Abstract
Serotonin/substance P synthesizing cells in the raphé nuclei of the brain are candidates for designation as central chemoreceptors that are stimulated by CO2/pH. We have previously demonstrated that these neurons are CO2-stimulated in situ. Evidence also suggests that CO2-inhibited raphé neurons recorded in vitro and in situ synthesize γ-aminobutyric acid (GABA). Unknown is whether there are other types of chemosensitive cells in the raphé. Here, we showed that a previously unrecognized pool of raphé neurons also exhibit chemosensitivity, and that they are not serotonergic. We used extracellular recording of individual raphé neurons in the unanesthetized juvenile rat in situ perfused decerebrate brainstem preparation to assess chemosensitivity of raphé neurons. Subsequent juxtacellular labeling of individually recorded cells, and immunohistochemistry for the serotonin synthesizing enzyme tryptophan hydroxylase (TPH) and for neurokinin-1 receptor (NK1R; the receptor for substance P) indicated a group of CO2-stimulated cells that are not serotonergic, but express NK1R and are closely apposed to surrounding serotonergic cells. CO2-stimulated serotonergic (5-HT) and non-5-HT cells constitute distinct groups that have different firing characteristics and hypercapnic sensitivities. Non-5-HT cells fire faster and are more robustly stimulated by CO2 than are 5-HT cells. Thus, we have characterized a previously unrecognized type of CO2-stimulated medullary raphé neuron that is not serotonergic, but may receive input from neighboring serotonin/substance P synthesizing chemosensitive neurons. The potential network properties of the three types of chemosensitive raphé neurons (the present non-5-HT cells, serotonergic cells, and CO2-inhibited cells) remain to be elucidated.
Keywords: raphé, breathing, chemosensitivity, serotonin
Introduction
The brainstem raphé nuclei play a critical role in maintaining homeostasis and autonomic control of processes including respiration, thermoregulation, nociception, and stress responses (Richerson, 2004; Hellman et al., 2007; Hodges et al., 2008; Hodges and Richerson, 2010a). Raphé dysfunction is implicated in various pathologies (Paterson et al., 2006; Kinney, 2009; Kinney and Thach, 2009; Hilaire et al., 2010; Broadbelt et al., 2011). The raphé nuclei are best known as the major population of serotonin synthesizing (5-HT) cells in the brain. Serotonin is a known modulator of ventilation and serotonergic neurons in the raphé nuclei are proposed to be chemonsensors (1st order sensory neurons, responsive to arterial CO2/pH; Corcoran et al., 2009; Hodges and Richerson, 2010b). Indeed, some 5-HT neurons demonstrate sensitivity to CO2 /pH in vitro, in vivo, and in situ (Veasey et al., 1995; 1997; Wang et al., 2001; Severson et al., 2003; Iceman et al., 2013). Although considerations of this region often focus on serotonergic cells, the raphé nuclei also include non-serotonergic cells and are heterogeneous with respect to neurotransmitter phenotype, response of cells to hypercapnia, developmental origin, and other physiological function (Millhorn et al., 1988; Charara and Parent, 1998; Kirby et al., 2003; Jensen et al., 2008; Kiyasova and Gaspar, 2011; Calizo et al., 2011; Mason, 2011; Gaspar and Lillesaar, 2012; Iceman et al., 2013).
Many of these non-5-HT neurons in the raphé have been characterized as glutamatergic and γ-aminobutyric acid (GABA) synthesizing (GABAergic; Calizo et al., 2011). GABA raphé cells are thought to be involved in processes including CO2 sensation, pain, temperature, stress, heart rate, and blood pressure (Zaretsky et al., 2003a; b; Hodges et al., 2005; Cao and Morrison, 2005; Cao et al., 2006; DiMicco et al., 2006; Winkler et al., 2006).
Some non-5-HT raphé cells have been implicated in the hypercapnic ventilatory response. Non-5-HT (likely GABAergic) cells in the raphé are inhibited by CO2 in vitro and in situ (Wang et al., 1998; Wang and Richerson, 1999; Severson et al., 2003; Hodges et al., 2005; Iceman et al., 2012) and may play some role in the hypercapnic ventilatory response. Raphé neurokinin-1 receptor (NK1R) expressing cells do not synthesize 5-HT and toxic lesioning of these cells results in a blunted hypercapnic response in rats and goats in vivo (Nattie et al., 2004; Hodges et al., 2004; Commons, 2009). As substance P is the endogenous ligand for NK1R, these cells would presumably be receiving input from substance P synthesizing neurons. Serotonin synthesizing cells are the main source of substance P, and these neurochemicals are co-expressed in the 5-HT cells of the raphé (Ljungdahl et al., 1978). No single population of cells appears to be primarily responsible for the hypercapnic ventilatory response, and this complex process likely includes many cell types and nuclei and relies heavily on redundancy.
The present study was designed to identify CO2-stimulated cells in the medullary raphé, and to determine if they universally expressed markers for serotonin synthesis. We tested the hypothesis that a portion of medullary raphé cells are stimulated by hypercapnia in situ and possess a variety of phenotypes. We identify a previously unrecognized class of chemosensitive raphé neuron: non-serotonergic cells stimulated by hypercapnia.
Experimental procedures
Experimental animals and surgery
All experiments were done in accordance with the guidelines of the “Guide for the Care and Use of Laboratory Animals” of the National Institutes of Health and were approved by the University of Alaska Fairbanks Institutional Animal Care and Use Committee. Experiments were conducted in preparations derived from juvenile (P20–P30; 60–150g) male Simonsen albino rats; (Sprague-Dawley derived; Simonsen Laboratories) in situ using the perfused decerebrate juvenile rat brainstem preparation, as per published methods (Toppin et al., 2007; Corcoran et al., 2013; Iceman et al., 2013). Briefly, animals were heparinized (0.3 mL of 1,000 i.u./mL; i.p.; Baxter, Deerfield, IL), then deeply anesthetized with isoflurane. Preparations were transected below the diaphragm, immersed in ice-chilled perfusate, and decerebrated rostral to the superior colliculi. Subsequent procedures were conducted in the absence of anesthesia as decerebration renders animals insensitive to pain. Preparations were placed prone in a stereotaxic head frame and the descending aorta was cannulated retrogradely with a double-lumen catheter and perfused with solution at a temperature of 31 °C. The perfusate contained the following (in mM): MgSO4 (1.0); NaH2PO4 (1.25); KCl (4.0); NaHCO3 (24); NaCl (115); CaCl2 (2.0); D-glucose (10); Ficoll 70 (0.18). Under baseline conditions, perfusing solutions were equilibrated with 95% O2 – 5% CO2 (PCO2 33 mmHg; pH 7.4). The neuromuscular blocker gallamine triethidodide (60 mg/L) was added to the perfusate to eliminate movement. The pressure of aortic perfusion (measured with a blood pressure transducer attached to the second lumen) was increased gradually to 50–75 mmHg and then held constant. Perfusate was collected and recirculated. Neuronal recordings were always initiated under baseline conditions, followed by brief (5 min) hypercapnic challenges (91% O2 – 9% CO2 ; PCO2 60 mmHg; pH 7.2; 5 min) before a return to baseline. Wilson et al. (2001) previously demonstrated that these procedures lead to brainstem tissue equilibration with the perfusate. The levels of O2 and CO2 in the perfusate were maintained by equilibrating a perfusate reservoir with gas mixtures produced with a precision gas mixer (GSM-2, CWE) and verified with a CO2 analyzer (CD-3A, Applied Electrochemistry). Lacking hemoglobin, solution hyperoxia (PO2 ≈ 600 mmHg) is necessary to maintain O2 content sufficient to meet tissue metabolic demands. This unavoidable hyperoxia was constant under all conditions. Baseline perfusate conditions approximated normocapnic plasma in vivo, and hypercapnic challenges produced conditions similar to those of plasma during a 4% elevation in inspired CO2.
Extracellular recording
Extracellular recordings of medullary raphé neurons were made using pulled glass capillary electrodes (15 – 40 MΩ), filled with biotinamide hydrobromide (Life Technologies) dissolved at 5% in 0.5M sodium acetate. We targeted regions of the medullary raphé (including the raphé obscurus, raphé magnus, and raphé pallidus) along the midline (0–0.1 mm lateral) between 0 and 3.25 mm caudal to interaural line, 10–12 mm below the dorsal surface. These are areas from which CO2 sensitive neurons have been identified in vitro. Electrodes were placed above raphé target areas and driven into the tissue using a fine stepping motor (2 µm steps; Burleigh Inchworm) held in a stereotaxic 5-axes micropositioner integrated with a digital brain atlas (Benchmark Angle Two; MyNeuroLab). Baseline firing was recorded for each unit in normocapnia, followed by a 5 min hypercapnic challenge, and then a 5 min minimum normocapnic recovery period. Electrodes were connected to an Axon Multiclamp 700B intracellular amplifier (Molecular Devices) with high pass filter at 300 Hz and low pass filter at 1 kHz bessel via an Axon CV7B high impedance headstage (Molecular Devices). Signals were digitized using Spike 2 (CED) or LabChart (AD Instruments), sampled (> 10 kHz) and stored as computer data files for subsequent analysis.
Biotinamide fills
Extracellular recordings were made with an intracellular amplifier (Axon Multiclamp 700B) in current clamp mode, so that current could be injected through the electrode while action potentials (extracellular field potentials) were monitored. Neurons were indiscriminately assessed for response to hypercapnia. After the completion of the trial, if the neuron’s signal amplitude was sufficiently large, we attempted to individually fill the neuron with biotinamide using the juxtacellular labeling method (Pinault, 1996; Winkler et al., 2006). Recorded neurons were individually filled with biotinamide by applying positive-current pulses (400 ms duration, 50% duty cycle) of gradually increasing intensity (0–10 nA max in 0.2 nA steps) to each cell through the bridge circuit of the recording amplifier until entrainment of cell discharge to the current pulse was achieved. Cell entrainment was maintained for at least 30 s. These current pulses trigger the iontophoretic ejection of biotinamide and entrainment facilitates uptake of this marker by the recorded and entrained cell. Entrainment was never initiated when multiple units were visible, and double neuron or ectopic labeling was not observed. 30 min after termination of entrainment were allowed for biotinamide to disperse within the neuron before tissue fixation. The stereotaxic coordinates of the recording site were noted. During recording of single cells, fluctuations in spike amplitude were sometimes observed due to slight changes in relative extracellular position of the electrode, and had no correlation with gas treatment or cell discharge. Spike height, width, and shape were monitored before, during, and after juxtacellular entrainment to ensure that only one cell was recorded and labeled (Pinault, 1996; Winkler et al., 2006).
Neurons were selected for recording and hypercapnic challenges when we were confident that the electrode tip was located within the medullary raphé, the recorded unit was firing spontaneously, and the unit’s signal amplitude was sufficiently large to ensure a satisfactory recording. No discrimination was made based on unit firing characteristics or chemosensitivity. Chemosensitivity was assessed offline, and was not always obvious during the hyper-capnic trials. In this sense, cells were recorded and filled indiscriminately, based only on likelihood of being in the medullary raphé and exhibiting adequate spontaneous discharge. Filled cells included a wide variety of sizes and morphology, and we observed no obvious bias concerning which cells were successfully visualized versus those that were not. Therefore, visualized cells should comprise a population representative of the targeted medullary raphé regions.
Immunohistochemistry
After juxtacellular labeling, rats were perfused through the descending aorta with fixative, 4% paraformaldehyde in 0.1M PBS. Brainstems were removed and stored overnight in the fixative, cry-oprotected with 30% sucrose/PBS until infiltrated, and frozen in hexanes cooled with an ethanol/dry ice slurry. A series of coronal sections (30 µm) were cut through the medulla using a freezing microtome (cryostat), and mounted directly onto slides. Biotinamide introduced into single neurons by juxtacellular labeling was revealed with a streptavidin-Alexa 546 conjugate (Life Technologies #S-11225; 4µg/mL). Sections were incubated in blocking buffer for 1 h (0.3% Triton X-100, 5% normal goat serum in 0.1M PBS) then overnight in antibody for the 5-HT-synthesizing enzyme tryptophan hydroxylase (TPH). We used mouse anti-TPH monocolonal primary antibody (Sigma #T0768); 1:1000 dilution in blocking buffer) followed by 1 h incubation in a secondary Alexa 488-labeled goat anti-mouse antibody (Life Technologies #A11029; 1:500 dilution in 0.1M PBS with 5% normal goat serum). Some sections were also separately incubated with a rabbit polyclonal anti-neurokinin 1 receptor (NK1R) antibody (Advanced Targeting Systems #AB-N33AP; 1:500) followed by secondary Alexa 647-labeled goat anti-rabbit (Life Technologies #A21244; 1:500 dilution). Immunohistochemical controls included incubation of medullary sections without primary antibody to rule out non-specific binding of the fluorophores and incubation without fluorophores to rule out autofluorescence. We did not observe TPH or NK1R immmunoreactivity in areas known to lack these markers. Sections were air-dried, mounted with Vectashield (Vector Labs) and coverslipped. Low-magnification (10×) images were used to determine the location of biotinamide-labeled cells in relation to anatomical landmarks (ventral surface, pyramids, etc.), this placement was correlated with areas of the raphé and cells were mapped onto the brain atlas at the appropriate location. Local biotinamide-, TPH- and NK1R-related fluorescence was visualized to identify absence or presence of colocalization of TPH in the soma or NK1R on the membrane of biotinamide-labeled neurons. Fluorophores were individually excited and emission spectra were collected separately to minimize interference using a Zeiss LSM510 confocal microscope: biotin-filled neuron, Alexa 546, 543nm laser, filter BP 560–615; anti-TPH, Alexa 488, 488nm laser, filter BP505–530; anti-NK1R, Alexa 647, 633nm laser, filter LP650. Images at one focal plane were collected with a 10× objective and z-stacks were collected with a 40× objective. 40× images are presented as a collapsed projection of a z-stack.
Data analysis
We discriminated individual extracellular unit activity using computer spike sorting software (Spike 2, CED; Spike Histogram, AD Instruments). Stable 1- to 3-min periods of single unit firing were analyzed before, during, and after hypercapnic challenge (“baseline”, “hypercapnia”, and “recovery”, respectively) to provide a mean value for unit firing frequency (spikes/s), mean interspike interval (ms), standard deviation and standard error of mean interspike interval, and spike width. If a neuron responded to hypercapnic perfusate with a change in firing frequency greater than 20% relative to baseline and returned toward baseline upon return to normocapnia, the neuron was considered chemosensitive and the recording continued (Wang et al., 1998). We recorded 323 individual cells throughout gas challenges. Preliminary accounts of CO2-inhibited cells have been reported and are not considered here (Iceman et al., 2012). The regularity and frequency of neuronal spikes were assessed over the 1–3 min of observation using a modification of a method developed for identification of 5-HT neurons during extracellular recordings from anesthetized rats (Mason, 1997). A modification of these along with other criteria for unanesthetized cats (Veasey et al., 1995) can identify 5-HT neurons based on electrophysiological characteristics with approximately 90% accuracy in vivo (Mulkey et al., 2004). Statistical differences were calculated using a two-way repeated measures ANOVA (one factor repetition) with Holm-Sidak pairwise multiple comparison procedures or a Mann-Whitney Rank Sum test. Overall significance level was set to p ≤0.05 (SigmaPlot 12). Values are expressed as means ± standard error of the mean.
Results
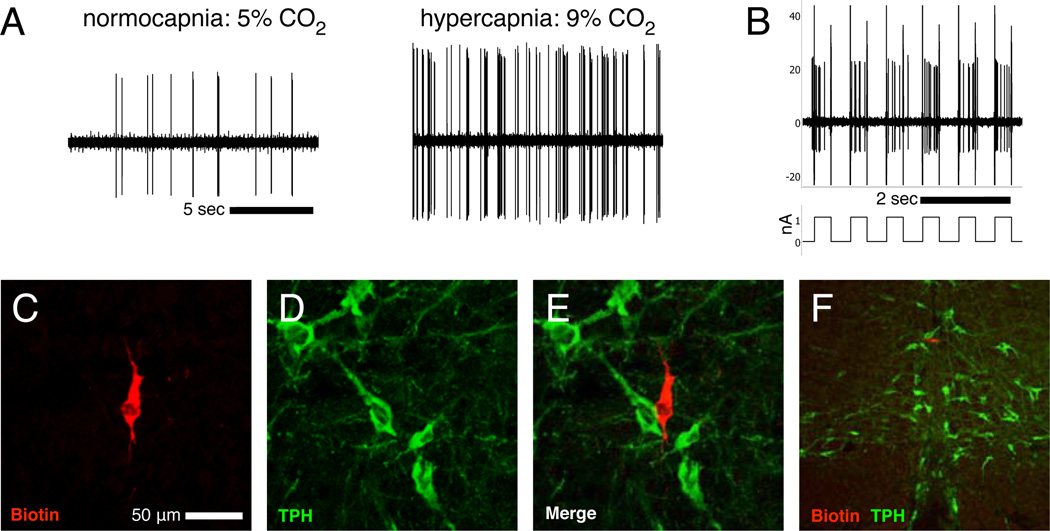
Electrophysiological characterization of neuronal chemosensitivity was obtained by extracellular recording of 323 spontaneously active individual medullary raphé neurons during CO2 challenges. We designated 70 cells (22%) as CO2-stimulated and 63 cells (19%) as CO2-inhibited (changed firing rate during hypercapnia >20% relative to baseline). The 190 remaining cells (59%) were classified as insensitive to our CO2 manipulations. 23 of the stimulated cells were subsequently juxtacellularly filled, histologically processed, and successfully visualized to determine positive or negative immunoreactivity for the 5-HT-synthesizing enzyme tryptophan hydroxylase (TPH-ir). A representative CO2-stimulated non-5-HT neuron is illustrated in Figure 1.
Figure 1. Extracellular recording of a CO2-stimulated non-5-HT neuron.
Recordings (A) during normocapnia and hypercapnia demonstrate a CO2-stimulated cell, which was juxtacellularly entrained (B) with 400 ms positive current pulses of ~1.2 nAmp and filled with biotinamide as a result. The biotinamide fill (C; red) and TPH-ir (D; green) were visualized after histological processing to reveal the recorded neuron as non-serotonergic (E). A low magnification photomicrograph of a coronal section (F; ventral surface visible at bottom) shows the cell location within the raphé magnus, near the midline. Height and width analysis of individual spikes (not shown) confirmed recording of the same individual neuron throughout CO2 exposure and juxtacellular labeling.
The medullary raphé contains a variety of CO2-stimulated neuron types
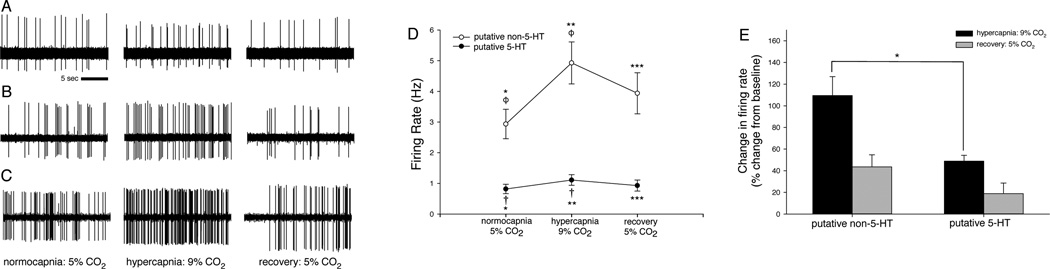
Single unit extracellular recordings from individual medullary raphé neurons demonstrated distinct subclasses of neurons based on firing characteristics and responses to hypercapnia. Cell firing frequency and regularity (Fornal et al., 1985; Mason 1997) were used to classify neurons and categorize them into putative 5-HT (n=24) and non-5-HT (n=46) groups. Representative recordings (Fig. 2) illustrate chemosensitivity of a 5-HT neuron and two non-5-HT neurons (characterized as putative 5-HT or non-5-HT based on firing characteristics and confirmed as such by juxtacellular labeling). The 5-HT neuron (Fig 2a) had baseline, hypercapnic, and recovery firing rates of 0.72 Hz, 0.99 Hz, and 0.68 Hz, respectively: a 38% hypercapnic response. The non-5-HT neuron in Fig. 2b had firing rates of 1.24 Hz, 1.93 Hz, and 0.73 Hz: a 56% hypercapnic response. Another non-5-HT neuron (Fig. 2c) had firing rates of 2.74 Hz, 5.53 Hz, and 1.93 Hz: a 101% hypercapnic response.
Figure 2. CO2-stimulated putative 5-HT and non-5-HT cells are distinct, and putative non-5-HT cells are more robustly stimulated by CO2 than are putative 5-HT cells.
(A–C) Recordings from single CO2-stimulated raphé neurons show spontaneous firing during exposure to 5% arterial CO2 (left column), a >20% increase in firing frequency with exposure to 9% CO2 (center column), and recovery with return to 5% CO2 conditions (right column). (A) A 5-HT neuron increased firing by 38% during hypercapnia. (B) A non-5-HT neuron had a 56% hypercapnic response, and another non-5-HT neuron (C) had a 101% hypercapnic response. (D) In CO2-stimulated cells, hypercapnia caused a mean 49% increase in firing rate of putative 5-HT cells (filled circles, †), and a mean 110% increase in firing rate of putative non-5-HT cells (open circles, ϕ). Normocapnic recovery returned firing frequencies to baseline levels. Firing frequencies differed between CO2-stimulated putative 5-HT and non-5-HT neuron groups during all gas conditions (∗, ∗∗, ∗ ∗ ∗). (E) Hypercapnic firing frequencies normalized to respective baseline firing frequencies confirm differences in hypercapnic responses between the CO2-stimulated putative 5-HT and non-5-HT neuron groups (∗). Symbols denote p <0.05 between means (e.g. means labeled “*” differ from each other, means labeled “†” differ from each other, etc.).
CO2-stimulated putative 5-HT and non-5-HT cells are distinct, and have different baseline, hypercapnic, and recovery firing frequencies
The average firing frequencies during baseline, hypercapnia, and recovery were 1.03 ± 0.19 Hz, 1.41 ± 0.23 Hz, and 1.18 ± 0.23 Hz, respectively for the putative 5-HT group (n=24), and 2.94 ± 1.48 Hz, 4.93 ± 0.69 Hz, and 3.99 ± 0.67 Hz for the putative non-5-HT group (n=46; Fig. 2e). Mean hypercapnic firing frequencies differed from baseline frequencies within each group, confirming our designation of these cells as CO2-stimulated (F2,135 = 17.559, p < 0.001). Mean baseline, hypercapnic, and recovery firing frequencies differed between the groups, indicating distinction in firing characteristics between the putative 5-HT and non-5-HT groups (F1,68 = 10.443, p = 0.002).
Putative non-5-HT cells are more robustly stimulated by CO2 than putative 5-HT cells
Results of a two-way repeated measures ANOVA indicate a difference in hypercapnic response between the groups, illustrating that the putative non-5-HT cells had a more robust hypercapnic sensitivity on average (F2,135 = 8.133, p < 0.001). Figure 2e shows firing frequencies expressed proportional to baseline normocapnic firing frequency (0% = baseline) for each treatment (baseline normocapnic, hypercapnic, and normocapnic recovery). Putative 5-HT neurons increased firing by a mean 49% during hypercapnia, while putative non-5-HT neurons increased firing by a mean 110%, displaying twice the hypercapnic reactivity of the putative 5-HT group. The difference (∗, U = 333.00, p = 0.007) in proportional hypercapnic response between the groups confirms the greater hypercapnic responsiveness of the putative non-5-HT cells.
The medullary raphé contains CO2-stimulated non-5-HT neurons that embed with raphé 5-HT neurons
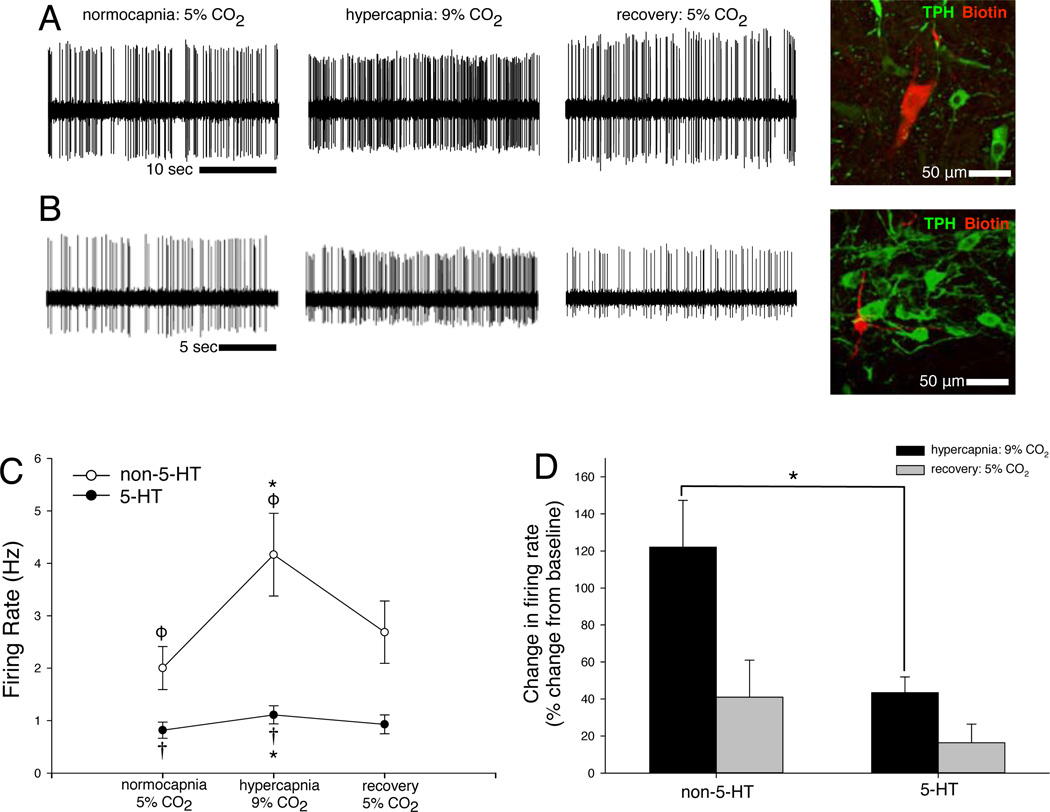
Of the 23 electrophysiologically characterized CO2-stimulated cells that were juxtacellularly filled, subsequently recovered, and successfully visualized, 7 were serotonergic, based on immunoreactivity for tryptophan hydroxylase (TPH-ir; Iceman et al., 2013). 16 cells were negative for TPH-ir, identifying them as non-serotonergic. CO2-stimulated non-5-HT cells, suggested not to be 5-HT based on firing analysis and confirmed as non-5-HT by juxtacellular labeling, were always revealed among a bed of raphé TPH-ir (5-HT) cells. Two such cells are illustrated in Figure 3a–b.
Figure 3. Definitively phenotyped CO2-stimulated 5-HT and non-5-HT cells are distinct, and non-5-HT cells are more robustly stimulated by CO2 than are 5-HT cells.
A neuron (row A) had normocapnic and hypercapnic firing rates of 4.26 Hz and 8.45 Hz, respectively (a 98% increase with hypercapnia), and was subsequently filled and stained negative for TPH-ir. Another neuron (row B) had firing rates of 0.45 Hz and 1.21 Hz (a 123% increase), and was also negative for TPH-ir. Both cells were within the raphé and closely apposed to several TPH-ir 5-HT cells. (D) In definitively phenotyped CO2-stimulated cells, hypercapnia caused a mean 44% increase in firing rate of 5-HT cells (filled circles, †), and a mean 125% increase in firing rate of non-5-HT cells (open circles, ϕ). Normocapnic recovery returned firing frequencies to baseline levels. Firing frequencies differed between CO2-stimulated putative 5-HT and non-5-HT neuron groups during hypercapnia (∗). (E) Hypercapnic firing frequencies normalized to respective baseline firing frequencies confirm differences in hypercapnic responses between the CO2-stimulated 5-HT and non-5-HT neuron groups (∗). Symbols denote p < 0.05 between means (e.g. means labeled “*” differ from each other, means labeled “†” differ from each other, etc.).
Definitively phenotyped CO2-stimulated non-5-HT cells have different hypercapnic and recovery firing frequencies and more robust CO2 sensitivity than CO2-stimulated 5-HT cells
When we considered only the 23 cells that were juxtacellularly filled and immunostained (Fig. 3c–d), similar trends to those illustrated in Figure 2d–e are apparent for definitively phenotyped non-5-HT and 5-HT cells. In definitively phenotyped cells, mean firing rates during baseline, hypercapnia, and recovery were 2.00 ± 0.41 Hz, 4.17 ± 0.79 Hz, and 2.69 ± 0.60 Hz for the non-5-HT group (n=16), and 0.88 ± 0.15 Hz, 1.11 ± 0.17 Hz, and 0.93 ± 0.18 Hz for the 5-HT group (n=7; Fig 3d).
As was evident in the previous classification (Fig. 2d), mean hypercapnic firing frequencies differed from baseline frequencies within each group, confirming a significant hypercapnic response within both non-5-HT and 5-HT groups (F2,42 = 8.252, p < 0.001). Also, in both groups, recovery firing frequencies differed from hypercapnic frequencies (p < 0.01) and were no different from baselines, confirming a return to baseline firing rate after 5 min of recovery normocapnia. Mean firing frequencies were also different between groups (F1, 21 = 5.126, p = 0.034).
As was evident in the previous classification (Fig. 2e), there was a difference in hypercapnic response between the non-5-HT (125% increase with hypercapnia) and 5-HT (44% increase with hypercapnia) groups (F2,42 = 4.828, p = 0.013). Figure 3d shows hypercapnic firing frequencies of definitively phenotyped non-5-HT (n=16) and 5-HT (n=7) cells expressed as a proportion of their baseline firing frequencies. These data illustrate that the non-5-HT group display almost triple the hypercapnic response of the 5-HT group (∗, U = 20.00, p = 0.018). Overall, the trends apparent in the subset of cells classified as non-5-HT or 5-HT by juxtacellular filling and staining are the same as those derived from the larger set of cells classified by firing pattern alone, confirming the utility of classifying unlabeled cells into “putative non-5-HT” and “putative 5-HT” groups by firing pattern characteristics (Mason 1997).
TPH and NK1R are expressed in the medullary raphé but do not co-localize and the medullary raphé contains CO2-stimulated non-5-HT cells that express NK1R
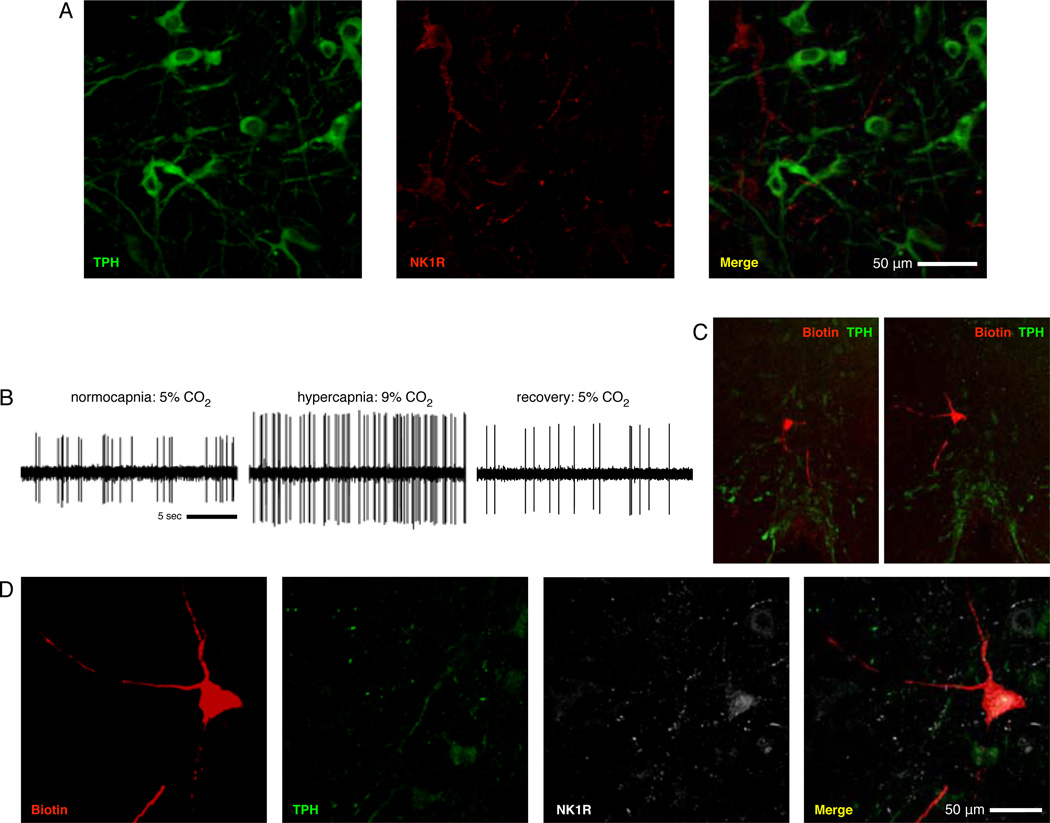
We found TPH-ir and NK1R-ir cells in the medullary raphé, but these markers did not co-localize within individual cells (Fig. 4a). Of the CO2-stimulated cells that were negative for TPH-ir, we subsequently stained a subset (n=6) for NK1R-ir. We identified CO2-stimulated raphé neurons (Fig. 4b–c) negative for TPH-ir but positive for NK1R-ir (n=6/6; Fig. 4d). These neurons neighbored raphé 5-HT/substance P synthesizing cells (Fig. 4c). This pattern of receptor expression and dendritic arborization suggests the CO2-stimulated non-5-HT cells may be interneurons receiving input from neighboring raphé 5-HT/substance P synthesizing cells.
Figure 4. TPH and NK1R do not colocalize in the medullary raphé, and the medullary raphé contains CO2-stimulated non-5-HT cells that express NK1R.
(A) Shown are TPH (green) and NK1 receptor (red) immunostaining in the raphé magnus. Immunoreactivity of these two markers were never observed to colocalize in any of our tested sections. (B) Shown is a cell that increased firing frequency by 82% with hypercapnia. 10× views of two adjacent sections (C; ventral surface visible at bottom) show the juxtacellular fill and extensive dendritic processes of the recorded neuron (red) in raphé magnus and tissue staining for TPH-ir (green). 40× views of the filled cell (D) demonstrate that the recorded cell lacks TPH-ir seen in neighboring cells (green) but is NK1R-ir (white), indicating expression of NK1R by the recorded cell.
Discussion
We describe for the first time individually characterized CO2-stimulated non-5-HT neurons in the medullary raphé in situ, and we show that they express receptors for substance P. These non-serotonergic cells had faster firing frequencies and larger hypercapnic responses than raphé 5-HT neurons in situ. The non-5-HT cells had high baseline firing frequencies, with firing rates up to 6.1 Hz for cells known not to synthesize 5-HT based on negative TPH-ir (Fig. 3c). Baseline firing frequencies of CO2-stimulated 5-HT (TPH-ir) cells were never observed in excess of 1.49 Hz. All labeled 5-HT cells displayed spontaneous slow, regular firing, stereotypical of 5-HT neurons (Mason, 1997). In no case did a cell displaying spontaneous firing characteristic of a non-5-HT neuron subsequently present immunoreactivity for TPH. CO2-stimulated non-5-HT neurons were located throughout the medullary raphé and, unlike CO2-stimulated 5-HT neurons, they do not appear to be preferentially located in a particular nucleus or area of the raphé (Ray et al., 2011; Iceman et al., 2013).
Our staining indicates that markers for 5-HT synthesis do not colocalize with NK1R (the receptor for substance P) in the medullary raphé (Fig. 4a), consistent with reports of others (Léger et al., 2002; Nattie et al., 2004; Commons, 2009). Substance P is a known modulator of breathing (Ptak et al., 2009; Doi and Ramirez, 2010), and NK1R are highly expressed in brain regions known to be important in the hypercapnic response, including the retrotrapezoid nucleus, locus coeruleus, and the preBötzinger complex/rostral ventral respiratory group (Chen et al., 2000; Gray et al., 2001; Stornetta et al., 2006). Toxic lesion of NK1R expressing neurons in the ventral medulla caused hypoventilation, reduced hypercapnic ventilatory response, and perturbed hypoxic responses (Nattie and Li, 2006). Therefore, NK1R expressing neurons compose a group of cells that contribute to normal ventilation and chemoresponses.
Hodges et al. (2004) injected either SP-SAP (targeting NK1R expressing neurons) or ibotenic acid (targeting glutamate receptors and producing nonspecific neurotoxicity) into medullary raphé of goats. SP-SAP reduced the hypercapnic response by 24% (resulting from specific loss of NK1R-expressing cells) while ibotenic acid reduced the hypercapnic response by 27% (resulting from the loss of multiple raphé neuron types, including both NK1R-ir and TPH-ir cells). These results suggest that partial destruction of NK1R expressing raphé neurons is sufficient to induce a deficit in CO2 responsivity comparable to the deficit induced by more general raphé destruction.
Nattie and colleagues (2004) injected either SP-SAP, or an anti-serotonin reuptake transporter conjugated to SAP (anti-SERT-SAP, targeting 5-HT releasing cells), or a combination of both toxin conjugates into the medullary raphé of rats. SP-SAP or anti-SERT-SAP each decreased the hypercapnic response during both wakefulness and sleep. SP-SAP and anti-SERT-SAP coapplication produced similar reductions in NK1R-ir and TPH-ir neuron numbers as did individual treatments, but did not enhance the hypercapnic response deficit from that produced by either individual treatment. These results demonstrated that partial destruction of 5-HT neurons and NK1R expressing neurons in the raphé was not additive. The authors concluded that both groups of neurons are important for the hypercapnic response. As with destruction of any single putative CO2-sensitive nucleus or cell type, selective impairment of the raph é or its individual components does not eliminate the ventilatory response to hypercapnia, because it is a complex response to which many cell types contribute. We propose that the two groups of neurons identified in our current study represent the same groups as those identified by Nattie et al. (2004).
Non-5-HT CO2-inhibited neurons (GABAergic) are plentiful in raphé culture, acute slice, and in situ (Richerson 1995; Wang et al., 1998; 1999; 2001; Hodges et al., 2005; Iceman et al., 2012). The CO2-stimulated non-5-HT cells described here are surrounded by 5-HT neurons and are likely also surrounded by GABA neurons. Given this, and because they express receptors for substance P, it is reasonable to hypothesize that they may be receiving input from the CO2-stimulated 5-HT/substance P cells and might receive input from the CO2-inhibited cells. Since raphé CO2 -stimulated 5-HT and CO2-inhibited cells are intrinsically chemosensitive in acute dissociated culture conditions, they are candidates for 1st order sensory neurons. A seemingly universal feature of all other sensory systems is the convergence of 1st order sensory neurons (responsible for sensory transduction) onto 2nd order neurons (consolidating sensory inputs) prior to transmission to higher sensors and effector systems. Raphé 5-HT and GABA neurons do project to major homeostatic integration and respiratory control centers of the midbrain, brainstem, and spinal cord (Skagerberg and Björklund, 1985; Cao et al., 2006), but the potential targets of the CO2-stimulated non-5-HT cells are unknown. Nattie et al. (2004) suggested one possible relationship in which chemosensitive 5-HT neurons affect NK1R expressing neurons downstream, ultimately enhancing the hypercapnic ventilatory response. When either or both of these cell types were silenced, the same attenuation of the hypercapnic response was observed, suggesting a serial (1st order and 2nd order) rather than an additive (both cell types 1st order) relationship.
Rice and colleagues (2009) applied rabies virus to the diaphragm of cats in a retrograde tracing study. Unsurprisingly, serotonergic cells in the brainstem (including raphé) were labeled, identifying them as one source of efferent diaphragmatic innervations. However, the majority of labeled raphé cells were non-serotonergic. It is reasonable to suppose that whatever their identity, those cells modulate breathing. Pete et al. (2002) found hypercapnic c-Fos activation in raphé cells that express preprotachykinin mRNA, a precursor for substance P. c-Fos staining was also found in other raphé neurons that did not express this marker (did not synthesize substance P). Haxhiu et al. (2001) found similar hypercapnic c-Fos activation in the medullary raphé, occurring in both 5-HT and in non-5-HT cells. It is possible that the latter cells are the same population of hypercapnia-activated 5-HT neurons described here, and by Nattie et al., (2004) and Hodges et al. (2004). The above studies support our interpretation that at least two distinct groups of raphé cells are activated by hypercapnia: 5-HT/SP, and non-5-HT/SP, and that the raphé provides both non-5-HT and 5-HT innervation to the diaphragm. The functional inputs to and outputs from the non-5-HT cells we report remain to be tested.
The response of conclusively identified 5-HT cells to CO2 in culture is much greater than that observed in situ, in acute slice, or in anesthetized preparations (Richerson, 1995; Mulkey et al., 2004; DePuy et al., 2011). In these less responsive preparations, 5-HT neurons display a relatively modest average response to CO2 (all less than 100% increase from baseline). Wang et al. (1998; 2001) report that 75–90% of 5-HT neurons in culture are stimulated by CO2, and exhibit a two- to three-fold increase in firing rate. In contrast, just under half of neurons that we have conclusively identified as 5-HT (TPH-ir) were CO2-stimulated in situ, with only a 43% mean increase in firing rate (Iceman et al., 2013). Furthermore, most putative 5-HT neurons (not conclusively identified as such) do not exhibit chemosensitivity in situ, in acute slice, or in vivo (Richerson, 1995; Veasey et al., 1995; 1997; Wang and Richerson, 1999; Iceman et al., 2013). It is unknown which preparation(s) are most likely to represent the endogenous activity of 5-HT neurons, but the relevance of neuronal behavior observed in situ can be validated by comparison with single unit recordings in chronically instrumented awake, freely moving animals (Veasey et al., 1995; 1997). 31% of raphé neurons are stimulated by CO2 in situ and 22% are stimulated by CO2 in vivo. Of those CO2-stimulated neurons, the average degree of responsiveness is 43% in situ (in response to a 4% increase in arterial PCO2), and ~35% in vivo (in response to a similar 4% increase in inspired CO2).
We propose that variation in GABAergic inhibition is responsible in part for the discrepancy of these results. Raphé 5-HT neurons express GABAA and GABAB receptors, and are tonically inhibited by their activation (Gallager and Aghajanian, 1976; Bowery et al., 1987; Tao et al., 1996; Abellán et al., 2000; Bagdy et al., 2000; Boothman et al., 2006; Templin et al., 2012). Inhibitory postsynaptic currents in raphé 5-HT cells are mediated by GABAA receptors (Inyushkin et al., 2010). Allers and Sharp (2003) described dorsal raphé GABA neurons that branch extensively amongst multiple raphé nuclei, sometimes crossing the midline, and contacting various raphé 5-HT neurons. Thus, both anatomic and functional data illustrate that raphé GABAergic neurons inhibit 5-HT neurons. Raphé 5-HT and GABA neurons also share a reciprocal connection, which would provide a negative feedback loop to inhibit 5-HT release (Bagdy et al., 2000; Richardson-Jones et al., 2011). When the CO2 response of raphé neurons was tested in culture, recordings were made with antagonists for NMDA, AMPA, and GABAA receptors. In culture, firing of putative 5-HT raphé neurons increased by 87% on average after GABAA antagonism, indicating that firing was suppressed by GABAA-mediated inhibition (Wang et al., 1998). We propose that 5-HT neurons may have a “ceiling” firing rate, which they are unlikely to exceed endogenously (Iceman et al., 2013). Tonic GABA inhibition could be partly responsible for this ceiling effect and be one means by which 5-HT neuron discharge frequency is kept within the narrow range that is characteristic of serotonergic neurons in vivo. Removal of tonic GABA inhibition could also account for the more robust chemosensitivity observed in 5-HT raphé cells in vitro, as GABAA receptors are antagonized in those recordings. In the present study, 23% of the CO2-stimulated cells were TPH-ir (7 of 23 immunostained cells). In raphé culture, 100% of CO2-stimulated neurons are TPH-ir. The CO2-stimulated non-5-HT neurons we observe either do not thrive in culture, or culture conditions (synaptic blockade) prevent such cells from demonstrating chemoresponsiveness (Wang and Richerson, 1999; Wang et al., 2001; Bradley et al., 2002; Severson et al., 2003).
The varied functions of the raphé in maintaining homeostasis and the body of raphé literature suggest that collectively, raphé neurons could function as a precisely tuned, highly feedback-regulated tonic modulator of many physiological processes. In addition to inhibition by GABA neurons, 5-HT neurons are thought to interact with raphé NK1R expressing neurons in a reciprocal fashion (reviewed in Valentino and Commons, 2005) to fine-tune the serotonin system, eventually culminating in 5-HT autoinhibition (Liu et al., 2002; Valentino et al., 2003; Soiza-Reilly and Commons, 2011). In this way, serotonergic tone can be tightly regulated, and the negative consequence of ungoverned excitation of 5-HT neurons prevented (Sternbach, 1991). Network-mediated governance could explain the relative sensitivities of raphé cells and the modest chemoresponsiveness of 5-HT neurons in situ and in vivo.
We have demonstrated that CO2-stimulated cells are present in the medullary raphé, and that these include two distinct classes: modestly stimulated 5-HT, and robustly stimulated non-5-HT neurons. The CO2-stimulated non-5-HT neurons constitute a previously unrecognized class of chemosensitive raphé neuron. The functional outputs of chemosensitive raphé cells and their relationships with each other remain to be discovered.
Some raphé serotonergic neurons are stimulated by CO2
We describe non-serotonergic raphé neurons that are robustly CO2-stimulated
Distinct groups of chemosensitive raphé neurons may influence respiration
Acknowledgments
Research supported by NIH 2U54NS041069-06A1 and P20GM103395 (M.B.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abellán MT, Jolas T, Aghajanian GK, Artigas F. Dual control of dorsal raphe serotonergic neurons by GABAA receptors. Electrophysiological and microdialysis studies. Synapse. 2000;36:2134. doi: 10.1002/(SICI)1098-2396(200004)36:1<21::AID-SYN3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Allers KA, Sharp T. Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphé nucleus using juxtacellular labelling methods in vivo. Neuroscience. 2003;122:193–204. doi: 10.1016/s0306-4522(03)00518-9. [DOI] [PubMed] [Google Scholar]
- Bagdy E, Kiraly I, Harsing LG. Reciprocal innervation between serotonergic and GABAergic neurons in raphé nuclei of the rat. Neurochem Res. 2000;25:1465–1473. doi: 10.1023/a:1007672008297. [DOI] [PubMed] [Google Scholar]
- Boothman LJ, Sharp T. A role for midbrain raphé gamma aminobutyric acid neurons in 5-hydroxytryptamine feedback control. Neuroreport. 2005;16:891–896. doi: 10.1097/00001756-200506210-00004. [DOI] [PubMed] [Google Scholar]
- Boothman LJ, Allers KA, Rasmussen K, Sharp T. Evidence that central 5-HT2A and 5-HT2B/C receptors regulate 5-HT cell firing in the dorsal raphé nucleus of the anaesthetised rat. British Journal of Pharmacology. 2003;139:998–1004. doi: 10.1038/sj.bjp.0705328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothman L, Raley J, Denk F, Hirani E, Sharp T. In vivo evidence that 5-HT2C receptors inhibit 5-HT neuronal activity via a GABAergic mechanism. British Journal of Pharmacology. 2006;149:861–869. doi: 10.1038/sj.bjp.0706935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. NSC. 1987;20:365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat Neurosci. 2002;5:401–402. doi: 10.1038/nn848. [DOI] [PubMed] [Google Scholar]
- Broadbelt KG, Paterson DS, Belliveau RA, Trachtenberg FL, Haas EA, Stanley C, Krous HF, Kinney HC. Decreased GABAA receptor binding in the medullary serotonergic system in the sudden infant death syndrome. J. Neuropathol. Exp. Neurol. 2011;70:799–810. doi: 10.1097/NEN.0b013e31822c09bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calizo LH, Akanwa A, Ma X, Pan Y-Z, Lemos JC, Craige C, Heemstra LA, Beck SG. raphé serotonin neurons are not homogenous: electrophysiological, morphological and neurochemical evidence. Neuropharmacology. 2011;61:524–543. doi: 10.1016/j.neuropharm.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W-H, Morrison SF. Brown adipose tissue thermogenesis contributes to fentanyl-evoked hyperthermia. Am J Physiol Regul Integr Comp Physiol. 2005;288:R723–R732. doi: 10.1152/ajpregu.00669.2004. [DOI] [PubMed] [Google Scholar]
- Cao Y, Fujito Y, Matsuyama K, Aoki M. Effects of electrical stimulation of the medullary raphé nuclei on respiratory movement in rats. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:497–505. doi: 10.1007/s00359-005-0087-0. [DOI] [PubMed] [Google Scholar]
- Charara A, Parent A. Chemoarchitecture of the primate dorsal raphé nucleus. J Chem Neuroanat. 1998;15:111–127. doi: 10.1016/s0891-0618(98)00036-2. [DOI] [PubMed] [Google Scholar]
- Chen LW, Wei LC, Liu HL, Rao ZR. Noradrenergic neurons expressing substance P receptor (NK1) in the locus coeruleus complex: a double immunofluorescence study in the rat. Brain Res. 2000;873:155–159. doi: 10.1016/s0006-8993(00)02494-x. [DOI] [PubMed] [Google Scholar]
- Commons KG. Locally collateralizing glutamate neurons in the dorsal raphé nucleus responsive to substance P contain vesicular glutamate transporter 3 (VGLUT3) J Chem Neuroanat. 2009;38:273–281. doi: 10.1016/j.jchemneu.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran AE, Richerson GB, Harris MB. Serotonergic mechanisms are necessary for central respiratory chemoresponsiveness in situ. Respir Physiol Neurobiol. 2013;186:214–220. doi: 10.1016/j.resp.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol. 2009;168:49–58. doi: 10.1016/j.resp.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG. Control of breathing by raphé obscurus serotonergic neurons in mice. J Neurosci. 2011;31:1981–1990. doi: 10.1523/JNEUROSCI.4639-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMicco JA, Sarkar S, Zaretskaia MV, Zaretsky DV. Stress-induced cardiac stimulation and fever: common hypothalamic origins and brainstem mechanisms. Auton Neurosci. 2006;126–127:106–119. doi: 10.1016/j.autneu.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Doi A, Ramirez J-M. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J Neurosci. 2010;30:8251–8262. doi: 10.1523/JNEUROSCI.5361-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornal C, Auerbach S, Jacobs BL. Activity of serotonin-containing neurons in nucleus raphé magnus in freely moving cats. Exp Neurol. 1985;88:590–608. doi: 10.1016/0014-4886(85)90074-3. [DOI] [PubMed] [Google Scholar]
- Gallager DW, Aghajanian GK. Effect of antipsychotic drugs on the firing of dorsal raphé cells. II. Reversal by picrotoxin. Eur J Pharm. 1976;39:357–364. doi: 10.1016/0014-2999(76)90145-x. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Lillesaar C. Probing the diversity of serotonin neurons. Philos Trans R Soc Lond, B, Biol Sci. 2012;367:2382–2394. doi: 10.1098/rstb.2011.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxhiu MA, Tolentino-Silva F, Pete G, Kc P, Mack SO. Monoaminergic neurons, chemosensation and arousal. Respir Physiol. 2001;129:191–209. doi: 10.1016/s0034-5687(01)00290-0. [DOI] [PubMed] [Google Scholar]
- Hellman KM, Brink TS, Mason P. Activity of murine raphé magnus cells predicts tachypnea and on-going nociceptive responsiveness. J Neurophysiol. 2007;98:3121–3133. doi: 10.1152/jn.00904.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. The role of medullary serotonin (5-HT) neurons in respiratory control: contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. J Appl Physiol. 2010a;108:1425–1432. doi: 10.1152/japplphysiol.01270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Medullary serotonin neurons and their roles in central respiratory chemoreception. Respir Physiol Neurobiol. 2010b;173:256–263. doi: 10.1016/j.resp.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Opansky C, Qian B, Davis S, Bonis J, Bastasic J, Leekley T, Pan LG, Forster HV. Transient attenuation of CO2 sensitivity after neurotoxic lesions in the medullary raphé area of awake goats. J Appl Physiol. 2004;97:2236–2247. doi: 10.1152/japplphysiol.00584.2004. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen Z-F, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Wang W, Richerson GB. Acidosis-inhibited raphé neurons are GABAergic. FASEB J. 2005;19:369.21. [Google Scholar]
- Iceman KE, Richerson GB, Harris MB. Medullary serotonin neurons are CO2-sensitive in situ. J Neurophysiol. doi: 10.1152/jn.00288.2013. Published ahead of print September 18, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iceman KE, Richerson GB, Harris MB. GABAergic neurons in the medullary raphé possess network independent chemosensitivity in situ. FASEB J. 2012;26:894.13. [Google Scholar]
- Inyushkin AN, Merkulova NA, Orlova AO, Inyushkina EM. Local GABAergic modulation of the activity of serotoninergic neurons in the nucleus raphe magnus. Neurosci Behav Physiol. 2010;40:885–893. doi: 10.1007/s11055-010-9337-x. [DOI] [PubMed] [Google Scholar]
- Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM. Redefining the serotonergic system by genetic lineage. Nat Neurosci. 2008;11:417–419. doi: 10.1038/nn2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC. Brainstem mechanisms underlying the sudden infant death syndrome: evidence from human pathologic studies. Dev Psychobiol. 2009;51:223–233. doi: 10.1002/dev.20367. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Thach BT. The sudden infant death syndrome. N. Engl. J. Med. 2009;361:795–805. doi: 10.1056/NEJMra0803836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Pernar L, Valentino RJ, Beck SG. Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphé nucleus: electrophysiological and immunohistochemical studies. Neuroscience. 2003;116:669–683. doi: 10.1016/s0306-4522(02)00584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyasova V, Gaspar P. Development of raphé serotonin neurons from specification to guidance. Eur J Neurosci. 2011;34:1553–1562. doi: 10.1111/j.1460-9568.2011.07910.x. [DOI] [PubMed] [Google Scholar]
- Léger L, Gay N, Cespuglio R. Neurokinin NK1- and NK3-immunoreactive neurons in serotonergic cell groups in the rat brain. Neurosci Lett. 2002;323:146–150. doi: 10.1016/s0304-3940(01)02543-5. [DOI] [PubMed] [Google Scholar]
- Liu R, Jolas T, Aghajanian G. Serotonin 5-HT2 receptors activate local GABA inhibitory inputs to serotonergic neurons of the dorsal raphé nucleus. Brain Res. 2000;873:34–45. doi: 10.1016/s0006-8993(00)02468-9. [DOI] [PubMed] [Google Scholar]
- Liu R, Ding Y, Aghajanian GK. Neurokinins activate local glutamatergic inputs to serotonergic neurons of the dorsal raphé nucleus. Neuropsychopharmacology. 2002;27:329–340. doi: 10.1016/S0893-133X(02)00305-6. [DOI] [PubMed] [Google Scholar]
- Ljungdahl A, Hökfelt T, Nilsson G. Distribution of substance P-like immunoreactivity in the central nervous system of the rat–I. Cell bodies and nerve terminals. Neuroscience. 1978;3:861943. doi: 10.1016/0306-4522(78)90116-1. [DOI] [PubMed] [Google Scholar]
- Mason P. Physiological identification of pontomedullary serotonergic neurons in the rat. J Neurophysiol. 1997;77:1087–1098. doi: 10.1152/jn.1997.77.3.1087. [DOI] [PubMed] [Google Scholar]
- Mason P. From descending pain modulation to obesity via the medullary raphé. Pain. 2011;152:S20–S24. doi: 10.1016/j.pain.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhorn DE, Hökfelt T, Seroogy K, Verhofstad AA. Extent of colocalization of serotonin and GABA in neurons of the ventral medulla oblongata in rat. Brain Res. 1988;461:169–174. doi: 10.1016/0006-8993(88)90736-6. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Neurokinin-1 receptor-expressing neurons in the ventral medulla are essential for normal central and peripheral chemoreception in the conscious rat. J Appl Physiol. 2006;101:1596–1606. doi: 10.1152/japplphysiol.00347.2006. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A, Richerson GB, Richerson GB, Lappi DA. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol (Lond) 2004;556:235–253. doi: 10.1113/jphysiol.2003.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Pete G, Mack SO, Haxhiu MA, Walbaum S, Gauda EB. CO2-induced c-Fos expression in brainstem preprotachykinin mRNA containing neurons. Respir Physiol Neurobiol. 2002;130:265–274. doi: 10.1016/s0034-5687(02)00013-0. [DOI] [PubMed] [Google Scholar]
- Pinault D. A novel single-cell staining procedure performed in vivo under electrophysiological control: morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin. J Neurosci Methods. 1996;65:113–136. doi: 10.1016/0165-0270(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphé neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci. 2009;29:3720–3737. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CD, Lois JH, Kerman IA, Yates BJ. Localization of serotoninergic neurons that participate in regulating diaphragm activity in the cat. Brain Res. 2009;1279:71–81. doi: 10.1016/j.brainres.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Nguyen TH, Kung HF, Gardier AM, Dranovsky A, David DJ, Guiard BP, Beck SG, Hen R, Leonardo ED. Serotonin-1A Autoreceptors Are Necessary and Sufficient for the Normal Formation of Circuits Underlying Innate Anxiety. J Neurosci. 2011;31:6008–6018. doi: 10.1523/JNEUROSCI.5836-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB. Response to CO2 of neurons in the rostral ventral medulla in vitro. J Neurophysiol. 1995;73:933–944. doi: 10.1152/jn.1995.73.3.933. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat. Rev. Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Roberts C, Thomas DR, Bate ST, Kew JNC. GABAergic modulation of 5-HT7 receptor-mediated effects on 5-HT efflux in the guineapig dorsal raphé nucleus. Neuropharmacology. 2004;46:935–941. doi: 10.1016/j.neuropharm.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci. 2003;6:1139–1140. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- Sharp T, Boothman L, Raley J, Quérée P. Important messages in the “post”: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol. Sci. 2007;28:629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Skagerberg G, Björklund A. Topographic principles in the spinal projections of serotonergic and non-serotonergic brainstem neurons in the rat. NSC. 1985;15:445–480. doi: 10.1016/0306-4522(85)90225-8. [DOI] [PubMed] [Google Scholar]
- Soiza-Reilly M, Commons KG. Glutamatergic drive of the dorsal raphé nucleus. J Chem Neuroanat. 2011 doi: 10.1016/j.jchemneu.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148:705–713. doi: 10.1176/ajp.148.6.705. [DOI] [PubMed] [Google Scholar]
- Stornetta RL. Neurochemistry of bulbospinal presympathetic neurons of the medulla oblongata. J Chem Neuroanat. 2009;38:222–230. doi: 10.1016/j.jchemneu.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Ma Z, Auerbach SB. Differential regulation of 5-hydroxytryptamine release by GABAA and GABAB receptors in midbrain raphé nuclei and forebrain of rats. British Journal of Pharmacology. 1996;119:1375–1384. doi: 10.1111/j.1476-5381.1996.tb16049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templin JS, Bang SJ, Soiza-Reilly M, Berde CB, Commons KG. Patterned expression of ion channel genes in mouse dorsal raphé nucleus determined with the Allen Mouse Brain Atlas. Brain Res. 2012;1457:1–12. doi: 10.1016/j.brainres.2012.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppin VAL, Harris MB, Kober AM, Leiter JC, St John WM. Persistence of eupnea and gasping following blockade of both serotonin type 1 and 2 receptors in the in situ juvenile rat preparation. J Appl Physiol. 2007;103:220–227. doi: 10.1152/japplphysiol.00071.2007. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Commons KG. Peptides that fine-tune the serotonin system. Neuropeptides. 2005;39:1–8. doi: 10.1016/j.npep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Bey V, Pernar L, Commons KG. Substance P Acts through local circuits within the rat dorsal raphé nucleus to alter serotonergic neuronal activity. J Neurosci. 2003;23:7155–7159. doi: 10.1523/JNEUROSCI.23-18-07155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphé neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic dorsal raphé neurons to specific motor challenges in freely moving cats. NSC. 1997;79:161–169. doi: 10.1016/s0306-4522(96)00673-2. [DOI] [PubMed] [Google Scholar]
- Wang W, Richerson GB. Development of chemosensitivity of rat medullary raphé neurons. NSC. 1999;90:1001–1011. doi: 10.1016/s0306-4522(98)00505-3. [DOI] [PubMed] [Google Scholar]
- Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphé neurones in primary tissue culture. J Physiol (Lond) 1998;511(Pt 2):433–450. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphé are serotonergic. J Neurophysiol. 2001;85:2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- Wilson RJ, Remmers JE, Paton JF. Brain stem PO2 and pH of the working heart-brain stem preparation during vascular perfusion with aqueous medium. Am J Physiol Regul Integr Comp Physiol. 2001;281:R528–R538. doi: 10.1152/ajpregu.2001.281.2.R528. [DOI] [PubMed] [Google Scholar]
- Winkler CW, Hermes SM, Chavkin CI, Drake CT, Morrison SF, Aicher SA. Kappa opioid receptor (KOR) and GAD67 immunoreactivity are found in OFF and NEUTRAL cells in the rostral ventro-medial medulla. J Neurophysiol. 2006;96:3465–3473. doi: 10.1152/jn.00676.2006. [DOI] [PubMed] [Google Scholar]
- Zaretsky DV, Zaretskaia MV, DiMicco JA. Stimulation and blockade of GABAA receptors in the raphé pallidus: effects on body temperature, heart rate, and blood pressure in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2003a;285:R110–R116. doi: 10.1152/ajpregu.00016.2003. [DOI] [PubMed] [Google Scholar]
- Zaretsky DV, Zaretskaia MV, Samuels BC, Cluxton LK, DiMicco JA. Microinjection of muscimol into raphé pallidus suppresses tachycardia associated with air stress in conscious rats. J Physiol (Lond) 2003b;546:243–250. doi: 10.1113/jphysiol.2002.032201. [DOI] [PMC free article] [PubMed] [Google Scholar]