corda AJC. (1842) Icones Fungorum hucusque cognitorum. Prague: J. G. Calve [Google Scholar]
- Cochrane G, Karsch-Mizrachi I, Nakamura Y. (2011) The International Nucleotide Sequence Database Collaboration. Nucleic Acids Research 39: D15–D18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvaux NA. (1809) Observations sur quelques genres à établir dans famille des Champignons. Journal de Botanique, Paris 2: 38–105 [Google Scholar]
- Fangfuk W, Petchang R, To-anun C, Fukuda M, Yamada A. (2010) Identification of Japanese Astraeus, based on morphological and phylogenetic analyses. Mycoscience 51: 291–299 [Google Scholar]
- Fries EM. (1829) Systema Mycologicum. Vol. 3 (1). Gryphiswald: Ernst Mayritius [Google Scholar]
- Gardes M, Bruns TD. (1993) ITS primers with the enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Molecular Ecologist 2: 113–118 [DOI] [PubMed] [Google Scholar]
- Guzmán G. (1970) Monografia del género Scleroderma Pers. emed Fr. Darwinia 16: 233–407 [Google Scholar]
- Huelsenbeck JP, Rannala B, Masly JP. (2000) Accommodating phylogenetic uncertainty in evolutionary studies. Science 288: 2349–2350 [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–455 [DOI] [PubMed] [Google Scholar]
- Kreisel H. (1969) Grundzüge eines natürlichen Systems der Pilze. Jena: Gustav Fischer [Google Scholar]
- Larget B, Simon DL. (1999) Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees. Molecular Biology and Evolution 16: 750–759 [Google Scholar]
- Lloyd CG. (1901) Geaster hygrometricus var. giganteus. Mycological Notes 1: 68 [Google Scholar]
- Lloyd CG. (1902) The Geastreae. Mycological Writings 1: 1–44 [Google Scholar]
- Lloyd CG. (1908) The gasteromycetes of Schwienitz’s herbarium. Mycological Notes 30: 395–405 [Google Scholar]
- Martín MP. (1988) Aportación al conocimiento de las higroforáceas y los gasteromicetes de Cataluña. Edicions especials de la Societat Catalana de Micologia 2: 1–508 [Google Scholar]
- Martín MP, Winka K. (2000) Alternative methods of extracting and amplifying DNA from lichens. Lichenologist 32: 189–196 [Google Scholar]
- Massee G. (1889) New or imperfectly known gasteromycetes. Grevillea 19: 94–98 [Google Scholar]
- May TW, Milne J, Shingles S, Jones JH. (2003) Fungi of Australia. Vol. 2B. Catalogue and Bibliography of Australian Fungi. 2. Basidiomycota p.p. & Myxomycota p.p. Canberra, ACT: Australian Biological Resources Study [Google Scholar]
- Morgan AP. (1889) North American Fungi. The Gasteromycetes Order II – Lycoperdaceae. Journal of the Cincinnati Society of Natural History 11: 36–45 [Google Scholar]
- Persoon CH. (1801) Synopsis Methodica Fungorum. Gottingen: Dieterich [Google Scholar]
- Phosri C, Martín MP, Sihanonth P, Whalley AJS, Watling R. (2007) Molecular study of the genus Astraeus. Mycological Research 111: 275–286 [DOI] [PubMed] [Google Scholar]
- Phosri C, Martín MP, Suwannasai N, Sihanonth P, Watling R. (2012) Pisolithus: a new species from Southeast Asia and a new combination. Mycotaxon 120: 195–208 [Google Scholar]
- Phosri C, Martín MP, Watling R, Jeppson M, Sihanonth P. (2009) Molecular phylogeny and re-assessment of some Scleroderma spp. (gasteromycetes). Anales del Jardín Botánico de Madrid 66 (S1): 83–91 [Google Scholar]
- Phosri C, Watling R, Martín MP, Whalley AJS. (2004) The genus Astraeus in Thailand. Mycotaxon 89: 453–463 [Google Scholar]
- Pilát A. (1958) (ed.) Gasteromycetes. [Flora CSR, Serie B.] Prague: Ceskoslovenke Akademie [Google Scholar]
- Rambaut A. (2002) Se-Al. Sequence alignment editor. Version 2.0a11. Oxford: Department of Zoology, University of Oxford [Google Scholar]
- Schweinitz LD. (1822) Sypnosis fungorum Carolinae superioris. Schriften der naturforschenden Gesellschaft zu Leipzig 1: 20–131 [Google Scholar]
- Shear CL. (1902) Mycological notes and new species. Bulletin of the Torrey Botanical Club 29: 451 [Google Scholar]
- Swofford DL. (2002) PAUP*: phylogenetic analysis using parsimony (*and other methods). Version 4. Sunderland, MA: Sinauer Associates [Google Scholar]
- Telleria MT, Dueñas M, Melo I, Hallenberg N, Martín MP. (2010) A re-evaluation of Hypochnicium (Polyporales) based on morphological and molecular characters. Mycologia 102: 1426–1436 [DOI] [PubMed] [Google Scholar]
- White T, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR Protocols: a guide to methods and applications: 315–322 San Diego: Academic Press [Google Scholar]
- Wilson AW, Binder M, Hibbett DS. (2012) Diversity and evolution of ectomycorrhizal host associations in the Sclerodermatineae (Boletales, Basidiomycota). New Phytologist 194: 1079–1095 [DOI] [PubMed] [Google Scholar]
- Woodward TJ. (1794) An essay towards a history of the British stellated Lycoperdons. Transactions of the Linnean Society of London 2: 32–62 [Google Scholar]
- Zeller SM. (1948) Notes on certain gasteromycetes, including two new orders. Mycologia 41: 36–58 [PubMed] [Google Scholar]